Published online Jul 7, 2005. doi: 10.3748/wjg.v11.i25.3842
Revised: September 3, 2004
Accepted: September 9, 2004
Published online: July 7, 2005
AIM: To determine if Fourier-transform infrared (FT-IR) spectroscopy of endoscopic biopsies could accurately diagnose gastritis and malignancy.
METHODS: A total of 123 gastroscopic samples, including 11 cases of cancerous tissues, 63 cases of chronic atrophic gastritis tissues, 47 cases of chronic superficial gastritis tissues and 2 cases of normal tissues, were obtained from the First Hospital of Xi’an Jiaotong University, China. A modified attenuated total reflectance (ATR) accessory was linked to a WQD-500 FT-IR spectrometer for spectral measurement followed by submission of the samples for pathologic analysis. The spectral characteristics for different types of gastroscopic tissues were summarized and correlated with the corresponding pathologic results.
RESULTS: Distinct differences were observed in the FT-IR spectra of normal, atrophic gastritis, superficial gastritis and malignant gastric tissues. The sensitivity of FT-IR for detection of gastric cancer, chronic atrophic gastritis and superficial gastritis was 90.9%, 82.5%, 91.5%, and specificity was 97.3%, 91.7%, 89.5% respectively.
CONCLUSION: FT-IR spectroscopy can distinguish gastric inflammation from malignancy.
- Citation: Li QB, Sun XJ, Xu YZ, Yang LM, Zhang YF, Weng SF, Shi JS, Wu JG. Use of Fourier-transform infrared spectroscopy to rapidly diagnose gastric endoscopic biopsies. World J Gastroenterol 2005; 11(25): 3842-3845
- URL: https://www.wjgnet.com/1007-9327/full/v11/i25/3842.htm
- DOI: https://dx.doi.org/10.3748/wjg.v11.i25.3842
Fourier-transform infrared (FT-IR) spectroscopy can effectively provide chemical variation information of the structure and composition of biologic materials at molecular level[1]. Therefore, vibrational spectroscopy is becoming an increasingly powerful tool for the research on biochemistry of cancer[2-5]. Our research group has successfully used FT-IR spectroscopy to diagnose carcinomas, such as carcinoma of stomach, colon, esophagus, lung, salivary gland since 1995[6-9]. There are significant differences between the spectra of malignant and corresponding normal tissues[10-12]. In addition, FT-IR spectroscopy could detect molecular abnormalities which occur before the change in morphology seen under light microscope[13]. Therefore, FT-IR technology makes it possible to detect inflammatory and precancerous changes. Rapid, accurate and convenient detection of gast-roscopic tissues can be performed using FT-IR spectroscopy if mid-infrared fiber optics technology and stomach endoscopy technology are combined, however the flexible mid-infrared optical fiber and mini probe are not yet available[14].
Informed consent was obtained from each patient prior to the study. A total of 123 fresh surgically resected gastric tissue specimens were obtained from the First Hospital of Xi’an Jiaotong University, China. There were 47 women and 76 men, aged between 18 and 80 years (mean 50.3 years). One endoscopic pinch biopsy, 1-3 mm in diameter, was obtained from each patient. The detected samples consisted of 11 cases of cancerous tissues, 63 cases of chronic atrophic gastritis tissues, 47 cases of chronic superficial gastritis tissues and 2 cases of normal tissues.
As the size of samples was too small to obtain an FT-IR spectrum with high quality, the modified ATR accessory linked to a WQD-500 FT-IR spectrometer was used. The FT-IR spectrometer was equipped with a liquid nitrogen cooled mercury cadmium telluride (MCT) detector.
The fresh tissue specimens were obtained in gastroscopy detection, and then immediately and non-invasively measured using the mobile FT-IR spectrometer near the operation room. The sample was put on ATR accessory to measure the spectrum. The background spectrum was collected. For the collection of each spectrum, 32 scans at a resolution of 4/cm, with a normal range from 4 000/cm to 800/cm were applied. It took about 1-2 min to non-invasively measure the spectrum. After measurement, the samples were stored in liquid nitrogen and sent for the pathologic examination of H&E staining as the reference in the spectral analysis.
