Published online May 1, 2004. doi: 10.3748/wjg.v10.i9.1315
Revised: September 20, 2003
Accepted: October 12, 2003
Published online: May 1, 2004
AIM: To investigate the pathway via which 17β-estradiol (β-Est) exerts suppressive effects on rat hepatic fibrosis.
METHODS: In vivo study was done in CCl4-induced female hepatofibrotic rats. Fibrosis-suppressive effect of β-Est (20 μg/kg·d) was evaluated in intact and ovariectomized rat models. Six weeks after the treatment, all the rats were sacrificed and specimens of serum or liver tissue were collected for the studies. Serum liver enzymes, fibrosis markers and estradiol levels were determined by standard enzymatic methods, ELISA and RIA, respectively. Degrees of fibrosis and areas of hepatic stellate cells (HSCs) positive for alpha-smooth muscle actin (α-SMA) in the liver were determined by van Gieson (VG) stain and immunohistochemistry. In vitro studies, HSCs were isolated by a combination of pronase-collagenase perfusion and density gradient centrifugation. First-passage HSCs were randomly divided into 10 groups, and different concentrations of β-Est, 2-hydroxyestradiol (2OHE) or 2-methoxyestradiol (2MeOE) were separately added to the cell groups. After incubation for 72 h, the degree of cell proliferation, collagen production, α-SMA or estrogen receptor (ER) expression was determined by MTT assay, ELISA and immunohistochemistry, respectively.
RESULTS: β-Est treatment reduced aspartate aminotransfer-ase (AST), alanine aminotransferase (ALT), hyaluronic acid (HA) and type IV collagen (C IV) in sera, suppressed hepatic collagen content, decreased the areas of HSCs positive for α-SMA significantly in both intact and ovariectomized female hepatofibrotic rats. There was a negative correlation between the percentage of fibrotic area of liver tissue and the serum estradiol level; the calculated correlation coefficient was -0.57 (P < 0.01). β-Est and its metabolites concentration-dependently (10-9 mol/L-10- 7 mol/L) inhibited HSC proliferation and collagen synthesis. At the concentration of 10-7 mol/L, they could inhibit α-SMA expression. The order of potency was 2MeOE > 2OHE > β-Est.
CONCLUSION: β-Est may suppress hepatic fibrosis probably via its biologically active metabolites.
- Citation: Liu QH, Li DG, Huang X, Zong CH, Xu QF, Lu HM. Suppressive effects of 17β-estradiol on hepatic fibrosis in CCl4-induced rat model. World J Gastroenterol 2004; 10(9): 1315-1320
- URL: https://www.wjgnet.com/1007-9327/full/v10/i9/1315.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i9.1315
Several epidemiological studies demonstrated a lower incidence of hepatic cirrhosis and its complications in women than in men[1-3]. But as menopause comes, the morbidity of the disease increases gradually. So, endogenous estrogen may take part in the protective effects on hepatic fibrosis. In vivo, 17β-estradiol (β-Est) is the most active estrogen and can be converted to several metabolites, some of which are known to possess biological activities. Of the various metabolites, 2-hydroxyestradiol (2OHE) and 2-methoxyestradiol (2MeOE) are the biologically active substances, which have been investigated in depth[4,5]. 2OHE is one of the hydroxylated metabolites of β-Est converted by cytochrome P-450 enzymes (CYP450) and can be rapidly catalyzed by enzyme catechol-O-methyl-transferase (COMT) to 2MeOE[6-8]. Recent studies have shown that 2OHE and 2MeOE could inhibit DNA synthesis, cell proliferation, collagen synthesis and mitogen activated protein kinase activity in vascular smooth muscle cells (VSMCs), cardiac fibroblasts (CFs) and glomerular mesangial cells (GMCs) with the potency stronger than β-Est[9-13]. Hence, the fibrosis-suppressive effects of β-Est not only reflect the biological effects of β-Est per se but also those of its biologically active metabolites.
Hepatic stellate cells (HSC), the key cells responsible for hepatic fibrogenesis, are VSMC, CF or GMC analogs and belong to the pericytes generically[14]. So, they may have many properties in common, including a prominent role in fibrosing injury and the same reaction to metabolites of β-Est.
The present study was initiated to investigate the role of β-Est in the female rat model of carbon tetrachloride (CCl4)-induced hepatic fibrosis. Moreover, the effects of β-Est, 2OHE and 2MeOE on the first-passage rat HSCs were assessed.
