Published online Nov 7, 2009. doi: 10.3748/wjg.15.5200
Revised: August 4, 2009
Accepted: August 11, 2009
Published online: November 7, 2009
AIM: To examine the protective effect of green tea extract (GT) on hepatic fibrosis in vitro and in vivo in dimethylnitrosamine (DMN)-induced rats.
METHODS: HSC-T6, a rat hepatic stellate cell line, was used as an in vitro assay system. Cell proliferation, collagen content, and type 1 collagen expression were examined in activated HSC-T6 cells. Collagen was determined by estimating the hydroxyproline content. In rats with DMN-induced hepatic fibrosis, serum aspartate aminotransferase and alanine aminotransferase concentrations, liver hydroxyproline and lipid peroxides were determined. Pathologic changes were examined by hematoxylin & eosin staining.
RESULTS: GT administration prevented the development of hepatic fibrosis in the rat model of DMN-induced liver fibrosis. These results were confirmed both by liver histology and by quantitative measurement of hepatic hydroxyproline content, a marker of liver collagen deposition. Accordingly, inhibition of proliferation, reduced collagen deposition, and type 1 collagen expression were observed in activated HSC-T6 cells following GT treatment. These results imply that GT reduced the proliferation of activated HSC and down regulated the collagen content and expression of collagen type 1, thereby ameliorating hepatic fibrosis.
CONCLUSION: This study demonstrates that green tea administration can effectively improve liver fibrosis caused by DMN, and may be used as a therapeutic option and preventive measure against hepatic fibrosis.
-
Citation: Kim HK, Yang TH, Cho HY. Antifibrotic effects of green tea on
in vitro andin vivo models of liver fibrosis. World J Gastroenterol 2009; 15(41): 5200-5205 - URL: https://www.wjgnet.com/1007-9327/full/v15/i41/5200.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.5200
Hepatic fibrosis is a consequence of severe liver damage and occurs in many forms of chronic liver damage, including virus infection, autoimmune liver diseases and sustained alcohol abuse[1]. Hepatic stellate cells (HSC) are recognized as the primary cellular source of matrix components in chronic liver diseases, and therefore play a critical role in the development and maintenance of liver fibrosis[2]. The key cellular and molecular events involved in the pathogenesis of liver fibrosis include activation of HSC to a myofibroblast-like phenotype, production of excess matrix proteins, and increased cell proliferation[2]. Overproduction of extracellular matrix (ECM) components, particularly collagen, is a characteristic of activated HSC, and activation and proliferation of HSC have been implicated in the pathogenesis of liver fibrosis[3]. Therefore, suppression of HSC activation has been proposed as a therapeutic target against hepatic fibrosis[4].
Acetaldehyde, a highly reactive compound produced by alcohol metabolism, stimulates the deposition of ECM proteins. Acetaldehyde also stimulates type 1 collagen synthesis and gene transcription in cultured rat and human HSC[5] and in human liver fibroblasts[6].
Several studies have shown that lipid peroxidation stimulates collagen production in fibroblasts and HSC[7], and plays an important role in the development of liver fibrosis. Lipid peroxidation has been shown to stimulate the expression of collagen gene transcripts[8]. It has recently been shown that stellate cells are activated by free radicals as well as by malondialdehyde (MDA), a product of lipid peroxidation[9]. In addition, stellate cell activation by type 1 collagen has been shown to be blocked by antioxidants[9], suggesting that lipid peroxidation may play a role in hepatofibrogenesis.
