Published online Sep 28, 2014. doi: 10.3748/wjg.v20.i36.12781
Revised: April 17, 2014
Accepted: June 20, 2014
Published online: September 28, 2014
Helicobacter pylori (H. pylori) is an extremely common, yet underappreciated, pathogen that is able to alter host physiology and subvert the host immune response, allowing it to persist for the life of the host. H. pylori is the primary cause of peptic ulcers and gastric cancer. In the United States, the annual cost associated with peptic ulcer disease is estimated to be $6 billion and gastric cancer kills over 700000 people per year globally. The prevalence of H. pylori infection remains high (> 50%) in much of the world, although the infection rates are dropping in some developed nations. The drop in H. pylori prevalence could be a double-edged sword, reducing the incidence of gastric diseases while increasing the risk of allergies and esophageal diseases. The list of diseases potentially caused by H. pylori continues to grow; however, mechanistic explanations of how H. pylori could contribute to extragastric diseases lag far behind clinical studies. A number of host factors and H. pylori virulence factors act in concert to determine which individuals are at the highest risk of disease. These include bacterial cytotoxins and polymorphisms in host genes responsible for directing the immune response. This review discusses the latest advances in H. pylori pathogenesis, diagnosis, and treatment. Up-to-date information on correlations between H. pylori and extragastric diseases is also provided.
Core tip:Helicobacter pylori (H. pylori) infection remains a common cause of morbidity and mortality. Evidence for additional H. pylori-mediated diseases, as well as potentially beneficial effects of H. pylori infection, complicates decisions regarding when testing for and treating of H. pylori infections is appropriate. In the meantime, eradication of H. pylori is becoming more difficult due to increasing antibiotic resistance. This review summarizes recent findings on H. pylori pathogenesis, testing, and treatment.
-
Citation: Testerman TL, Morris J. Beyond the stomach: An updated view of
Helicobacter pylori pathogenesis, diagnosis, and treatment. World J Gastroenterol 2014; 20(36): 12781-12808 - URL: https://www.wjgnet.com/1007-9327/full/v20/i36/12781.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i36.12781
Helicobacter pylori (H. pylori) has infected humans for more than 58000 years[1], yet it largely escaped notice until it was cultured by Marshall and Warren[2]. Research on H. pylori changed paradigms regarding disease causation. Physicians previously attributed ulcers to stress or anxiety and did not believe that bacteria could cause cancer. H. pylori was the first bacterial species proven to cause cancer and is classified as a group I carcinogen by the International Agency for Research on Cancer. This is the same category as smoking, radiation, and asbestos. Many patients with H. pylori-associated MALT lymphoma can be cured by antibiotic treatment without the need for surgery or chemotherapy.
H. pylori-associated gastric cancer comprises about 5.5% of all cancers globally and accounts for 25% of all infection-associated cancers[3]. If H. pylori increases the risk of developing other diseases, the cost of H. pylori-associated morbidity could be much higher. An effective H. pylori vaccine is not yet on the horizon, so eradication must be accomplished using antibiotics. As with many other organisms, H. pylori antibiotic resistance is increasing[4,5].
Researchers continue to discover new associations between H. pylori and idiopathic diseases, as well as potential benefits of H. pylori infection. The systemic effects of gastric colonization can lead to extragastric pathology; however new sites of H. pylori colonization have also been identified. More research is needed to clarify which extragastric diseases are caused by H. pylori and whether the bacteria must be present at the disease site.
Unlike most bacterial pathogens, H. pylori typically colonizes the host for life unless specific treatment is given. H. pylori has co-evolved with humans for approximately 58000 years, and strain types that predominate within certain regions of the world correlate with human migration patterns[1,6]. Most infected individuals do not develop overt disease, leading to the hypothesis that some H. pylori strains are harmless or even beneficial[7]; however, the list of diseases potentially caused or worsened by H. pylori has been growing in recent years, making it premature to conclude that any strain is commensal.