Fifty female Sprague-Dawley rats aged 10 wk (Laboratory Animal Center affiliated to Chinese Academy of Sciences, Shanghai, China) were divided into 5 groups (n = 10). All the rats were initiated with either a bilateral ovariectomy (Ovx) or a sham operation 3 wk before the studies. Control group received subcutaneous injection of olive oil (3 mL/kg every 3 d and first dosage doubled), CCl4 group was given the same dose of 400 g/L CCl4, Ovx + CCl4 group was treated with the same dose of 400 g/L CCl4 after ovariectomy, β-Est + CCl4 group along with CCl4 injection described above was treated with 0.002% β-Est (1 mL/kg per day, Sigma-Aldrich Corporation, St Louis, Missouri, United States), Ovx + β-Est + CCl4 group was treated the same as β-Est + CCl4 group with the addition of ovariectomy.
At the end of 6 wk, the number of alive rats in the groups was 10, 7, 8, 10, 9 respectively. Several rats died of infection at the site of injection or hepatic crack by unsuitable handling. After an overnight fast, all the rats were sacrificed. Serum samples obtained were treated with proteinase inhibitor and stored at -20 °C. Liver tissue specimens were rinsed with normal saline containing 0.1 g/L DEPC, some were stored at -80 °C, and the others were fixed in neutral formalin and embedded in paraffin.
Serum activities of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were assayed by standard enzymatic methods, hyaluronic acid (HA) and type IV collagen (C IV) concentrations were measured by enzyme-linked immunosorbent assay (ELISA) using a commercial kit (Navy Medical Institute, Shanghai, China).
The embedded liver specimens were sliced 5 μm in thickness, mounted on slides, deparaffinised in xylene, and dehydrated in alcohol. For histopathological studies, the liver sections were stained with van Gieson (VG). For alpha-smooth muscle actin (α-SMA) immunohistochemistry, the sections were incubated with 3% (v/v) hydrogen peroxide in methanol for 15 min to block endogenous peroxidase, and then with 1 g/L Triton-X plus 1 g/L BSA in phosphate-buffered saline (PBS) to block non-specific antigens. After absorption of surplus liquid, the samples were incubated with a 1:100 dilution of a polyclonal antibody against α-SMA (Boster Biological Technology Corporation, Wuhan, China) at 37 °C for 2 h. Then the samples were rinsed and incubated with a 1:100 dilution of biotin-conjugated goat anti-rabbit IgG (Boster Biological Technology Corporation, Wuhan, China) at 37 °C for 30 min. Finally, the antigen-antibody complexes were visualized with 3,3’-diaminobenzidine (DAB).
For morphometric analysis, the mean value of collagen content or α-SMA positive cells in six ocular fields per specimen was used as the percentage area at 100x magnification using Zeiss KS400 image analysis system (Kontron Electronics, Eching, Germany).
Serum estradiol concentrations were measured with radioimmunoassay (RIA) using a commercial kit (Diagnostic Products Corporation, Tianjin, China).
HSCs were isolated and purified from the livers of normal female rats weighing about 400 g to 450 g by a combination of pronase-collagenase perfusion and density gradient centrifugation[15-19]. Briefly, the liver was perfused in situ through the portal vein, first with Ca2+/Mg2+-free Krebs-Ringer (KR) solution at a flow rate of 20 mL/min, followed by pronase and then with collagenase (Sigma-Aldrich Corporation, St Louis, Missouri, USA) in KR solution. The digested liver was excised, minced with scissors, and incubated in KR solution containing pronase and DNase (Sigma-Aldrich Corporation, St Louis, Missouri, United States) for 30 min. The resulting suspension was filtered through nylon mesh (200 μm in diameter) and then washed 3 times by centrifugation in D-Hanks solution at 1700 r/min for 7 min. An HSC-enriched fraction was obtained by centrifugation of the filtrate in an 180 g/L nycodenz (Sigma-Aldrich Corporation, St Louis, Missouri, United States) solution with the volume ratio of 1:2 at 3 200 r/min for 17 min. The cells in the upper layer were washed by centrifugation at 1700 r/min for 7 min and suspended in Dulbecco’s modified Eagle medium (DMEM, GIBCO BRL Life Technologies Incorporation, United States) supplemented with 200 mL/L fetal bovine serum (FBS, GIBCO BRL Life Technologies Incorporation, United States). Both cell viability and purity were over 90%, examined by trypan blue (Sigma-Aldrich Corporation, St Louis, Missouri, United States) exclusion and autofluorescence respectively.