Green tea, which is a widely consumed drink, has received much attention due to its beneficial biological effects. Polyphenols, often collectively referred to as catechins, account for up to 30% of the dry weight and serve as a major effective component of green tea. The effects of green tea have been widely studied and antioxidative, antiallergic, antimutagenic/anticarcinogenic, and antibacterial effects have been documented[10-12]. It has been shown that an aqueous extract of polyphenols from green tea (Camellia sinensis) reduces liver fibrosis in rats induced by bile duct ligation, and epigallocatechin gallate (EGCG), the major component in green tea, was implicated as the main active ingredient[13]. EGCG has been reported to suppress cell proliferation and collagen production in HSC[14]. In addition, the hepatoprotective effects of green tea against carbon tetrachloride, cholestasis and alcohol induced liver fibrosis were reported in many studies[13,15,16]. However, the hepatoprotective effect of green tea in dimethylnitrosamine (DMN)-induced models has not been studied. The DMN-induced liver fibrosis model can reproduce most of the features observed during human liver fibrosis[17]. Furthermore, this model has other advantages such as progressive and remarkable pathological alterations, a high fibrosis reproduction rate, and a low mortality rate in experimental animals[18]. This model is also stable even after termination of DMN administration and is a reliable tool for screening antifibrotic agents[19]. Therefore, the aim of the present study was to examine the protective effect of green tea extract (GT) on hepatic fibrosis in a rat HSC line and in a rat model of DMN-induced hepatic fibrosis.
Green tea, cultivated from Cheju island, Korea, was extracted with 80% methanol and freeze-dried.
Cell culture: HSC-T6 cells, an immortalized rat HSC line, were cultured in Dulbecco’s minimal essential medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% FBS (Gibco) and 0.5% antibiotics. Cultures were placed in a humidified atmosphere of 5% CO2 at 37°C, and the medium was changed twice a week. Acetaldehyde (175 μmol/L) was added to induce collagen type 1 and morphological features of activated stellate cell.
Cell viability: HSC-T6 cells were seeded into 96-well plates at a density of 1.5 × 104 cells/well until 50% confluence. Cells treated with GT (10, 50, 100 μg/mL) for 48 h were incubated with MTT (1 mg/mL) in a medium for 3 h at 37°C. The supernatant was removed and 100 μL of DMSO was added to each well to dissolve the formazan product. Absorbance at 570 nm was measured using a microplate reader.
Hydroxyproline content: Collagen was determined by estimating the hydroxyproline content, an amino acid characteristic of collagen. HSC-T6 cells were lysed after treatment with GT (100 μg/mL) for 24 h. The lysates were hydrolyzed in 6 mol/L HCl for 16 h at 110°C and evaporated to dryness to remove the acid. The residue, dissolved in distilled water, was mixed with 50% isopropanol and chloramine-T solution and left for 10 min at room temperature. Finally, p-dimethylaminobenzaldehyde in 60% perchloric acid was added and heated to 60°C for 25 min. The absorbance was measured at 558 nm.
Expression of collagen type 1: The expression of collagen type 1 was observed by ELISA. HSC-T6 cells, seeded on 24-well plates at a density of 1.5 × 105 and cultured until 90% confluency, were treated with serum-free DMEM with or without 175 μmol/L acetaldehyde. Ascorbic acid (50 μg/L), and 3-aminopropionitrile fumarate (100 μg/L) were also added to increase the collagen proline hydroxylation and to prevent collagen cross-linking. After 24 h of treatment with GT (100 μg/L), aliquots of medium were transferred into immunowell plates, and glutaraldehyde (0.01%) was added and incubated at room temperature for 1 h. Collagen type 1 antibody (1:4000, Abcam Co., Cambridge, UK) was added and further incubated for 2 h at 37°C. The antigen-coated plates were blocked with casein and incubated with the secondary antibody (1:8000) linked to peroxidase, and subsequently re-incubated with substrate (TMB 10 mg/mL, 3% H2O2, 50 mmol/L sodium acetate buffer, pH 5.1) for 15 min. The enzymatic reaction was stopped by adding 1 mol/L H2SO4, and the absorbance at 450 nm was measured with a microplate reader.
Animals and treatments: Male albino rats (235-250 g) were purchased from Samtako (Kyunggi-do, Korea) and housed in controlled temperature and relative humidity, and a 12 h light/dark cycle. All experiments were performed according to National guidelines for the use of animals in biomedical research. The rats were randomly assigned to four groups of eight rats each: the normal control group without any treatment (NC), the hepatic fibrosis control group (FC), and hepatic fibrosis with 100 mg/kg GT treated group (FG). Hepatic fibrosis was induced by intraperitoneal injections of 10 mg/kg dimethylnitrosamine (DMN, Sigma, St. Louis, USA) for 3 consecutive days each week over a period of 4 wk. Normal saline was given to NC rats. GT was administered in drinking water which was calculated according to the amount of water consumed the previous day. At the end of the 4 wk experimental period, all rats were killed under ether anesthesia. Blood was obtained from the inferior vena cava, and the liver was excised. The liver was immediately frozen for biochemical measurements or fixed in formalin for histochemical examination.