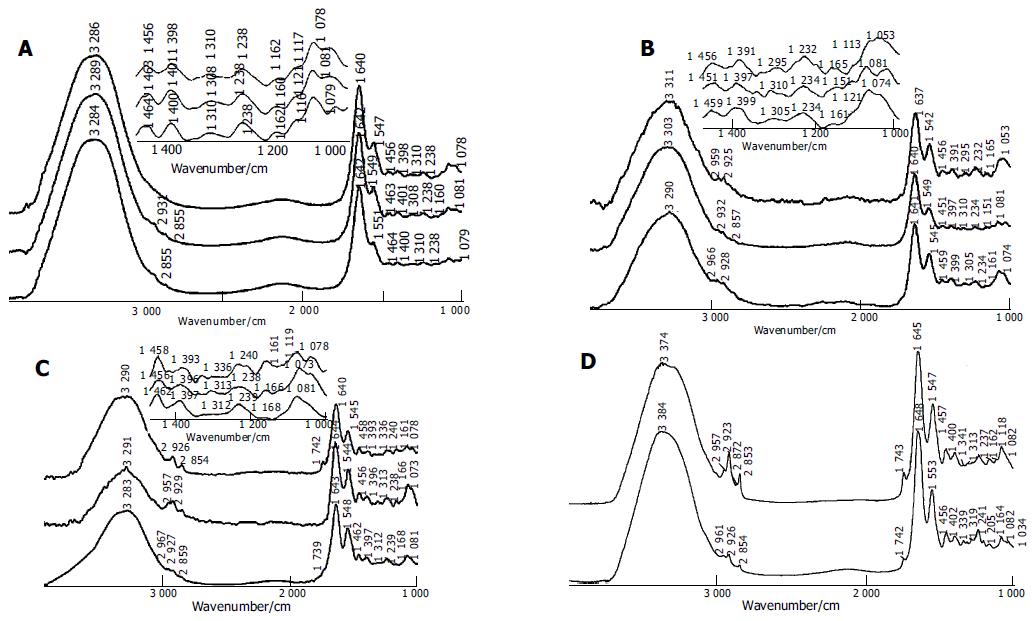
The spectral differences among chronic superficial gastritis, atrophic gastritis and malignant gastric endoscopy samples were studied. For the sharper difference in the ratio of peak intensity, baseline corrections of the spectra were performed at first. Several typical spectra of malignant gastric tissues obtained by endoscopy are illustrated in Figure 1A. The spectral characteristics were similar to those of spectra of gastric tissues, which were obtained during surgical operation in our previous research[15]. The spectral features of malignant gastric tissues were in good agreement with the criteria established in our previous work[15]. For example, the broad -3 300/cm absorption band, assigned to N-H stretching vibration of protein and OH stretching vibration of water, was more intense in the malignant stomach samples because of the higher content of water in malignant tissues. C = O band near 1741/cm was assigned to the fat in tissues, and C-H stretching vibration bands near 2 961, 2 927, and 2 853/cm were related to lipid and fat content and these bands usually disappeared in the spectra of malignant gastric tissues. -1641/cm absorption peak belonged to amide I band of protein and H-O-H variable-angle vibration of water. In addition, amide II band of protein was located near 1550/cm. The intensity of amide II band decreased in the spectra of malignant gastric tissues. Thus, the ratio of intensity of -1641/cm band to -1550/cm band was higher in the malignant gastric samples. The intensity of -1400/cm peak was stronger than that of -1460/cm peak in the spectra of cancerous samples. According to the statistical results, the relative intensity of I1460/I1400 was less than 1 for about 90% of malignant gastric samples. The intensity of absorption peak at about 1308/cm increased. The peak position shifted to a low wave number. The peak position was lower than 1310/cm for 72% of the malignant tissues. The intensity of -1160/cm was often less than that of -1120/cm in the spectra of stomach cancer samples.
The spectra of chronic atrophic gastritis were close to those of malignant gastric tissues (Figure 1B), and exhibited partial characteristic bands as those of malignant tissues. CH stretching vibrational band near 3 000-2 800/cm and C = O vibrational band about 1740/cm were still weak in the spectra of atrophic gastritis samples. Compared with the spectra of malignant gastric tissues, the amide II band in the spectra of atrophic gastritis tissues was broader, and the relative intensity of amide II band to amide I band became higher. The decrease in the extent of the intensity of absorption peak near 1460/cm was not significant, i.e., the intensity of band at about 1460/cm became a little less than that at 1400/cm in the spectra of atrophic gastritis samples. In the spectra of about 81% of atrophic gastritis samples, the relative intensity of I1460/I1400 was less than or equal to 1. Compared with the spectra of malignant tissues, the absorption peak near 1310/cm was weaker in the spectra of atrophic gastritis samples. Similar to gastric cancerous tissues, the peak position of this band at about 1310/cm often shifted to a low wave number, which was different from the situation of chronic superficial gastritis, indicating that the peak position was lower than 1310/cm for 73% of atrophic gastritis tissues.