Cells were plated at a density of 1 × 106 cells in 1 mL culture medium on uncoated plastic culture dishes. The culture medium was changed after 24 h and then every 3 d. After cultured for 14 d, the cells were passaged.
The first-passage HSCs were plated at a density of 2 × 105 cells in 1 mL DMEM supplemented with 100 g/L FBS on an uncoated plastic culture dish. Subconfluent HSCs were divided into 10 groups, and different concentrations of β-Est, 2OHE, 2MeOE (Sigma-Aldrich Corporation, St Louis, Missouri, United States) or vehicle were added to the cells. After incubated for 24 h, experiments were done to assess the functional state of HSCs.
HSC proliferation was measured by MTT assay. The cells were continually cultivated with 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT, Sigma-Aldrich Corporation, St Louis, Missouri, United States) in a 96-well plate for 4 h. After the supernatant in each well was aspirated and discarded, the cells were agitated with dimethylsulfoxide (DMSO, Sigma-Aldrich Corporation, St Louis, Missouri, United States) 150 μLfor 10 min. Finally, the optical density of each well was read in an auto reader using a 492 nm filter with a reference at 630 nm.
The levels of HA and C IV in the supernatants of cell were messured as afore-metioned.
For immunohistochemical examination of α-SMA, estrogen receptor (ER)α and ERβ, the cells were mounted on slides and fixed with 40 g/L paraformaldehyde for 5 min at 4 °C. Following incubation with 30 mL/L H2O2 for 15 min, the cells were blocked by 1 g/L Triton-X plus 1 g/L BSA in PBS for 15 min at room temperature. They were incubated for 2 h at 37 °C with a 1:100 dilution of polyclonal antibody against α-SMA, ERα or ERβ (Santa Cruz Biotechnology Incorporation, California, United States) in a humid chamber. After the samples were rinsed, they were incubated with a 1:200 dilution of biotin-conjugated goat anti-rabbit IgG, and then visualized with DAB. Integral light density (ILD) analysis was performed on 20 cells in up, down, left, right quadrant of each section, data were calculated from average of 4 ILDs.
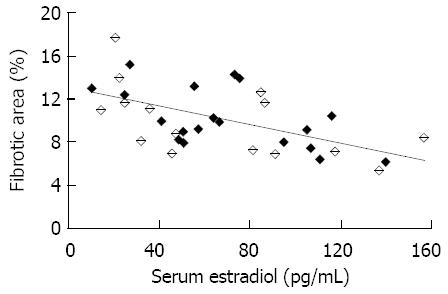
Data are presented as mean ± SE unless otherwise indicated. Parametric data were compared using one-way analysis of variance, followed by multiple pair-wise comparisons according to the least-significant difference t-test. Pearson’s analysis was performed to compare the correlation between serum estradiol and fibrotic area percentage. A P value less than 0.05 was accepted as statistically significant.
Table 1 shows the liver enzymes and fibrosis markers in CCl4-treated female rats. Treatment with CCl4 caused a significant increase in the activities of serum ALT and AST compared with the control animals. The enzyme levels in ovariectomized model rats were higher than those in CCl4 model group, whereas CCl4 plus β-Est group showed a significant decrease in enzyme levels compared with CCl4 group. There was no significant difference between CCl4 group and Ovx + β-Est + CCl4 group.
| ALT (nkat/L) | AST (nkat/L) | HA (μg/L) | C IV (μg/L) | ||
| Control | 275.50 ± 32.05 | 84.80 ± 11.83 | 155.50 ± 10.41 | 17.10 ± 1.77 | |
| CCl4 | 1 | 070.71 ± 82.95b | 806.00 ± 82.43b | 481.43 ± 24.68b | 49.57 ± 2.47b |
| Ovx + CCl4 | 1 | 403.88 ± 120.30ab | 1 102.88 ± 84.91bd | 583.75 ± 37.50ab | 61.00 ± 3.30ab |
| CCl4 + β-Est | 789.40 ± 58.61ab | 606.70 ± 45.68ab | 389.30 ± 29.77ab | 38.80 ± 2.59ab | |
| Ovx + CCl4 + β-Est | 1 | 008.44 ± 93.07b | 794.89 ± 70.93b | 474.56 ± 32.57b | 46.22 ± 3.06b |
The changes of fibrosis markers, HA and C IV, were in parallel with those of the enzyme levels in all groups.