Hepatotoxicity and lipid peroxidation: Hepatotoxicity was assessed by quantifying the activities of serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using a spectrophotometric diagnostic kit (Youngdong Pharmaceutical Co., Korea). Lipid peroxidation in the liver and serum were determined by measuring the levels of MDA, an end product of lipid metabolism. For the serum sample, 3 mol/L sulfuric acid and 100 g/L phosphotungstic acid were added and incubated at room temperature for 10 min, and then centrifuged. For the liver sample, homogenates of liver in potassium phosphate buffer were prepared. MDA contents in the serum and liver samples were determined using a colorimetric reaction with thiobarbituric acid.
Hepatic hydroxyproline content: A portion of liver tissue (200 mg) was homogenized in 10 volumes of 0.5 mol/L potassium phosphate (KP) buffer and hydroxyproline content was measured as described above.
Histology of liver: Liver tissues were fixed in 10% neutral buffered formalin, dehydrated with 50%-100% ethanol, and embedded in paraffin. Five micrometer sections were cut and stained with hematoxylin-eosin.
All data were analyzed and expressed as mean ± SD. Comparisons were performed by Student’s t-test to detect differences between the groups. A level of P < 0.05 was considered statistically significant.
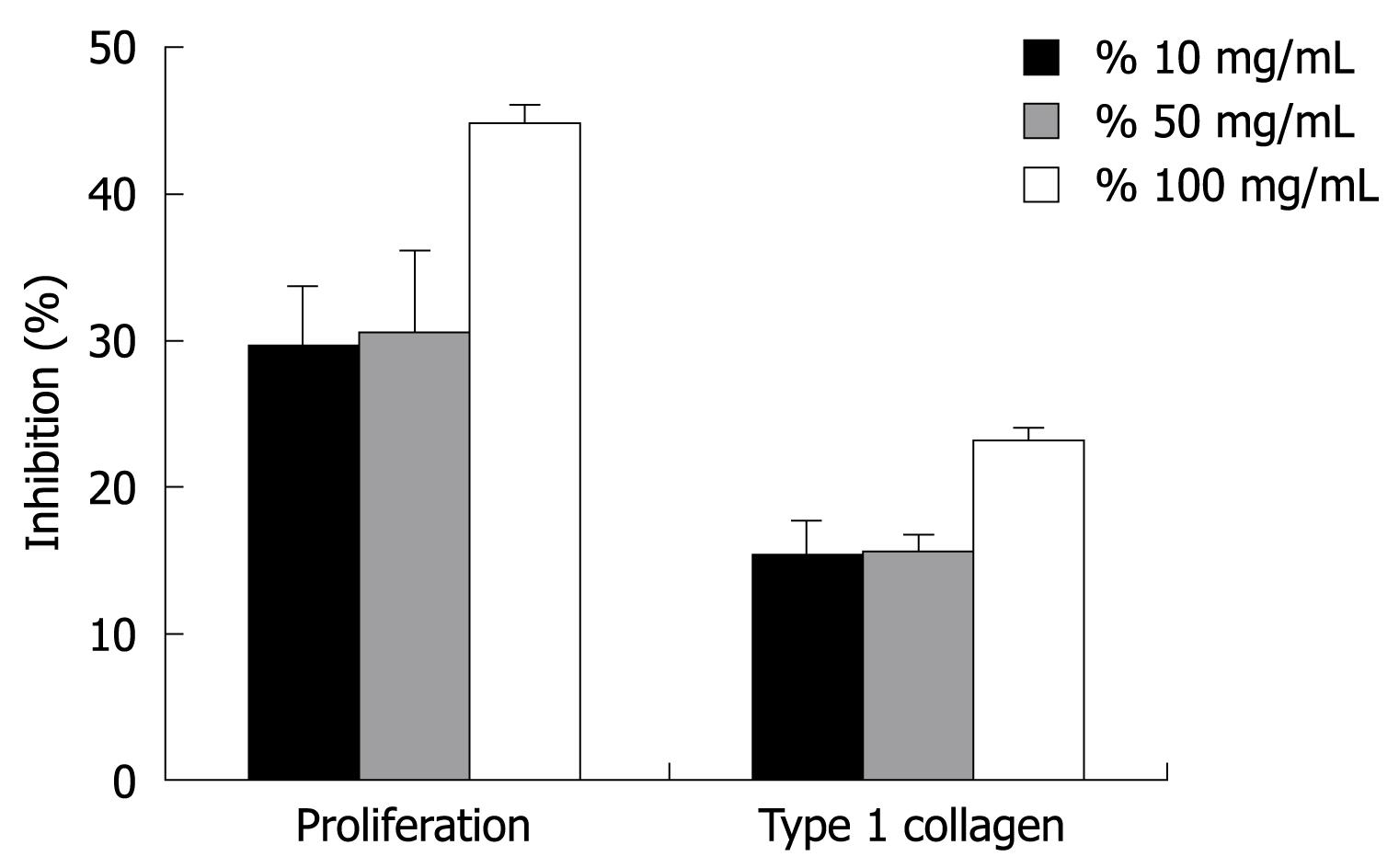
The anti-proliferative activity in HSC-T6 cells was determined by cell viability using the MTT assay. As shown in Figure 1, HSC-T6 cell proliferation was dose-dependently inhibited by GT. GT at 10, 50 and 100 μg/mL caused dose-dependent inhibition of HSC-T6 cell proliferation by 29.5% ± 4.2%, 30.6% ± 5.6%, and 44.8% ± 1.2 %, respectively (P < 0.05, Figure 1). The antiproliferative effects were not related to the nonspecific cytotoxic effects of green tea because cells showed normal morphology (data not shown).