Several unique properties contribute to H. pylori persistence. All H. pylori clinical isolates express urease. Urease converts urea to ammonia plus carbon dioxide, raising the pH of the surrounding area. This provides temporary protection against gastric acid, but H. pylori is not an acidophile. It requires the near-neutral pH found in the mucus layer directly adjacent to the gastric surface epithelium. The helical shape of H. pylori makes it easier for its polar flagella to propel H. pylori through viscous mucus. Chemotaxis systems direct H. pylori towards some amino acids, bicarbonate, and cholesterol, while acidic pH serves as a repellent[8]. This system keeps the organisms in the favorable milieu close to the surface epithelium.
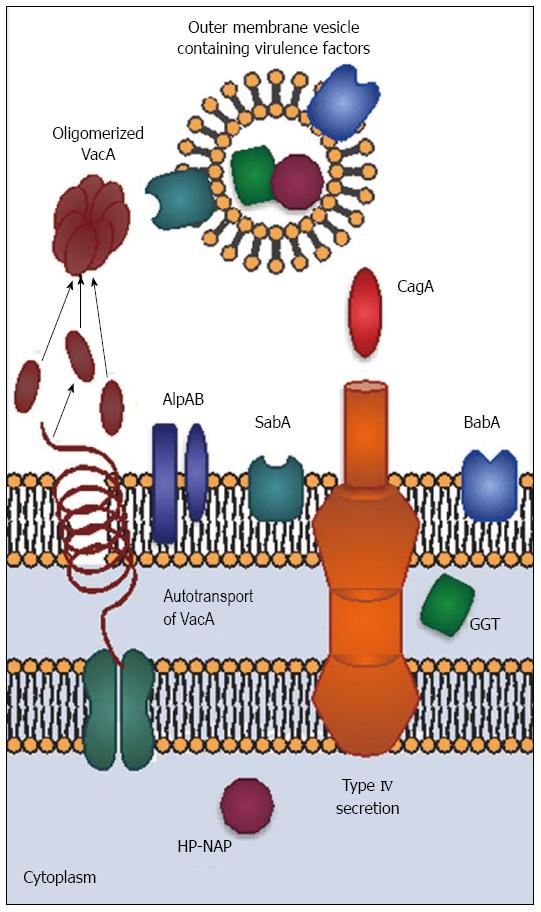
The outer surface of H. pylori also has unique properties. H. pylori glycosylates host cholesterol and inserts it into its outer membrane. The function of glycosylated cholesterol is not entirely known, but H. pylori lacking cholesterol are extremely sensitive to environmental stress[9,10]. Lack of glucosylated cholesterol reduces CagA-mediated activities and interactions with T calls[10,11]. Another interesting membrane feature is the relatively non-toxic lipopolysaccharide (LPS) found in H. pylori, which may contribute to persistence by limiting the host inflammatory response. Unlike LPS from other species, H. pylori LPS is recognized by TLR-2 rather than TLR-4[12]. In some strains, side chains on the LPS O antigen mimic the Lewis blood group antigens Lex and Ley. The membrane extends to form a sheath covering the flagella. The combination of sheathed flagella, hypoinflammatory LPS, and molecular mimicry reduce the host response, allowing the organism to persist with minimal pathology. Furthermore, H. pylori forms outer membrane blebs that pinch off from the surface of the bacterium. These outer membrane vesicles contain both membrane-associated and cytoplasmic components, including CagA and other virulence factors[13]. In addition to being an alternative virulence factor-delivery mechanism, outer membrane vesicles may serve as “dummy targets” for the immune system, thus facilitating immune evasion.
H. pylori has a number of virulence factors which influence colonization and disease severity (Figure 1). CagA, encoded by cytotoxin-associated gene A (cagA) is the most important and best-studied virulence factor; however, it does not function in isolation. CagA-positive H. pylori strains are associated with greater inflammation and increased risk of ulcers and cancer in both humans and animals[14]. The cagA gene is part of a pathogenicity island that also encodes components of a type IV secretion apparatus. The secretion apparatus is a syringe-like structure that was once thought to have little importance other than its ability to inject CagA; however, recent studies suggest CagA-independent functions. For example, the structural protein CagL mimics fibronectin[15] and peptidoglycan is injected into host cells along with CagA[16]. Finally, it is important to note that the presence of cagA usually coincides with the presence of other virulence factors, including vacA, babA and oipA[17]. Thus, H. pylori pathogenesis is multifactorial and cannot be boiled down to one gene.