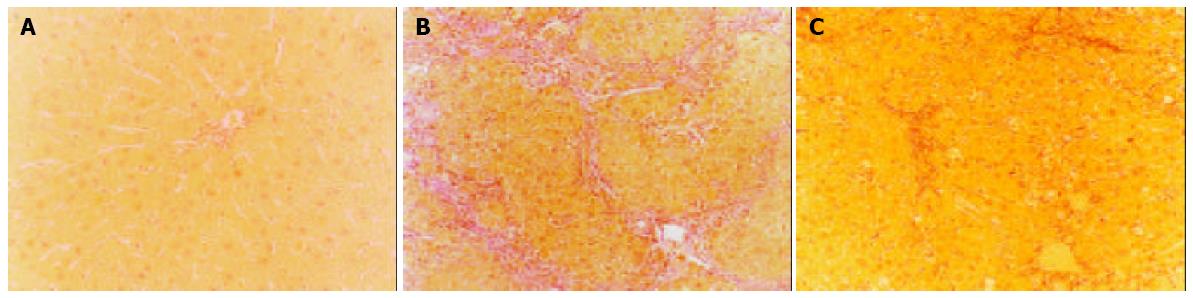
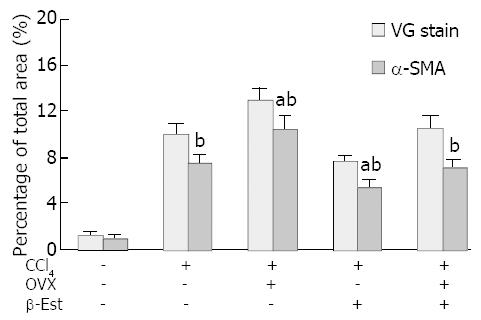
The control livers showed a distinct lobular architecture with collagens only surrounding the central and portal veins (Figure 1A). Administration of CCl4 induced inflammation, necrosis and collagen deposition in the livers. Ovariectomy significantly increased hepatic fibrosis induced by CCl4 (Figure 1B), whereas β-Est had the opposite effect. The fibrotic area percentage of Ovx + CCl4 + β-Est group was similar to that of CCl4 group (Figure 1C).
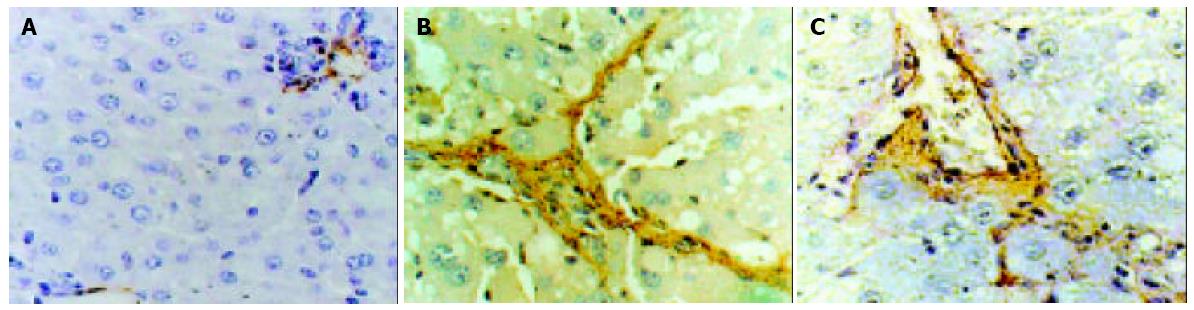
Immunohistochemistry of α-SMA, an activation marker of rat HSC[20-22], showed strong staining around vascular wall, but not the sinusoids in control female rat livers (Figure 2A). After administration of CCl4, fibrotic change was evident with significant increases of α-SMA positive cells in centrilobular and periportal fibrotic bands. Ovariectomy significantly increased the percentage area of staining (Figure 2B), whereas β-Est had the opposite effect. There was no significant difference in the fibrotic area percentage of staining between CCl4 and Ovx + CCl4 + β-Est groups (Figure 2C).
Figure 3 summarizes the histopathological and immunohist-ochemical changes in CCl4-treated female rats.