The spectra of chronic superficial gastritis tissues (Figure 1C) were similar to those of normal stomach tissues[15] (Figure 1D). The spectral features were as follows. CH stretching vibrational band near 3 000-2 800/cm and C = O vibrational band at about 1740/cm were strong in the spectra of superficial gastritis samples. In general, there existed strong and broad amide II bands in the spectra of superficial gastritis samples. The peak at 1460/cm was stronger than that at 1400/cm. The relative intensity of I1460/I1400 was higher than 1 in about 78% of superficial gastritis samples. The peak at about 1250/cm was stronger, and the band near 1308/cm disappeared or became weak and the position of this band often shifted to a high wave number, indicating that the peak position was higher than 1310/cm in 80% of superficial gastritis tissues. Similar to normal gastric tissues, the intensity of peak near 1160/cm increased and often became stronger than that at about 1120/cm.
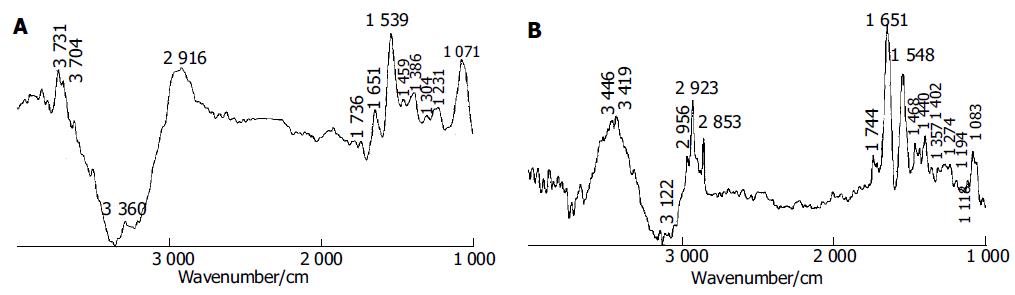
To enhance our understanding, the subtraction technique was performed in the spectral analysis[16]. The subtraction spectra (Figures 2A and B) could highlight spectral differences between chronic atrophic gastritis tissue and malignant gastric tissue, and between normal gastric tissue and chronic superficial gastritis tissue. From the two subtraction spectra, some new information could be observed.
Figure 2A illustrates the subtraction result of the spectrum of chronic atrophic gastritis tissue minus that of malignant gastric tissue. It verified that chronic atrophic gastritis tissues exhibited relatively stronger C-H stretching vibration, C = O stretching band, amide I, amide II than gastric cancer tissue. In addition, there was more water in gastric cancer tissue due to the strong negative band located near 3 360/cm in the spectrum of subtraction malignant tissue from chronic atrophic gastritis tissue.
Figure 2B shows the spectral differences between normal gastric tissue and chronic superficial gastritis tissue. The positive peaks in the region of 2 800-3 000/cm and near 1740/cm were observed in the subtraction spectrum, suggesting that normal gastric tissue contains more components of long-chain C-H and C = O bonds. However, these peaks often decrease and even disappear in the spectra of gastritis and malignant tissues. Because triglyceride contains a large proportion of methyl, methylene and carbonyl, and fat in the region of malignant tissue is consumed because of the necessary nutritional and energy requirement in the development of carcinoma. At the same time, amide I and amide II bands are stronger in the spectrum of normal gastric tissue than in that of chronic superficial gastritis tissue, indicating that normal gastric tissue has more regular protein secondary structures, such as α helical structure.
In conclusion, the results in our study demonstrate that the sensitivity of FT-IR detection to gastric cancer, chronic atrophic gastritis and superficial gastritis is 90.9%, 82.5%, 91.5%, and specificity is 97.3%, 91.7%, 89.5% respectively. FT-IR spectroscopy is effective in distinguishing gastric inflammation from malignancy.
