Published online Mar 14, 2008. doi: 10.3748/wjg.14.1575
Revised: November 28, 2007
Published online: March 14, 2008
AIM: To determine the inhibitory effect of the adenovirus-based angiopoietin-1 (Ang-1) targeted small interfering RNA expression system (Ad/Ang-1si) on the expression of the Ang-1 gene, cell growth and apoptosis in human esophageal cancer cell line Eca109.
METHODS: siRNA-expressing adenovirus targeting Ang-1 gene was constructed using the Ad Easy System. Cultured Eca109 cells were transfected with Ad/Ang-1si (Eca109/Ang-1si), and Ad/si was used to infect Eca109 cells as control (Eca109/si). Ang-1 gene expression and concentration was determined with RT-PCR and ELISA, respectively. Human umbilical vein endothelial cell (HUVEC) migration and proliferation were analyzed. After s.c. injection into athymic nu/nu mice, the tumor growth, vessel density and apoptosis of each group was also determined.
RESULTS: HUVEC migration induced by conditioned medium from Ang-1si-transfected Eca109 cells was significantly less than that induced by conditioned medium from Eca109 cells and control adenovirus-transfected Eca109 cells. Furthermore, after s.c. injection into athymic nu/nu mice, the tumor growth and cell apoptosis of Ad/Ang-1si -expressing Eca109 cells was significantly lower than that of parental or control adenovirus-transfected cells. Vessel density assessed by CD31 immunohistochemical analysis and Ang-1 expression by RT-PCR were also decreased.
CONCLUSION: The targeting Ang-1 may provide a therapeutic option for esophageal cancer.
- Citation: Liu XH, Bai CG, Yuan Y, Gong DJ, Huang SD. Angiopoietin-1 targeted RNA interference suppresses angiogenesis and tumor growth of esophageal cancer. World J Gastroenterol 2008; 14(10): 1575-1581
- URL: https://www.wjgnet.com/1007-9327/full/v14/i10/1575.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1575
Human esophageal cancer is the eighth most frequently diagnosed cancer and the sixth most frequent cause of cancer death in the world[1]. Esophageal cancer is one of the most lethal cancers of the digestive tract with a very low survival of 16% in the United States and 10% in Europe[2]. Most esophageal cancers arise from the middle and lower third of the esophagus. Despite the use of multimodal therapy (chemotherapy, radiation therapy, and surgery), the long-term disease-free survival rate of patients with esophageal cancer is still disappointingly low, particularly in the high-risk groups[3]. The identification of new therapeutic targets is therefore needed.
Angiogenesis has been specifically linked to increased growth and metastatic potential in human tumors[4–9]. Although numerous growth factors are involved, angiopoietin-1 (Ang-1) play a pivotal role in tumor angiogenesis[10–17]. Binding of Ang-1 to its ligand of the Tie-2 receptor reduces endothelial permeability and enhances vascular stabilization and maturation. All these events which contribute to angiogenesis and remodeling of blood vessels. However, Ang-1 contribution to tumor growth has received little attention.
Esophageal cancer is vascular in nature, with a high proliferation rate[18]. Several lines of evidence indicate a role of Ang-1 in the pathogenesis of esophageal cancer. Loges et al recently reported 94% of tumor specimens expressed Ang-1, implying a close association between Ang-1 expression and microvessel density (MVD)[19]. High levels of Ang-1 expression were also detected by immunohistochemical analysis in 42 of 45 esophageal cancer samples, and positive staining for Ang-1 at the time of diagnosis was correlated with VEGF121 and VEGF165 gene expression[20]. Taken together, these data indicate that Ang-1 is associated with neovascularization in the cancer stroma through VEGF networks in esophageal cancer, and inhibition of the expression or function of Ang-1 may improve in the disease outcome.
Our data in this study showed that adenoviruses-based siRNA expression system can be used to down-regulate Ang-1 expression, resulting in suppression of cell growth and induction of apoptosis through inhibiting angiogenesis in a nude mouse model of esophageal cancer. It can be concluded that Ang-1 is an alternative target in developing new therapeutic strategies for the treatment of esophageal cancers.
siRNA-expressing adenovirus targeting human Ang-1 was constructed according to the manufacturer's instructions. Briefly, one pairs of oligonucleotides targeting human Ang-1 mRNA was synthesized by Sagon DNA technologies (Sagon Biotechnology). The targeted Ang-1 sequences were 5’-GATCCCGAGGCTGGAAGGAATATAATTCAAGAGATTATATTCCTTCCAGCCTCTTTTTT-3’, 5’-AGCTAAAAAAGAGGCTGGAAGGAATATAATCTCTTGAATTATATTCCTTCCAGCCTCGG-3’. The dsDNA was ligated between the BamHI and HindIII sites on the pShuttle containing H1 promoter and GFP sequences. Adenoviruses were then constructed using the Ad Easy System (Strategene Biotechnology). The control vector was constructed by inserting a sequence that expresses a siRNA with limited homology to sequences in the human and mouse genomes. Adenoviral DNA was prepared on a large scale in Escherichia coli DH5α, generating Ad/Ang-1si and Ad/si, respectively. All adenoviruses were propagated in HEK293 cells and purified using BD Adeno-X™ purification kit (BD Biosciences Clontech). Viral titers were determined using BD Adeno X™ rapid titer kit (BD Biosciences Clontech).