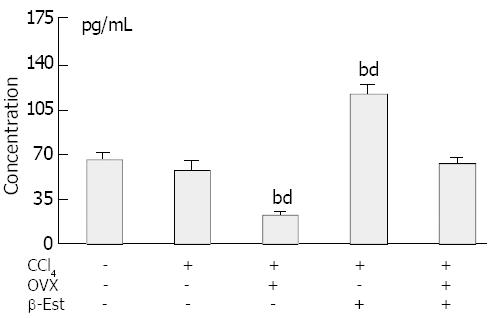
Figure 4 shows the serum estradiol levels in the rats. The levels did not change in animals treated with CCl4 alone. Ovariectomy produced significantly lower serum estradiol levels. In rats model treated with exogenous β-Est, the levels increased significantly as compared with those of control or CCl4 model groups. Twenty μg/kg per day β-Est could rectify the low serum estradiol levels in ovariectomized rats.
In 34 CCl4-treated rats, there was a negative correlation between the fibrotic area percentage and serum estradiol level, the calculated correlation coefficient was -0.57 (P < 0.01, Figure 5).
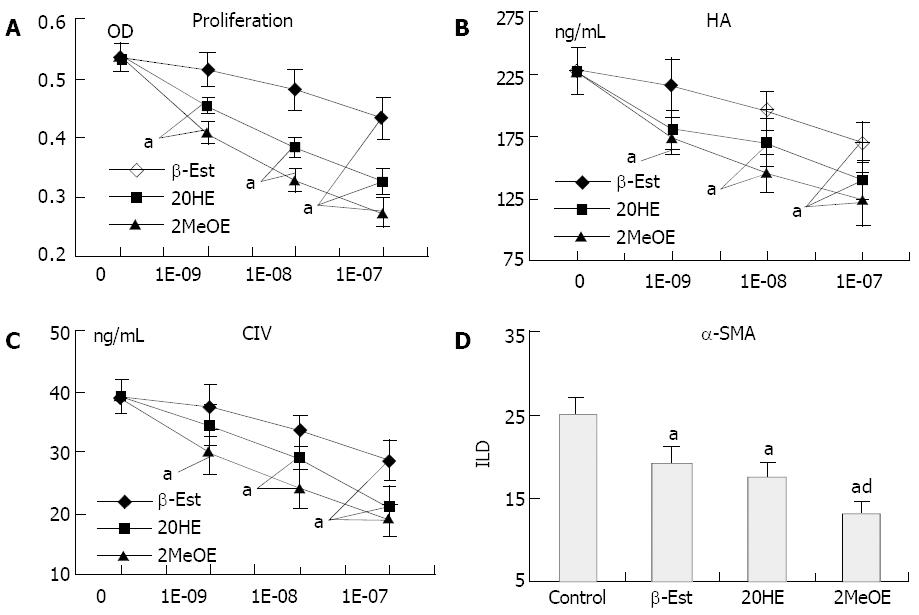
β-Est, 2OHE and 2MeOE (10-9-10-7 mol/L) inhibited HSC proliferation (Figure 6A) and ECM secretion (Figure 6B and C) in a dose-dependent manner. Only the high concentration (≥ 10-7 mol/L) of β-Est exerted inhibitory effects, while a concentration of 2MeOE as low as 10-9 mol/L had a similar effect. The order of potency was 2MeOE > 2OHE > β-Est. At a concentration of 10-7 mol/L, β-Est, 2OHE and 2MeOE all could inhibit HSCs in expressing α-SMA, and the potency of 2MeOE was significantly stronger than that of β-Est (Figure 6D)
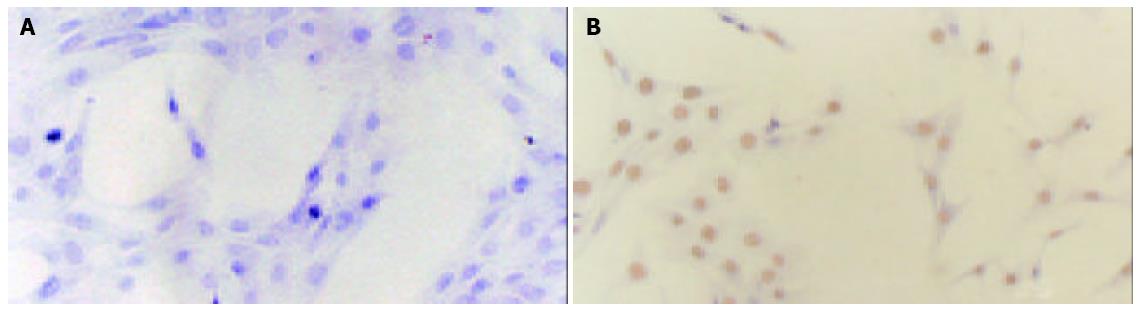
A representative immunohistochemistry showed the expression of ER-β (Figure 7B) but not ER-α (Figure 7A) on rat HSCs.