To assess the effect of GT on ECM production, hydroxyproline content and type 1 collagen expression, assessed by ELISA, were examined in activated HSC-T6 cells. Serum-starved HSC-T6 cells were cultured with acetaldehyde and GT treatment for 24 h. Treatment with 100 μg/mL GT significantly reduced cell hydroxyproline content by 23.0% ± 2.1% compared to the control group. Furthermore, the expression of type 1 collagen was up-regulated by acetaldehyde stimulation, and GT markedly reduced collagen type 1 expression in a dose-dependent manner. Acetaldehyde at a concentration of 175 μmol/L induced collagen type 1 expression by 17.4% ± 0.1%, and 10, 50 and 100 mg/mL GT reduced collagen type 1 expression by 15.2% ± 2.2%, 15.5% ± 1.3%, and 23.0% ± 1.1%, respectively (Figure 1).
As shown in Table 1, the liver index, which is the percent of liver weight at final body weight, was not significantly different among the experimental groups. In contrast, relative spleen weight was increased 3.5-fold by DMN treatment, and GT administration restored the relative spleen weight (P < 0.05).
AST and ALT concentrations in serum were used as biochemical markers to evaluate hepatic injury. ALT is a cytosolic enzyme, primarily present in the liver. An increase in plasma ALT indicates liver damage more specifically than AST. AST, which is a mitochondrial enzyme present in large quantities in the heart, liver, skeletal muscle, and kidney, in part indicates liver injury. Serum activities of ALT and AST were markedly increased with DMN treatment and GT supplementation attenuated the elevation of AST and ALT activities (Table 1).