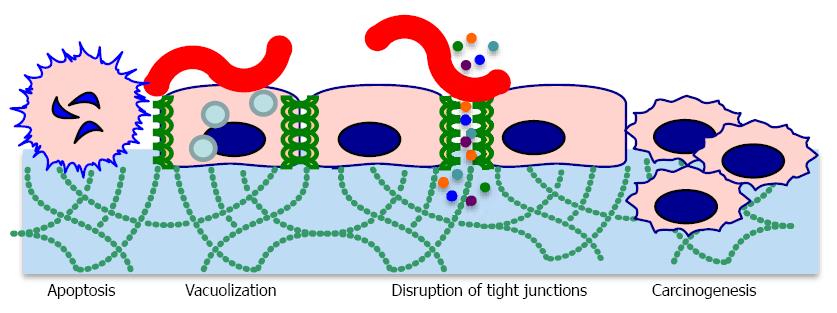
CagA is responsible for myriad signaling alterations, which profoundly influence host cell physiology. When H. pylori makes contact with host cells, CagA is directly injected into the host cell cytoplasm where it becomes phosphorylated and binds the SH2 domain of host SHP-2[18]. SHP-2 is a phosphatase involved in transduction of signaling from receptor tyrosine kinases[19]. Mutations in SHP-2 are found in some human tumors, providing a mechanistic link between CagA activity and tumor development. CagA also interacts with other proteins involved in signal transduction, such as c-Met[19]. CagA colocalizes with tight junction proteins, causing decreased cell-cell adhesion and loss of cell polarity (Figure 2)[20]. This, along with cytoskeletal rearrangements, contributes to increased invasiveness of host cells through the extracellular matrix. CagA also drives the transition from an epithelial to a mesenchymal cell phenotype[12]. All of these phenotypes are associated with carcinogenesis[21]. CagA positive strains are often associated with increased IL-8 production; however H. pylori induces IL-8 secretion through multiple mechanisms, some of which are CagA-independent[22]. CagA induces genes encoding defensin 2 and other antimicrobial peptides, which could explain why CagA positive strains appear to be easier to eradicate than CagA negative strains[23]. Dendritic cells intoxicated by CagA display impaired activation, leading to decreased inflammatory cytokine production and decreased stimulation of a Th-1 response[24].
Not all CagA proteins are created equal; some cagA alleles encode additional SH2 binding sites, further suppressing the function of SHP-2[18]. A five amino acid motif (EPIYA motif) in the carboxy-terminal portion of CagA is the phosphorylation site, and clinical isolates differ in the number of EPIYA motifs present[25]. EPIYA motifs are of particular interest because certain EPIYA polymorphisms are more strongly associated with cancer than others[26]. CagA lacking the EPIYA motif is not phosphorylated and activates the STAT3 pathway instead of the MAPK/ERK pathway, which is induced by SHP-2 binding[27]. STAT3 activation leads to increased cell migration, while MAPK/ERK activation leads to cell growth inhibition and morphological changes.Oddly, CagA reduces the cellular effects of VacA and vice versa. CagA reduces vacuolization and apoptosis caused by VacA and VacA reduces the cytoskeletal rearrangements caused by CagA[28]. These findings once again highlight the influence of H. pylori’s genetic variability and interactions between virulence factors on pathogenesis.
Vacuolating toxin A (VacA) was named for its ability to induce numerous large vacuoles in host cells. Unlike CagA, VacA forms an autotransporter structure to secrete itself without the need for host cell contact. VacA proteins oligomerize to form pore-like structures. VacA binds to receptor-like protein tyrosine phosphatases (RPTPα and RPTPβ) and other glycosylated transmembrane proteins on the host cell surface[29]. VacA is then endocytosed and forms anion-selective channels in vacuole membranes. The channels allow accumulation of chloride anions and weak bases, resulting in osmotic swelling[30]. VacA also inserts into mitochondrial membranes, causing mitochondrial dysfunction and subsequent apoptotic death of the cell[31]. Vacuolation is not the only effect of VacA intoxication. VacA disrupts the barrier function of epithelial cells, allowing leakage of crucial nutrients such as iron, nickel, and amino acids. This likely improves H. pylori growth[32]. In vitro, VacA inhibits antigen presentation and T cell activation, but it is not clear whether these activities occur in vivo[21].