Hepatic fibrosis, which is often associated with hepatocellular necrosis and inflammation, occurs as a repair process in many chronic liver diseases and is considered to be a forerunner of cirrhosis. HSCs are regarded as the primary target cells for inflammatory stimuli in the injured liver. Once activated, HSCs would be transformed into α-SMA positive myofibroblast-like cells, which are responsible for much of the collagen hypersecretion during hepatic fibrogenesis[23-28].
In vivo study showed that β-Est at physiological doses could suppress the development of hepatic fibrosis in CCl4- induced hepatofibrotic rat models along with decreased serum liver enzymes, reduced collagen content and smaller areas of fibrosis in the liver. There was a negative correlation between the percentage of fibrotic area and the serum estradiol level with a calculated correlation coefficient of -0.57. Additionally, in cultured rat HSCs, β-Est inhibited cell proliferation, ECM secretion and α-SMA expression in a dose-dependent manner. These findings support β-Est as a potent inhibitor of HSCs transformation, thus, playing an antifibrogenic role in the progression from hepatic fibrosis to cirrhosis.
The pathway by which β-Est inhibits HSC activation is not clear. As HSCs contain ERs, it is presumed that ERs mediate the fibrosuppressive effects of β-Est. However, several lines of evidence do not support the hypothesis. In this regard, high levels of ERβ but low or no levels of ERα expression were observed in normal and fibrotic livers and also in quiescent and activated HSCs from both male and female rats[29], but β-Est in physiological doses was unable to suppress hepatic fibrosis in male rats following dimethylnitrosamine or pig serum administration[30-32]. Our preliminary studies also suggested a sexually dimorphic response pattern of the injured liver to β-Est. Besides, the male patients expressed high serum levels of estradiol parallel to the stage of liver cirrhosis.
HSCs are VSMC, CF or GMC analogs that belong to the pericytes generically, so they may have many properties in common including a prominent role in repairing fibrosis against injury and similar reactions to β-Est. Recent studies by Iafrati et al[33] and Karas et al[34,35] showed that β-Est inhibited injury-induced VSMC proliferation in arteries of mice that lacked either ERα or ERβ as well as in double knockout mice that lacked both ERα and ERβ, suggesting that the effects might be mediated via an ER-independent pathway. Furthermore, 2OHE and 2MeOE, the biologically active metabolites of β-Est, could inhibit DNA synthesis, cell proliferation, collagen synthesis and mitogen activated protein kinase activity in VSMCs, CFs or GMCs with a potency stronger than β-Est[9-13,36,37]. Liver is the main locus where β-Est metabolizes, so it is possible that the antifibrogenic actions of β-Est are mediated in part by local conversion of β-Est to biologically active metabolites.
In the present study, we attempted to testify this hypothesis by comparing the inhibitory effects of β-Est and its metabolites on the activation of rat HSCs in vitro. We found that treatment of HSCs with β-Est or its metabolites differentially inhibited FCS-induced cell proliferation, ECM secretion and α-SMA expression in the following order of potency: 2MeOE > 2OHE > β-Est. Only the pharmacological concentration (> 10-7 mol/L) of β-Est could significantly inhibit HSC proliferation and collagen synthesis, while the concentration of 2MeOE or 2OHE as low as 10-9 mol/L gave similar effects. β-Est, 2OHE and 2MeOE at a concentration of 10-7 mol/L each inhibited α-SMA expression by 24%, 30% and 47%, respectively.
The findings of this study provided evidence in rat HSC experiments that the metabolites of β-Est with little or no affinity for ERs could mediate the fibrosuppressive effects of β-Est via an ER-independent pathway. The results suggest that β-Est metabolism in the liver may be an important determinant of fibrosuppressive effects of circulating estradiol and that sexual differences in the liver metabolism of β-Est could determine a sexually dimorphic response pattern of liver cirrhosis. Moreover, genetic or acquired differences in β-Est metabolism may determine the fibrosuppressive benefits a patient receives from β-Est replacement therapy. Finally, the results also imply that nonfeminizing β-Est metabolites may afford antifibrogenic protection regardless of gender.