Eca109 human esophageal cancer cell line was obtained from Shanghai Institute of Cell Biology, Chinese Academy of Sciences. Eca109 cells were cultured in DMEM with 10% fetal bovine serum. Eca109 cells were infected with the Ad/Ang-1si at 50 PFU/cell (Eca109/Ang-1si), Ad/si was used to infect Eca109 cells as control (Eca109/si). Adenovirus generation was confirmed by the expression of GFP. The ECV2304 endothelial cell line, derived from immortalized human umbilical vein endothelial cells (HUVEC) was purchased from Shanghai Institute of Cell Biology and grown in DMEM containing 10% FCS, 2 mmol/L glutamine, HAT (hypoxanthine 0.1 mmol/L, aminopterin 0.4 mmol/L, thymidine 16 mmol/L), and antibiotics. HUVECs (passage 3 or 4) with - 80% confluent were used for most experiments.
Eca109, Eca109/si and Eca109/Ang-1si cells were harvested and reseeded at 1 × 104 cells/well in 12-well plates. The total cell number was determined every two days with a hematocytometer and under an inverted microscope (Olympus). Cell viability was detected by trypan blue staining. Each value represents the average of triplicate wells.
Total RNA was isolated from Eca109, Eca109/si and Eca109/Ang-1si cells using the Trizol protocol (Invitrogen Biotechnology). Reverse transcription reactions were carried out for 1 h at 42°C with 1 &mgr;g of total RNA, 250 ng of oligo(dT), 1 × deoxynucleotide triphosphate mix, RNase inhibitor (Promega), 1 × RT buffer, and 200 units of SuperScript II RT (Invitrogen) in a total volume of 20 &mgr;L. Amplification of Ang-1 was performed in 50 &mgr;L of reaction mixture consisting of sense and antisense primers for Ang-1: 2 &mgr;g of cDNA, 1 × deoxynucleotide triphosphate mix, 1 × PCR buffer, 1.5 mmol/L MgCl2, and 2.5 units of AmpliTaq Gold DNA Polymerase (Perkin-Elmer, Wellesley, MA). The following primers were used: 5’-ATGACAGTTTTCCTTTCC-3’, 5’-TCAAAAATCTAAAGGTCG-3’ (Sagon Biotechnology).
Eca109, Eca109/si and Eca109/Ang-1si cells (2.5 × 105) were seeded into 24-well plates. Fresh medium was added after overnight culture. The cultured supernatants were collected 24 h later and centrifuged to eliminate cellular fragments. Ang-1 protein accumulated in the culture medium was analyzed using sandwich ELISA, wherein the supernatant of the culture was incubated with Ang-1 antibody (goat polyclonal anti-human Ang-1, Santa Cruz Biotechnology) and streptavidin alkaline phosphatase (Santa Cruz Biotechnology). The antigen-antibody complex was then incubated with p-nitrophenyl phosphate (Sigma Biotechnology) dissolved in pNPP buffer (Chemicon Biotechnology). Ang-1 concentrations in the samples were determined from the absorbance at 570 nm spectrophotometrically.
Cultured supernatants from Eca109, Eca109/si, and Eca109/Ang-1si cells were collected. Transwells (Costar, Cambridge, MA) were pretreated with serum-free medium at 37°C for 1 h before seeding with HUVECs at 1 × 105 per well in 100 &mgr;L endothelial basal medium with 0.1% fetal bovine serum. The transwells were then inserted into 24-well plates containing 600 &mgr;L conditioned medium and incubated at 37°C for 6 h to allow HUVEC cells to migrate. Cells on the upper side of the filter were removed with cotton swabs. Migrated cells on the lower side of the filter were fixed and stained with HE. The number of migrated cells was counted under a binocular microscope.