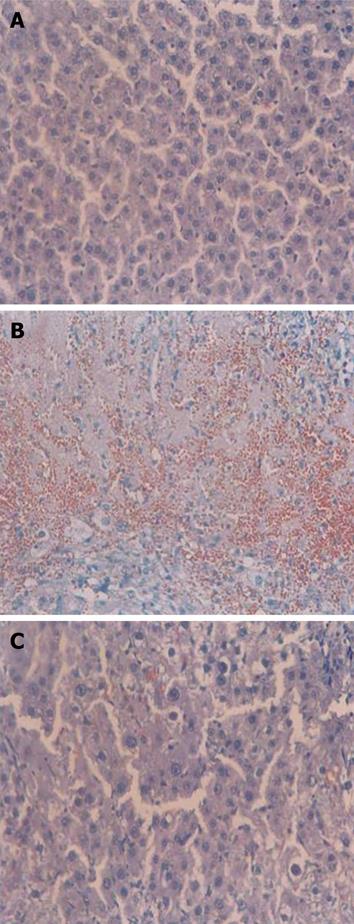
Liver fibrosis was evaluated by hematoxylin & eosin staining. The control group showed normal architecture (Figure 2A), whereas the DMN-treated group exhibited necrosis, congestion, hemorrhage, and destruction of the lobular architecture (Figure 2B). Red blood cells from blood vessels were found in liver tissue due to the collapse of the matrix structure. GT administration exhibited notable recovery effects (Figure 2C).
The histological findings were corroborated by biochemical parameters of liver tissue collagen content determined by hydroxyproline, and lipid peroxide determined by MDA.
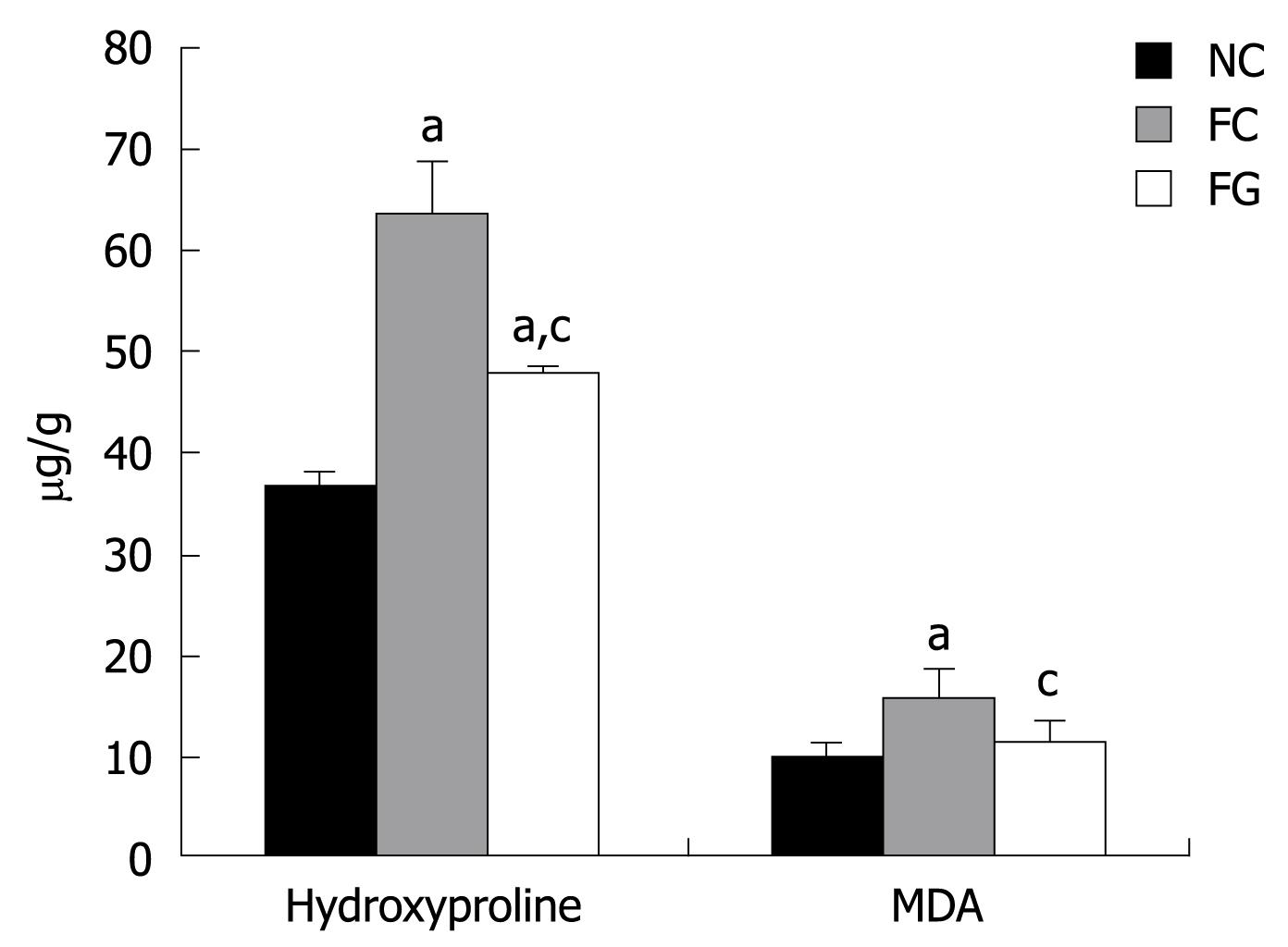
Hydroxyproline, a product of collagen metabolism, is an amino acid characteristic of collagen. The total collagen present in liver was, therefore, determined by estimating the hydroxyproline content. As shown in Figure 3, hydroxyproline content was significantly increased following DMN treatment (FC), indicating that the liver fibrosis model was successfully established. GT administration (FG, 100 mg/kg) restored the hydroxyproline content in fibrotic liver. Lipid peroxides, measured in terms of the formation of MDA, were significantly increased in DMN-induced rat liver. GT administration significantly reduced the lipid peroxide level.
Hepatic fibrosis is characterized by an abnormal accumulation of ECM proteins, particularly collagen[3,4]. When hepatic fibrosis occurs, collagen proliferation, mainly collagen type 1 and 3, accounts for 50% of the total protein in fibrotic liver[20], and collagens are the main components of ECM. Therefore, collagen type 1 is an important parameter reflecting the metabolism of collagen in liver. The main collagen producing cells in the liver are HSC, which proliferate and undergo a process of activation during the development of fibrosis resulting in increased capacity for collagen synthesis[21]. Changes in hydroxyproline content in the liver are considered an index for collagen metabolism and provide valuable information on the biochemical and pathological states of liver fibrosis. The present study demonstrated that consumption of GT prevented the development of hepatic fibrosis in a rat model of DMN-induced liver fibrosis. The results were confirmed both by liver histology and by quantitative measurement of hepatic hydroxyproline content, a marker of collagen deposition in liver.