Edited by Kumar M, Wang XL Proofread by Xu FM
| 1. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [Cited in This Article: ] |
| 2. | Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis c. J Hepatol. 2001;34:730-739. [Cited in This Article: ] |
| 3. | el-Serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87-107, vi. [Cited in This Article: ] |
| 4. | Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1-27. [Cited in This Article: ] |
| 5. | Zhu BT, Conney AH. Is 2-methoxyestradiol an endogenous estrogen metabolite that inhibits mammary carcinogenesis? Cancer Res. 1998;58:2269-2277. [Cited in This Article: ] |
| 6. | Cheng ZN, Shu Y, Liu ZQ, Wang LS, Ou-Yang DS, Zhou HH. Role of cytochrome P450 in estradiol metabolism in vitro. Acta Pharmacol Sin. 2001;22:148-154. [Cited in This Article: ] |
| 7. | Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. Characterization of the oxidative metabolites of 17beta-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology. 2003;144:3382-3398. [Cited in This Article: ] |
| 8. | Goodman JE, Jensen LT, He P, Yager JD. Characterization of human soluble high and low activity catechol-O-methyltransferase catalyzed catechol estrogen methylation. Pharmacogenetics. 2002;12:517-528. [Cited in This Article: ] |
| 9. | Dubey RK, Gillespie DG, Zacharia LC, Barchiesi F, Imthurn B, Jackson EK. CYP450- and COMT-derived estradiol metabolites inhibit activity of human coronary artery SMCs. Hypertension. 2003;41:807-813. [Cited in This Article: ] |
| 10. | Barchiesi F, Jackson EK, Gillespie DG, Zacharia LC, Fingerle J, Dubey RK. Methoxyestradiols mediate estradiol-induced antimitogenesis in human aortic SMCs. Hypertension. 2002;39:874-879. [Cited in This Article: ] |
| 11. | Dubey RK, Gillespie DG, Keller PJ, Imthurn B, Zacharia LC, Jackson EK. Role of methoxyestradiols in the growth inhibitory effects of estradiol on human glomerular mesangial cells. Hypertension. 2002;39:418-424. [Cited in This Article: ] |
| 12. | Dubey RK, Gillespie DG, Zacharia LC, Rosselli M, Imthurn B, Jackson EK. Methoxyestradiols mediate the antimitogenic effects of locally applied estradiol on cardiac fibroblast growth. Hypertension. 2002;39:412-417. [Cited in This Article: ] |
| 13. | Dubey RK, Gillespie DG, Zacharia LC, Rosselli M, Korzekwa KR, Fingerle J, Jackson EK. Methoxyestradiols mediate the antimitogenic effects of estradiol on vascular smooth muscle cells via estrogen receptor-independent mechanisms. Biochem Biophys Res Commun. 2000;278:27-33. [Cited in This Article: ] |
| 14. | Bissell DM. Sex and hepatic fibrosis. Hepatology. 1999;29:988-989. [Cited in This Article: ] |
| 15. | Friedman SL, Rockey DC, McGuire RF, Maher JJ, Boyles JK, Yamasaki G. Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology. 1992;15:234-243. [Cited in This Article: ] |
| 16. | Riccalton-Banks L, Bhandari R, Fry J, Shakesheff KM. A simple method for the simultaneous isolation of stellate cells and hepatocytes from rat liver tissue. Mol Cell Biochem. 2003;248:97-102. [Cited in This Article: ] |
| 17. | Ratziu V, Lalazar A, Wong L, Dang Q, Collins C, Shaulian E, Jensen S, Friedman SL. Zf9, a Kruppel-like transcription factor up-regulated in vivo during early hepatic fibrosis. Proc Natl Acad Sci USA. 1998;95:9500-9505. [Cited in This Article: ] |
| 18. | Reynaert H, Vaeyens F, Qin H, Hellemans K, Chatterjee N, Winand D, Quartier E, Schuit F, Urbain D, Kumar U. Somatostatin suppresses endothelin-1-induced rat hepatic stellate cell contraction via somatostatin receptor subtype 1. Gastroenterology. 2001;121:915-930. [Cited in This Article: ] |
| 19. | Williams EJ, Benyon RC, Trim N, Hadwin R, Grove BH, Arthur MJ, Unemori EN, Iredale JP. Relaxin inhibits effective collagen deposition by cultured hepatic stellate cells and decreases rat liver fibrosis in vivo. Gut. 2001;49:577-583. [Cited in This Article: ] |