HUVEC cells (2.5 × 105) were seeded into 96-well plates, and allowed to adhere for 5 h. The metabolic activity of HUVEC cells was determined every two days by methyl thiazoleterazolium (MTT) assay. Briefly, after light rinsing with PBS, 40 &mgr;L 5 g/L MTT (Sigma Biotechnology) was added. After 4 h of incubation at 37°C, HUVEC cells were washed with PBS, and then dissolved in 150 &mgr;L dimethyl sulfoxide (DMSO). The absorbance of each group was determined spectrophotometrically (λ = 570 nm).
Four- to 5-wk-old specific pathogen-free athymic (T-cell deficient) nude mice were purchased from Shanghai Experimental Animal Supply, China. The animal protocol was approved by the Institutional Animal Care and Utilization Committee of the Second Military Medical University. Eca109, Eca109/si, and Eca109/Ang-1si cells in mid-log-growth phase were harvested by trypsinization. Single-cell suspensions (2 × 106 cells in 0.1 mL HBSS) were injected s.c. into the nude mice. The tumors were measured every 4 d with a caliper, and the diameters were recorded. Tumor volume was calculated by the formula: a2b/2, where a and b are the two maximum diameters. When tumors reached 2 cm × 2 cm, the mouse was euthanized, and the tumor tissue was collected, fixed in formalin, embedded in paraffin, cut into 3 &mgr;m sections, and stained with HE.
Tissue sections stained with CD31 was used for evaluating MVD. Immunohistochemical staining was performed using the two step immunohistochemical technique with the DAKO EnVision system (EnVisionTM + Kits, HRP, DAKO Biotechnology). For antigen retrieval, the slides were treated with boiling 0.01 mol/L citrate buffer (pH 6.0) for 15 min. Tissue sections were incubated at room temperature for 1 h with CD31 antibody (goat polyclonal anti-human CD31, Santa Cruz Biotechnology), followed by incubation with horseradish peroxidase-labeled goat anti-mouse immunoglobulin for 30 min and developed with 3, 3'-diaminobenzidine. Then the slides were examined under × 100 magnification for the hot spots rich in vessels and MVDs were counted under × 200 magnification (0.708 mm2/field), so that every single brown-stained cell and cell cluster was calculated as a blood vessel, no matter whether a vessel lumen structure was seen. For vessels with large lumens, every 40 &mgr;m length of lumen was calculated as one vessel. Five different fields were chosen on each of the slides, and the stained vessels were counted simultaneously by 2 researchers under a multiocular microscope. The average of the 5 areas was recorded as the MVD score.