Accordingly, inhibition of proliferation, reduced collagen content, and type 1 collagen expression were observed in activated HSC-T6 cells following GT treatment. Activated HSC are the main source of ECM when liver fibrosis occurs[22]. Therefore, these results imply that GT inhibit the proliferation of activated HSC and down regulate the collagen content and expression of collagen type 1, thereby inhibiting hepatic fibrosis. The results of the present study are consistent with previous observations showing that EGCG, the major component in green tea, suppresses collagen production[23], and proliferation[24] in HSC.
Oxidative stress resulting from the increased production of reactive oxygen species and lipid peroxides is suggested to be associated with the proliferation and activation of stellate cells either directly or through paracrine stimulation of neighboring cells including injured hepatocytes[25]. Furthermore, oxidative stress has been shown to modulate collagen gene expression[7]. Therefore, a number of studies have focused on the pathogenetic significance of oxidative stress in liver injury, as well as on the therapeutic intervention of this process with antioxidant and metabolic scavengers. GT administration resulted in a reduction of lipid peroxide in HSC-T6 cells and DMN-treated fibrotic liver. Chen et al[26] have also shown that a single-dose of EGCG improved hepatic injury in rats induced by CCl4 administration through the inhibition of lipid peroxidation.
In conclusion, this study demonstrates that green tea administration can effectively improve liver fibrosis caused by DMN as shown by reduced levels of collagen, lipid peroxidation, HSC proliferation, and type 1 collagen expression in the liver. Therefore, green tea may protect liver cells and reduce the deposition of collagen fibers in the liver. Green tea provides a safe and effective strategy for improving hepatic fibrosis.