| 20. | Ikeda K, Wakahara T, Wang YQ, Kadoya H, Kawada N, Kaneda K. In vitro migratory potential of rat quiescent hepatic stellate cells and its augmentation by cell activation. Hepatology. 1999;29:1760-1767. [Cited in This Article: ] |
| 21. | Kim KY, Choi I, Kim SS. Progression of hepatic stellate cell activation is associated with the level of oxidative stress rather than cytokines during CCl4-induced fibrogenesis. Mol Cells. 2000;10:289-300. [Cited in This Article: ] |
| 22. | Sato M, Suzuki S, Senoo H. Hepatic stellate cells: unique characteristics in cell biology and phenotype. Cell Struct Funct. 2003;28:105-112. [Cited in This Article: ] |
| 23. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [Cited in This Article: ] |
| 24. | Gäbele E, Brenner DA, Rippe RA. Liver fibrosis: signals leading to the amplification of the fibrogenic hepatic stellate cell. Front Biosci. 2003;8:d69-d77. [Cited in This Article: ] |
| 25. | Mann DA, Smart DE. Transcriptional regulation of hepatic stellate cell activation. Gut. 2002;50:891-896. [Cited in This Article: ] |
| 26. | McCrudden R, Iredale JP. Liver fibrosis, the hepatic stellate cell and tissue inhibitors of metalloproteinases. Histol Histopathol. 2000;15:1159-1168. [Cited in This Article: ] |
| 27. | Dooley S, Delvoux B, Lahme B, Mangasser-Stephan K, Gressner AM. Modulation of transforming growth factor beta response and signaling during transdifferentiation of rat hepatic stellate cells to myofibroblasts. Hepatology. 2000;31:1094-1106. [Cited in This Article: ] |
| 28. | Brenner DA, Waterboer T, Choi SK, Lindquist JN, Stefanovic B, Burchardt E, Yamauchi M, Gillan A, Rippe RA. New aspects of hepatic fibrosis. J Hepatol. 2000;32:32-38. [Cited in This Article: ] |
| 29. | Zhou Y, Shimizu I, Lu G, Itonaga M, Okamura Y, Shono M, Honda H, Inoue S, Muramatsu M, Ito S. Hepatic stellate cells contain the functional estrogen receptor beta but not the estrogen receptor alpha in male and female rats. Biochem Biophys Res Commun. 2001;286:1059-1065. [Cited in This Article: ] |
| 30. | Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29:719-727. [Cited in This Article: ] |
| 31. | Shimizu I, Mizobuchi Y, Yasuda M, Shiba M, Ma YR, Horie T, Liu F, Ito S. Inhibitory effect of oestradiol on activation of rat hepatic stellate cells in vivo and in vitro. Gut. 1999;44:127-136. [Cited in This Article: ] |
| 32. | Xu JW, Gong J, Chang XM, Luo JY, Dong L, Hao ZM, Jia A, Xu GP. Estrogen reduces CCL4- induced liver fibrosis in rats. World J Gastroenterol. 2002;8:883-887. [Cited in This Article: ] |
| 33. | Iafrati MD, Karas RH, Aronovitz M, Kim S, Sullivan TR, Lubahn DB, O'Donnell TF, Korach KS, Mendelsohn ME. Estrogen inhibits the vascular injury response in estrogen receptor alpha-deficient mice. Nat Med. 1997;3:545-548. [Cited in This Article: ] |
| 34. | Karas RH, Hodgin JB, Kwoun M, Krege JH, Aronovitz M, Mackey W, Gustafsson JA, Korach KS, Smithies O, Mendelsohn ME. Estrogen inhibits the vascular injury response in estrogen receptor beta-deficient female mice. Proc Natl Acad Sci USA. 1999;96:15133-15136. [Cited in This Article: ] |
| 35. | Karas RH, Schulten H, Pare G, Aronovitz MJ, Ohlsson C, Gustafsson JA, Mendelsohn ME. Effects of estrogen on the vascular injury response in estrogen receptor alpha, beta (double) knockout mice. Circ Res. 2001;89:534-539. [Cited in This Article: ] |
| 36. | Dubey RK, Jackson EK. Cardiovascular protective effects of 17beta-estradiol metabolites. J Appl Physiol (1985). 2001;91:1868-1883. [Cited in This Article: ] |
| 37. | Dubey RK, Jackson EK. Estrogen-induced cardiorenal protection: potential cellular, biochemical, and molecular mechanisms. Am J Physiol Renal Physiol. 2001;280:F365-F388. [Cited in This Article: ] |