The TUNEL assay was performed for detection of in situ apoptotic cell death according to the manufacturer’s instructions (in situ cell death detection kit, HRP; Roche Biotechnology). Briefly, the sections were treated with proteinase K (20 mg/L) for 30 min and incubated at room temperature for 1 h with TUNEL reaction mixture, 50 &mgr;L peroxidase conjugate was added to each slide and the slides incubated for 30 min. For color reaction, 3, 3'-diaminobenzidine was used. The apoptotic index was expressed as the number of TUNEL-positive nuclei per 1000 cells counted for each sample.
The data were expressed as mean ± SE. Statistical comparison was made using SPSS10.0 statistical software package. The criterion for statistical significance was P < 0.05.
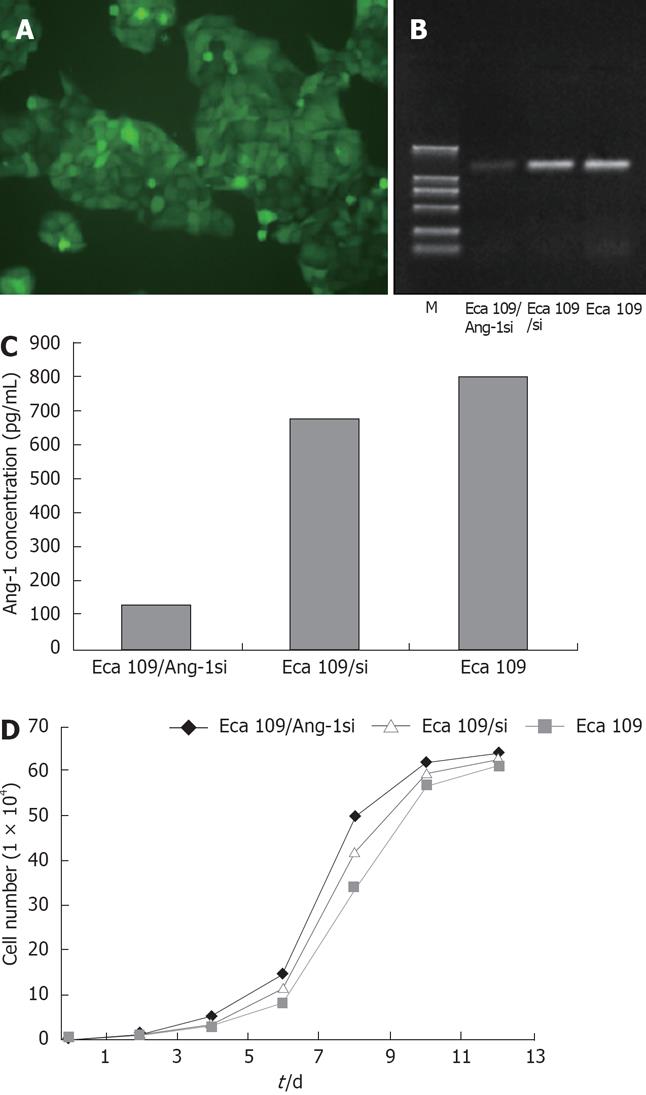
Adenovirus expressing siRNA to Ang-1 was constructed using the Ad Easy System to target human Ang-1 mRNA. Eca109 cells were stably transfected with this adenovirus, the transfection efficiency being approximately 100% (Figure 1A). The Ang-1 mRNA level was measured using RT-PCR, as shown in Figure 1B, Ang-1 expression was significantly inhibited by Ang-1si in Eca109/Ang-1si cells and Ang-1 level was reduced by 80% compared with Eca109 and Eca109/si cells. ELISA was performed using human Ang-1-specific antibody to quantify the amount of Ang-1 protein in the culture media. When cells were transfected with adenovirus expressing siRNA to Ang-1, the Ang-1 concentration in the media was decreased significantly (Figure 1C) compared with Eca109 and Eca109/si cells (P < 0.05). These results were in agreement with the amount of Ang-1 mRNA analyzed by RT-PCR experiments.
In addition, cell number was measured at various time points and no difference of cell growth curve was found among Eca109, Eca109/si, and Eca109/Ang-1si cells (Figure 1D).
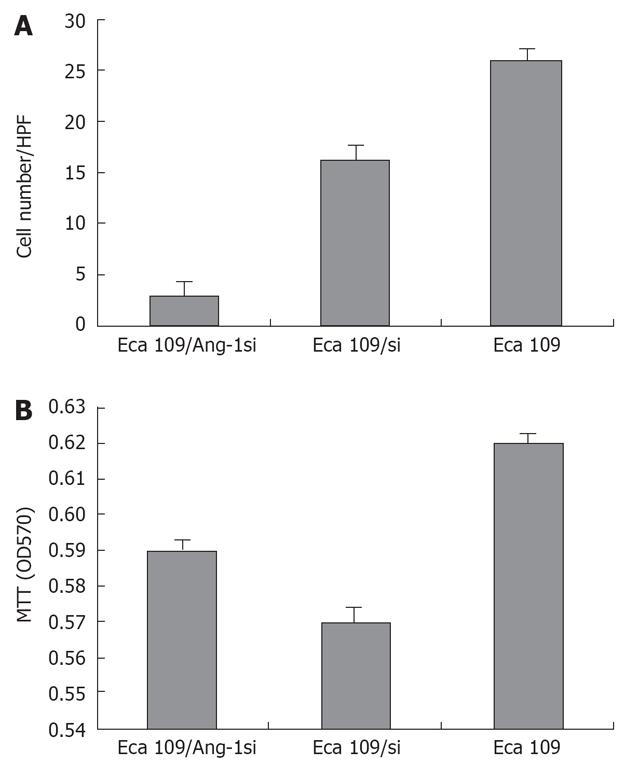
We collected cultured supernatants from Eca109, Eca109/si, and Eca109/Ang-1si cells. As shown in Figure 2A, the culture supernatants from Eca109 and Eca109/si cells induced robust endothelial cells migration. By contrast, few endothelial cells migrated when conditioned medium from Eca109/Ang-1si cells was used as the chemotactic stimulus.
To determine whether Ang-1 inhibition by siRNA influenced endothelial cells proliferation, the proliferative activity of each cell line was quantified by MTT. However, endothelial cells proliferation was not significantly lower when cells were cultured for 48 h in conditioned medium from Eca109/Ang-1si cells rather than from untransfected or control adenovirus-transfected cells (Figure 2B).