All H. pylori strains contain vacA genes, but not all strains produce functional VacA. This is due to polymorphisms within the VacA gene, particularly at the amino terminus (s region), in the middle of the gene (m region), and in an intermediate region (i region). The s2 polymorphism yields an inactive toxin[28]. Thus, strains with the s2 allele are often termed “VacA negative”. The i region polymorphisms were discovered more recently and influence vacuolating activity; vacA containing the i1 allele produces the most active toxin[33]. Strains harboring the s1m1 allele have been more commonly associated with ulcers and gastric cancer, but it now appears that the i1 allele is more strongly associated with ulcers and cancer than the presence of the s1m1 genotype[28]. In spite of the dramatic effects of VacA on epithelial cells, it is unclear whether VacA plays a causative role in these diseases. More likely, VacA facilitates nutrient acquisition, improving the ability of H. pylori to colonize the gastric epithelium[34].
H. pylori encodes two variably expressed sialic acid-binding adhesins, BabA and SabA. BabA, encoded by babA2, binds to the Lewis b ABO blood group antigen (Leb) and is present on red blood cells and certain epithelial cells. Expression of babA2 is variable, and can be turned “on” and “off” by slipped-strand mispairing mutations. BabA binding activity is most often present in CagA positive strains[34]. BabA-mediated binding to Leb contributes to formation of double stranded DNA breaks in host cell lines and may promote cancer-associated gene mutations[35]. Adherence via BabA also enhances the ability of the type IV secretion apparatus to contact host cells, leading to a stronger inflammatory response[36]. These data suggest that BabA may indirectly influence pathology by improving adherence to host cells.
SabA is a sialic acid-binding adhesin that recognizes the sialyl-Lewis A antigens sLex and sLea[37,38]. SabA increases colonization density in patients lacking Leb, indicating its importance as an adhesin. Sialylated Lewis antigens are more prevalent on inflamed or cancerous gastric tissue, leading to the hypothesis that SabA is involved in carcinogenesis; however, not all studies demonstrate correlations between the presence of SabA and cancer[39].
H. pylori has about 30 other outer membrane proteins which may serve as adhesins, but only a few have known functions. AlpA and AlpB are now known to bind host laminin. Their presence modulates the inflammatory response in gerbils through unknown mechanisms[40]. It is not known whether variability in alpAB gene sequence or expression influence human disease. OipA (Outer Inflammatory Protein; a.k.a HopH) is an outer membrane protein that functions as an adhesin[41]. The gene can be turned on and off via slipped strand mispairing. Interestingly, most cagA positive strains possess an expressed form of oipA, making it more difficult to separate the clinical effects of oipA from those of cagA[17]. In vitro and in vivo animal experiments clearly show that OipA is associated with increased IL-8 production and carcinogenicity[42]. Inactivating oipA in a carcinogenic H. pylori strain eliminated tumorigenesis in gerbils[43]. This may be partially explained by the decreased nuclear translocation of beta-catenin induced by mutant strains lacking OipA. Beta-catenin is an important player in the gastric carcinogenesis process[44].