Hepatic stellate cells (HSC) are recognized as the primary cellular source of matrix components in chronic liver diseases, and therefore play a critical role in the development and maintenance of liver fibrosis. Overproduction of extracellular matrix components, particularly collagen, is a characteristic of activated HSC, and activation and proliferation of HSC have been implicated in the pathogenesis of liver fibrosis.
Hepatoprotective effects of green tea against carbon tetrachloride, cholestasis and alcohol induced liver fibrosis were reported in many studies. However, the hepatoprotective effect of green tea in dimethylnitrosamine (DMN)-induced models has not been studied.
The present study demonstrates that consumption of green tea prevents the development of hepatic fibrosis in a rat model of DMN-induced liver fibrosis. These results were confirmed both by liver histology and by quantitative measurement of hepatic hydroxyproline content, a marker of collagen deposition in the liver. Accordingly, inhibition of proliferation, reduced collagen content, and type 1 collagen expression were observed in activated HSC cells following green tea treatment.
This study demonstrates that green tea may protect liver cells and reduces the deposition of collagen fibers in the liver. Green tea provides a safe and effective strategy for improving hepatic fibrosis.
HSC are the main collagen producing cells in the liver, which proliferate and undergo a process of activation during the development of fibrosis resulting in an increased capacity for collagen synthesis.
This study examined the protective effect of green tea extract on hepatic fibrosis in a HSC line and in a rat model of DMN-induced liver fibrosis. Green tea administration prevents the development of hepatic fibrosis in the rat model of liver fibrosis. Furthermore, inhibition of proliferation, reduced collagen deposition, and type 1 collagen expression were observed in activated HSC cells following green tea treatment. The results imply that green tea may protect liver cells and reduce the deposition of collagen fibers in the liver. Green tea provides a safe and effective strategy for improving hepatic fibrosis.
Peer reviewer: Fabio Grizzi, PhD, Laboratories of Quantitative Medicine, Istituto Clinico Humanitas IRCCS, Via Manzoni 56, 20089 Rozzano, Milan, Italy
S- Editor Li LF L- Editor Webster JR E- Editor Zheng XM
| 1. | Bissell DM. Hepatic fibrosis as wound repair: a progress report. J Gastroenterol. 1998;33:295-302. [Cited in This Article: ] |
| 2. | Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665-672. [Cited in This Article: ] |
| 3. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. [Cited in This Article: ] |
| 4. | Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol. 2005;45:605-628. [Cited in This Article: ] |
| 5. | Casini A, Cunningham M, Rojkind M, Lieber CS. Acetaldehyde increases procollagen type I and fibronectin gene transcription in cultured rat fat-storing cells through a protein synthesis-dependent mechanism. Hepatology. 1991;13:758-765. [Cited in This Article: ] |
| 6. | Savolainen ER, Leo MA, Timpl R, Lieber CS. Acetaldehyde and lactate stimulate collagen synthesis of cultured baboon liver myofibroblasts. Gastroenterology. 1984;87:777-787. [Cited in This Article: ] |
| 7. | Parola M, Pinzani M, Casini A, Albano E, Poli G, Gentilini A, Gentilini P, Dianzani MU. Stimulation of lipid peroxidation or 4-hydroxynonenal treatment increases procollagen alpha 1 (I) gene expression in human liver fat-storing cells. Biochem Biophys Res Commun. 1993;194:1044-1050. [Cited in This Article: ] |
| 8. | Lyons BL, Schwarz RI. Ascorbate stimulation of PAT cells causes an increase in transcription rates and a decrease in degradation rates of procollagen mRNA. Nucleic Acids Res. 1984;12:2569-2579. [Cited in This Article: ] |