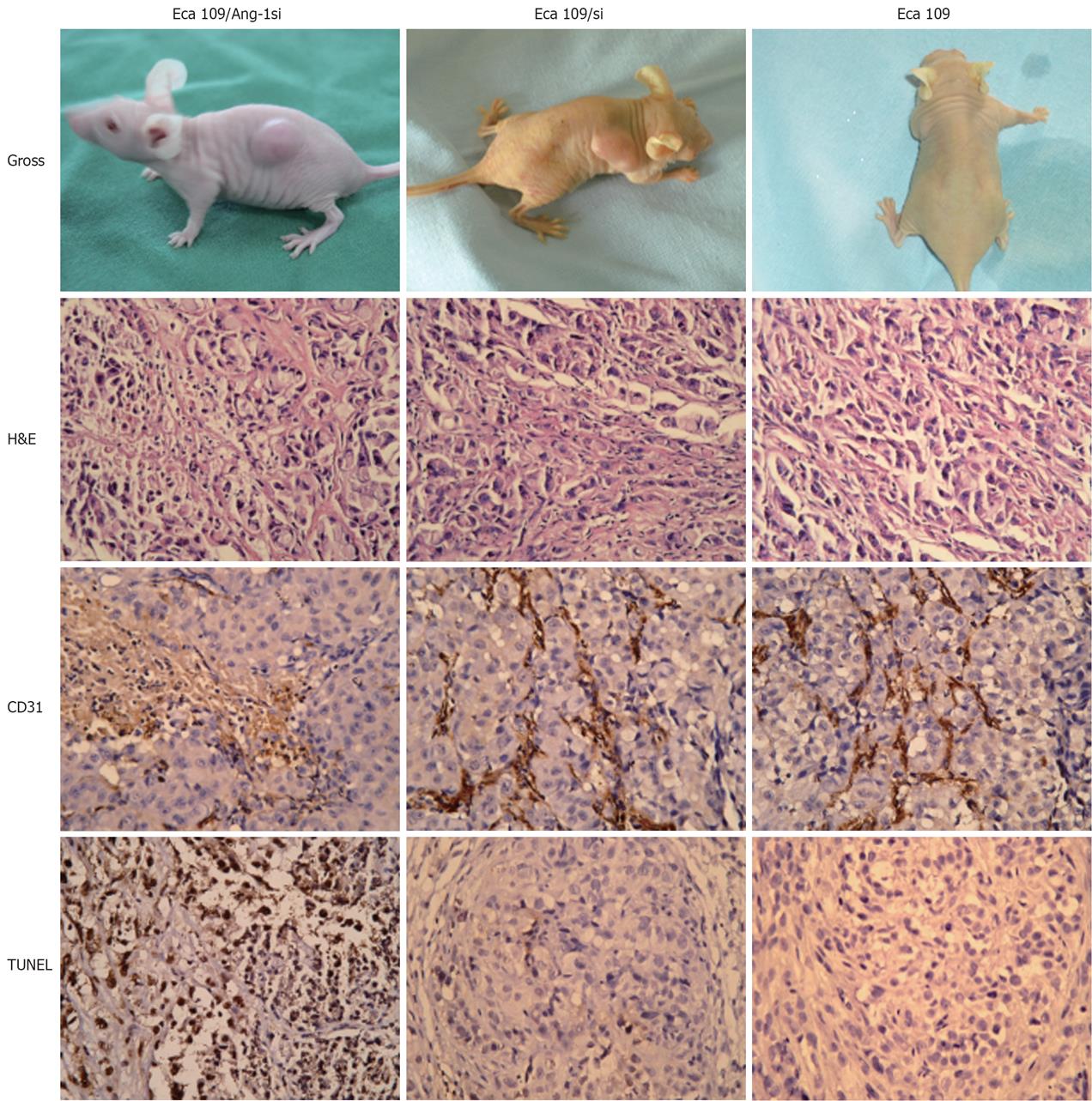
To determine whether inhibition of Ang-1 by siRNA had an effect on tumor growth, Eca109, Eca109/si, or Eca109/Ang-1si cells was inoculated s.c. into nu/nu mice. Eca109 and Eca109/si cells grew rapidly, resulting in palpable tumors 3-4 d following injection. By contrast, tumor formation was significantly slow after inoculation of Eca109/Ang-1si. The Eca109/Ang-1si tumors were significantly smaller than those in both control groups (Figure 3A).
H&E staining showed that Eca109/Ang-1si tumors were pale, with a massively necrotic center and a thin layer of tumor cells in the periphery (Figure 3B). On the contrary, control tumors appeared very vascularized.
As shown in Figure 3C, CD31-positive vessels were abundant in Eca109 and Eca109/si tumors. MVD was significantly decreased in tumors formed by Eca109/Ang-1si, although numerous vessels were seen in the normal tissues surrounding the tumor (Figure 3D). The MVD in tumors treated with Eca109/si was similar to that observed in the Eca109 tumors.
Terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling assay revealed the presence of massive apoptotic and necrotic cells in mice inoculated with Eca109/Ang-1si tumors. Eca109 and Eca109/si tumors showed only small areas of necrosis and apoptosis. Figure 3D shows that the apoptotic cells significantly increased in Eca109/Ang-1si tumors compared to Eca109 and Eca109/si tumors (Figure 3, P < 0.05).