Science Editor Wang XL and Guo SY Language Editor Elsevier HK
| 1. | Wong PT, Wong RK, Caputo TA, Godwin TA, Rigas B. Infrared spectroscopy of exfoliated human cervical cells: evidence of extensive structural changes during carcinogenesis. Proc Natl Acad Sci USA. 1991;88:10988-10992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 199] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Fujioka N, Morimoto Y, Arai T, Kikuchi M. Discrimination between normal and malignant human gastric tissues by Fourier transform infrared spectroscopy. Cancer Detect Prev. 2004;28:32-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Wong PT, Lacelle S, Fung Kee Fung M, Senterman M, Mikhael NZ. Characterization of exfoliated cells and tissues from hu-man endocervix and ectocervix by FTIR and ATR/FTIR spectroscopy. Biospectroscopy. 1995;1:357-364. [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Sindhuphak R, Issaravanich S, Udomprasertgul V, Srisookho P, Warakamin S, Sindhuphak S, Boonbundarlchai R, Dusitsin N. A new approach for the detection of cervical cancer in Thai women. Gynecol Oncol. 2003;90:10-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Argov S, Ramesh J, Salman A, Sinelnikov I, Goldstein J, Guterman H, Mordechai S. Diagnostic potential of Fourier-transform infrared microspectroscopy and advanced computational methods in colon cancer patients. J Biomed Opt. 2002;7:248-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Ling XF, Xu YZ, Soloway RD, Xu Z, Zhang TI, Zhou XS, Li WH, Yang ZL, Weng SF, Xu DF. Identification of colorectal and gastric cancer by Fourier transform Infrared (FT-IR) spectroscopy and separation from normal tissue. Gastroenterology. 2002;122:T1584. [Cited in This Article: ] |
| 7. | Li QB, Yang LM, Ling XF, Wang JS, Zhou XS, Shi JS, Wu JG. Application of the SIMCA method to cancer diagnosis with Fourier-transform infrared spectroscopy. Guangpuxue Yu Guangpu Fenxi. 2004;24:414-417. [PubMed] [Cited in This Article: ] |
| 8. | Ren Y, Xu YZ, Wang J, Zhang YF, Wang F, Shi JS, Wu JG. FTIR spectroscopic and statistical studies on the lung tissues. Guangpuxue Yu Guangpu Fenxi. 2003;23:681-684. [Cited in This Article: ] |
| 9. | Sun CW, Xu YZ, Sun KH, Wu QG, Li WH, Xu ZH, Wu JG. A study of the diagnosis of salivary gland tumors by means of mid infrared optical fiber technique. Guangpuxue Yu Guangpu Fenxi. 1996;16:22-25. [Cited in This Article: ] |
| 10. | Wu JG, Xu YZ, Sun CW, Soloway RD, Xu DF, Wu QG, Sun KH, Weng SF, Xu GX. Distinguishing malignant from normal oral tissues using FTIR fiber-optic techniques. Biopolymers. 2001;62:185-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Xu YZ, Soloway RD, Lin XF, Zhi X, Weng SF, Wu QG, Shi JS, Sun WX, Zhang TX, Wu JG. Fourier transform infrared (FT-IR) mid-IR spectroscopy separates normal and malignant tissue from the colon and stomach. Gastroenterology. 2000;118:A6438. [DOI] [Cited in This Article: ] |
| 12. | Wang JS, Xu YZ, Shi JS, Zhang L, Duan XY, Yang LM, Su YL, Weng SF, Xu DF, Wu JG. FTIR spectroscopic study on normal and cancerous tissues of esophagus. Guangpuxue Yu Guangpu Fenxi. 2003;23:863-865. [Cited in This Article: ] |
| 13. | Zhang L, Sun KH, Soloway RD, Ling XF, Xu YZ, Wu QG, Weng SF, Yang ZL, Zhang TL, Yao GQ. Intraoperative Fourier transform infrared spec-troscopy can guide individual resections in patients with gas-tric cancer. Gastroenterology. 2004;126:A626. [Cited in This Article: ] |
| 14. | Van Dam J. Novel methods of enhanced endoscopic imaging. Gut. 2003;52 Suppl 4:iv12-iv16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Peng Q, Xu Y, Li W, Wu J, Zhou X. [FTIR study on the normal and tumor gastrointestinal tissues]. Guangpuxue Yu Guangpu fenxi. 1998;18:528-531. [PubMed] [Cited in This Article: ] |
| 16. | Ling XF, Xu YZ, Weng SF, Li WH, Xu Z, Hammaker RM, Fateley WG, Wang F, Zhou XS, Soloway RD. Investigation of normal and malignant tissue samples from the human stomach using Fourier transform Raman spectroscopy. Appl Spectrosc. 2002;56:570-573. [DOI] [Cited in This Article: ] |