| 9. | Lee KS, Buck M, Houglum K, Chojkier M. Activation of hepatic stellate cells by TGF alpha and collagen type I is mediated by oxidative stress through c-myb expression. J Clin Invest. 1995;96:2461-2468. [Cited in This Article: ] |
| 10. | Erba D, Riso P, Bordoni A, Foti P, Biagi PL, Testolin G. Effectiveness of moderate green tea consumption on antioxidative status and plasma lipid profile in humans. J Nutr Biochem. 2005;16:144-149. [Cited in This Article: ] |
| 11. | Shiozaki T, Sugiyama K, Nakazato K, Takeo T. [Effect of tea extracts, catechin and caffeine against type-I allergic reaction]. Yakugaku Zasshi. 1997;117:448-454. [Cited in This Article: ] |
| 12. | Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea--a review. J Am Coll Nutr. 2006;25:79-99. [Cited in This Article: ] |
| 13. | Zhong Z, Froh M, Lehnert M, Schoonhoven R, Yang L, Lind H, Lemasters JJ, Thurman RG. Polyphenols from Camellia sinenesis attenuate experimental cholestasis-induced liver fibrosis in rats. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1004-G1013. [Cited in This Article: ] |
| 14. | Sakata R, Ueno T, Nakamura T, Sakamoto M, Torimura T, Sata M. Green tea polyphenol epigallocatechin-3-gallate inhibits platelet-derived growth factor-induced proliferation of human hepatic stellate cell line LI90. J Hepatol. 2004;40:52-59. [Cited in This Article: ] |
| 15. | Xiao J, Lu R, Shen X, Wu M. [Green tea extracts protected against carbon tetrachloride-induced chronic liver damage and cirrhosis]. Zhonghua Yufang Yixue Zazhi. 2002;36:243-246. [Cited in This Article: ] |
| 16. | Arteel GE, Uesugi T, Bevan LN, Gäbele E, Wheeler MD, McKim SE, Thurman RG. Green tea extract protects against early alcohol-induced liver injury in rats. Biol Chem. 2002;383:663-670. [Cited in This Article: ] |
| 17. | Wasser S, Tan CE. Experimental models of hepatic fibrosis in the rat. Ann Acad Med Singapore. 1999;28:109-111. [Cited in This Article: ] |
| 18. | Jézéquel AM, Mancini R, Rinaldesi ML, Ballardini G, Fallani M, Bianchi F, Orlandi F. Dimethylnitrosamine-induced cirrhosis. Evidence for an immunological mechanism. J Hepatol. 1989;8:42-52. [Cited in This Article: ] |
| 19. | George J, Rao KR, Stern R, Chandrakasan G. Dimethylnitrosamine-induced liver injury in rats: the early deposition of collagen. Toxicology. 2001;156:129-138. [Cited in This Article: ] |
| 20. | Gressner AM. The cell biology of liver fibrogenesis - an imbalance of proliferation, growth arrest and apoptosis of myofibroblasts. Cell Tissue Res. 1998;292:447-452. [Cited in This Article: ] |
| 21. | Friedman SL. Cellular sources of collagen and regulation of collagen production in liver. Semin Liver Dis. 1990;10:20-29. [Cited in This Article: ] |
| 22. | Alcolado R, Arthur MJ, Iredale JP. Pathogenesis of liver fibrosis. Clin Sci (Lond). 1997;92:103-112. [Cited in This Article: ] |
| 23. | Nakamuta M, Higashi N, Kohjima M, Fukushima M, Ohta S, Kotoh K, Kobayashi N, Enjoji M. Epigallocatechin-3-gallate, a polyphenol component of green tea, suppresses both collagen production and collagenase activity in hepatic stellate cells. Int J Mol Med. 2005;16:677-681. [Cited in This Article: ] |
| 24. | Chen A, Zhang L. The antioxidant (-)-epigallocatechin-3-gallate inhibits rat hepatic stellate cell proliferation in vitro by blocking the tyrosine phosphorylation and reducing the gene expression of platelet-derived growth factor-beta receptor. J Biol Chem. 2003;278:23381-23389. [Cited in This Article: ] |
| 25. | Svegliati Baroni G, D'Ambrosio L, Ferretti G, Casini A, Di Sario A, Salzano R, Ridolfi F, Saccomanno S, Jezequel AM, Benedetti A. Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology. 1998;27:720-726. [Cited in This Article: ] |
| 26. | Chen JH, Tipoe GL, Liong EC, So HS, Leung KM, Tom WM, Fung PC, Nanji AA. Green tea polyphenols prevent toxin-induced hepatotoxicity in mice by down-regulating inducible nitric oxide-derived prooxidants. Am J Clin Nutr. 2004;80:742-751. [Cited in This Article: ] |