Several studies have shown that Ang-1 is abundantly expressed in the primary tumors of esophageal cancer patients. In the present study, we showed that Ang-1 played a critical role in the growth of esophageal cancer. This was done by selectively inhibiting Ang-1 expression and protein production using RNA-mediated interference by siRNA.
RNA interference is a phenomenon that can be used to block specific gene expression, and has advantages over other strategies because of its high selectivity and potency. Delivery of small interfering RNA (siRNA) can be achieved through exogenous application of synthetic siRNA or through endogenous expression using plasmid or vector delivery to the target cells[2122]. Chemically or enzymatically synthesized siRNA is costly and has been shown to have a relatively short half-life with only transient inhibition of the target gene because of its low transfection rate and short duration of interference. Moreover, DNA vector-based siRNA technology has some advantages over chemically or enzymatically synthesized siRNA, as it induces more efficient and stable RNA interference[2324]. However, DNA plasmid vector induced siRNA still has limitations with respect to the clinical application of siRNA technology due to its low transfection efficiency.
To overcome these shortcomings, we constructed an adenovirus-based expression system in which sense and antisense strands of short Ang-1 sequence was transcribed into hairpin structures under the control of a H1 promoter and then processed into functional siRNAs by double strand-specific RNase. In this study, we used the highly selective siRNA approach to adenoviral constructs for genetic blockade of Ang-1 with a transfection efficiency of approximately 100%. Ang-1si specifically blocked Ang-1 expression in Eca109 human esophageal cancer cell line. Reduction in Ang-1 protein production was also documented by the finding that HUVEC cell migration induced by Eca109 conditioned medium was almost completely abolished following transfection with Ad/Ang-1si but it was not affected by the Ad/si control adenovirus. By contrast, HUVEC cell proliferation was unchanged in both Eca109/Ang-1si and Eca109/si cells. These results were in agreement with the reports of Koblizek et al that Ang-1 was not an endothelial cell mitogen[2526]; however, it stimulated endothelial cell migration[27–30].
Transfection of Ad/Ang-1si into Eca109 cells did not alter the cell growth in vitro. However, when these cells were injected either s.c. or into the bone of nude mice, tumor growth was slower than in parental and Ad/si control-transfected cells. Eca109/Ang-1si cells produced small tumors that were avascular in appearance with decreased vessel density and increased apoptotic and necrotic cells. Together, these data confirmed once again the specificity of our Ang-1si and indicated that Ang-1 played a pivotal role in esophageal cancer angiogenesis and tumor growth through promoting tumor neovascularization independently from promotion of tumor cell proliferation.
In summary, we have applied the highly selective siRNA approach to adenoviral constructs for genetic blockage of Ang-1 with a high transfection rate and long duration of interference. We found that siRNA technology can be used to specifically inhibit Ang-1 expression. In vitro promotion of angiogenesis in Ang-1 protein was documented by the finding that HUVEC cell migration induced by Eca109 conditioned medium was almost completely abolished following transfection with Ad/Ang-1si. In vivo cell transfection of Ad/Ang-1si resulted in selective inhibition of Ang-1 expression, leading to decreased tumor vascularity and growth in vivo. These data indicated that Ang-1 played a central role in esophageal cancer angiogenesis. Therefore, targeting Ang-1 with specific small-molecule inhibitors may have therapeutic benefit.
Angiopoietin-1 (Ang-1) has been shown to play a pivotal role in tumor angiogenesis. Several lines of evidence indicate that Ang-1 is associated with neovascularization in esophageal cancer, and inhibition of the expression or function of Ang-1 may improve the disease outcome. This study showed that adenoviruses-based small interfering RNA (siRNA) expression system can be used to down-regulate Ang-1 expression, resulting in suppression of cell growth and induction of apoptosis through inhibiting angiogenesis in a nude mouse model of esophageal cancer.
Angiogenesis has been found to be specifically linked to increased growth and metastatic potential in human tumors. Study in this field has become one of the hot spots at present. Previous studies demonstrated that growth factors, especial Ang-1, are responsible for the growth of esophageal cancer. Based on these findings, treatment targeting this gene has been designed and studied in in vivo and in vitro models. Preliminary results of these studies have shown beneficial and promising effects. Further experimental and clinical studies are needed before certain conclusions can be reached.
The association between angiopoietin-1 and angiogenesis and human cancer has been studied previously. However, the inhibitory effect of the adenovirus-based Ang-1 targeted small interfering RNA expression system (Ad/Ang-1si) on the expression of the Ang-1 gene, cell growth and apoptosis in Eca109 has not been explored. This study has bridged this gap and may provide additional targets for therapeutic development.
Since some basic evidence provided for angiopoietin-1, angiogenesis and their relationships in the development of human cancer, therapeutic approaches targeting angiopoietin-1 can be implemented in future studies and development of new methods for human cancers.
Angiopoietin-1 (Ang-1): Angiopoietin, a group of proteins including four molecules with similar structure, belongs to a growth factor family. Ang-1 is an endothelium- specific growth factor that can promote angiogenesis. As a 70 kDa glycoprotein, it is mainly produced by juxtavascular cells including pericytes, vascular smooth muscle cells and tumor cells, affecting the surrounding vascular endothelial cells through paracrine action. It can promote migration of endothelial cells, anti-apoptosis, and formation of luminal structure. Thus, it plays an important role in angiogenesis of human cancer.
This is an informative study demonstrating that Ang-1 played a central role in esophageal cancer angiogenesis. Therefore, targeting Ang-1 with specific small-molecule inhibitors may have therapeutic benefit. The preliminary conclusion is justified and substantiated by the results obtained.
| 1. | Sutter AP, Hopfner M, Huether A, Maaser K, Scherubl H. Targeting the epidermal growth factor receptor by erlotinib (Tarceva) for the treatment of esophageal cancer. Int J Cancer. 2006;118:1814-1822. [Cited in This Article: ] |
| 2. | Aklilu M, Ilson DH. Targeted agents and esophageal cancer--the next step? Semin Radiat Oncol. 2007;17:62-69. [Cited in This Article: ] |
| 3. | Zhang Z, Liao Z, Jin J, Ajani J, Chang JY, Jeter M, Guerrero T, Stevens CW, Swisher S, Ho L. Dose-response relationship in locoregional control for patients with stage II-III esophageal cancer treated with concurrent chemotherapy and radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:656-664. [Cited in This Article: ] |
| 4. | Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res. 2007;67:2729-2735. [Cited in This Article: ] |
| 5. | Hassouneh B, Islam M, Nagel T, Pan Q, Merajver SD, Teknos TN. Tetrathiomolybdate promotes tumor necrosis and prevents distant metastases by suppressing angiogenesis in head and neck cancer. Mol Cancer Ther. 2007;6:1039-1045. [Cited in This Article: ] |
| 6. | Prat A, Casado E, Cortes J. New approaches in angiogenic targeting for colorectal cancer. World J Gastroenterol. 2007;13:5857-5866. [Cited in This Article: ] |
| 7. | Inda AM, Andrini LB, Garcia MN, Garcia AL, Fernandez Blanco A, Furnus CC, Galletti SM, Prat GD, Errecalde AL. Evaluation of angiogenesis with the expression of VEGF and CD34 in human non-small cell lung cancer. J Exp Clin Cancer Res. 2007;26:375-378. [Cited in This Article: ] |
| 8. | Lee BL, Kim WH, Jung J, Cho SJ, Park JW, Kim J, Chung HY, Chang MS, Nam SY. A hypoxia-independent up-regulation of hypoxia-inducible factor-1 by AKT contributes to angiogenesis in human gastric cancer. Carcinogenesis. 2008;29:44-51. [Cited in This Article: ] |
| 9. | Lesko E, Majka M. The biological role of HGF-MET axis in tumor growth and development of metastasis. Front Biosci. 2008;13:1271-1280. [Cited in This Article: ] |
| 10. | Niedzwiecki S, Stepien T, Kopec K, Kuzdak K, Komorowski J, Krupinski R, Stepien H. Angiopoietin 1 (Ang-1), angiopoietin 2 (Ang-2) and Tie-2 (a receptor tyrosine kinase) concentrations in peripheral blood of patients with thyroid cancers. Cytokine. 2006;36:291-295. [Cited in This Article: ] |
| 11. | Caine GJ, Ryan P, Lip GY, Blann AD. Significant decrease in angiopoietin-1 and angiopoietin-2 after radical prostatectomy in prostate cancer patients. Cancer Lett. 2007;251:296-301. [Cited in This Article: ] |
| 12. | Wang J, Wu KC, Zhang DX, Fan DM. Antisense angiopoietin-1 inhibits tumorigenesis and angiogenesis of gastric cancer. World J Gastroenterol. 2006;12:2450-2454. [Cited in This Article: ] |
| 13. | Moon WS, Park HS, Yu KH, Jang KY, Kang MJ, Park H, Tarnawski AS. Expression of angiopoietin 1, 2 and their common receptor Tie2 in human gastric carcinoma: implication for angiogenesis. J Korean Med Sci. 2006;21:272-278. [Cited in This Article: ] |
| 14. | Kato Y, Asano K, Mizutani I, Konno T, Sasaki Y, Kutara K, Teshima K, Edamura K, Kano R, Suzuki K. Gene expressions of canine angiopoietin-1 and -2 in normal tissues and spontaneous tumours. Res Vet Sci. 2006;81:280-286. [Cited in This Article: ] |
| 15. | Tang D, Nagano H, Yamamoto H, Wada H, Nakamura M, Kondo M, Ota H, Yoshioka S, Kato H, Damdinsuren B. Angiogenesis in cholangiocellular carcinoma: expression of vascular endothelial growth factor, angiopoietin-1/2, thrombospondin-1 and clinicopathological significance. Oncol Rep. 2006;15:525-532. [Cited in This Article: ] |
| 16. | Fu YG, Sung JJ, Wu KC, Bai AH, Chan MC, Yu J, Fan DM, Leung WK. Inhibition of gastric cancer cells associated angiogenesis by 15d-prostaglandin J2 through the downregulation of angiopoietin-1. Cancer Lett. 2006;243:246-254. [Cited in This Article: ] |
| 17. | Zadeh G, Reti R, Koushan K, Baoping Q, Shannon P, Guha A. Regulation of the pathological vasculature of malignant astrocytomas by angiopoietin-1. Neoplasia. 2005;7:1081-1090. [Cited in This Article: ] |
| 18. | Matsumoto S, Yamada Y, Narikiyo M, Ueno M, Tamaki H, Miki K, Wakatsuki K, Enomoto K, Yokotani T, Nakajima Y. Prognostic significance of platelet-derived growth factor-BB expression in human esophageal squamous cell carcinomas. Anticancer Res. 2007;27:2409-2414. [Cited in This Article: ] |
| 19. | Loges S, Clausen H, Reichelt U, Bubenheim M, Erbersdobler A, Schurr P, Yekebas E, Schuch G, Izbicki J, Pantel K. Determination of microvessel density by quantitative real-time PCR in esophageal cancer: correlation with histologic methods, angiogenic growth factor expression, and lymph node metastasis. Clin Cancer Res. 2007;13:76-80. [Cited in This Article: ] |
| 20. | Nagata J, Kijima H, Hatanaka H, Tokunaga T, Kamochi J, Abe Y, Takagi A, Mine T, Yamazaki H, Nakamura M. Angiopoietin-1 and vascular endothelial growth factor expression in human esophageal cancer. Int J Mol Med. 2002;10:423-426. [Cited in This Article: ] |
| 21. | Allen D, Kenna PF, Palfi A, McMahon HP, Millington-Ward S, O'Reilly M, Humphries P, Farrar GJ. Development of strategies for conditional RNA interference. J Gene Med. 2007;9:287-298. [Cited in This Article: ] |
| 22. | Jiang Z, Zhao P, Zhou Z, Liu J, Qin L, Wang H. Using attenuated Salmonella typhi as tumor targeting vector for MDR1 siRNA delivery. Cancer Biol Ther. 2007;6:555-560. [Cited in This Article: ] |
| 23. | Du C, Ge B, Liu Z, Fu K, Chan WC, McKeithan TW. PCR-based generation of shRNA libraries from cDNAs. BMC Biotechnol. 2006;6:28. [Cited in This Article: ] |
| 24. | Best A, Handoko L, Schluter E, Goringer HU. In vitro synthesized small interfering RNAs elicit RNA interference in african trypanosomes: an in vitro and in vivo analysis. J Biol Chem. 2005;280:20573-20579. [Cited in This Article: ] |
| 25. | McCarter SD, Mei SH, Lai PF, Zhang QW, Parker CH, Suen RS, Hood RD, Zhao YD, Deng Y, Han RN. Cell-based angiopoietin-1 gene therapy for acute lung injury. Am J Respir Crit Care Med. 2007;175:1014-1026. [Cited in This Article: ] |
| 26. | Chen JX, Zeng H, Lawrence ML, Blackwell TS, Meyrick B. Angiopoietin-1-induced angiogenesis is modulated by endothelial NADPH oxidase. Am J Physiol Heart Circ Physiol. 2006;291:H1563-H1572. [Cited in This Article: ] |
| 27. | Nguyen VP, Chen SH, Trinh J, Kim H, Coomber BL, Dumont DJ. Differential response of lymphatic, venous and arterial endothelial cells to angiopoietin-1 and angiopoietin-2. BMC Cell Biol. 2007;8:10. [Cited in This Article: ] |
| 28. | Kim YM, Kim KE, Koh GY, Ho YS, Lee KJ. Hydrogen peroxide produced by angiopoietin-1 mediates angiogenesis. Cancer Res. 2006;66:6167-6174. [Cited in This Article: ] |