The neutrophil-activating protein (HP-NAP), encoded by napA, was first isolated from water extracts of H. pylori[45]. Although it is cytoplasmic, HP-NAP escapes via autolysis. Amino acid sequencing led to the napA gene, which is homologous to other bacterioferritins. Bacterioferritins are similar to mammalian ferritins in that they form multimeric, hollow structures that store iron and bind DNA. These proteins protect prevent iron-mediated chemical reactions from forming reactive oxygen species[46]. Further studies revealed that HP-NAP does not behave like other bacterioferritins. HP-NAP crosses both the epithelium and the endothelium, where it recruits neutrophils and stimulates both neutrophils and monocytes to produce IL-12 and IL-23[47,48]. These cytokines induce T cells to secrete interferon-γ and mediates the shift to a Th1 response[48]. HP-NAP binds to TLR-2, which typically recognizes bacterial cell wall components. Unlike other TLR-2 agonists, HP-NAP induces substantial IL-12 production[48]. HP-NAP also promotes clot formation and inhibits fibrin degradation by monocytes[49].
HP-NAP has been suggested as a tumor-fighting agent and an allergy modulator. Intratumoral injection of HP-NAP into mouse neuroendocrine or bladder tumors led to tumor regression and a shift towards cytotoxic T cells[50,51]. Since allergies are associated with a Th2-skewed immune response, inducing a shift towards a Th1 response could be beneficial[52]. Proof-of-principle has been demonstrated in a mouse model of ovalbumin-induced asthma. Injection of HP-NAP reduced eosinophilia and IgE concentrations in sensitized animals[53]. Enthusiasm for HP-NAP-based treatments is tempered by concerns regarding the potential for autoimmune reactions induced by homology between HP-NAP and human aquaporin in neural tissues[54].
The discovery that H. pylori gamma-glutamyl transpeptidase (GGT) functions as a virulence factor was surprising, since it hadn’t been identified as a virulence factor in other bacteria[55]. Moreover, it appears to be periplasmic rather than surface-associated or secreted. Its presence in outer membrane vesicles[13] could facilitate interactions with host cells. GGT increases IL-8 secretion and hydrogen peroxide production in epithelial cells and is associated with peptic ulcer development[56]. It also contributes to colonization, persistence, and tolerization of dendritic cells[57]. GGT-mediated degradation of glutathione produces pro-oxidant compounds that facilitate formation of reactive oxygen species. This mechanism could be responsible for host tissue damage[58].
In addition to H. pylori variability, several host factors also influence disease outcome. IL-1β is induced rapidly following infection. Polymorphisms in genes encoding IL-1β or its receptor antagonist increase the risk of gastric cancer in many populations; however this may not hold true for some Asian and white populations[21]. Polymorphisms in genes encoding TNFα, IL-10, and HLA are also implicated[21]. Reduced IL-10 expression results in a more inflammatory Th1-polarized host response. Smoking and a diet high in salty foods exacerbate the effects of H. pylori. All of these things could explain why some studies show clear correlations between H. pylori and a particular disease, while others do not.
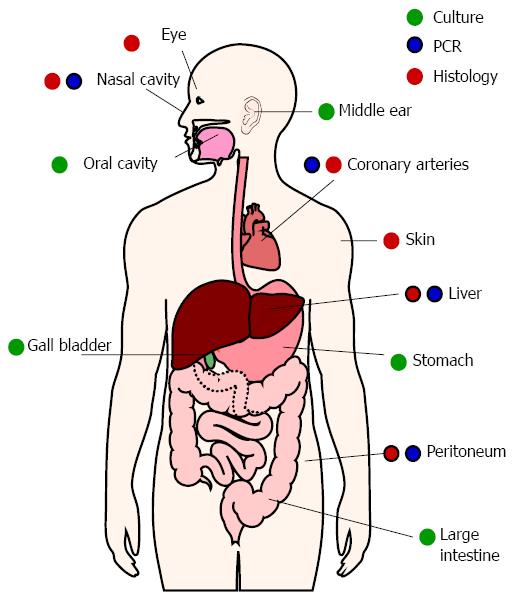
H. pylori primarily colonizes the stomach, but evidence suggests occasional or persistent colonization of other sites (Figure 3). Growth properties of H. pylori hamper detection of low-density colonization. Relatively slow growth and complex growth requirements have been barriers to identifying H. pylori colonization. H. pylori will not grow in many commonly-used solid or liquid media. H. pylori grows best with 1%-5% serum, but higher serum concentrations can be inhibitory[59,60]. H. pylori is also microaerophilic and is killed by atmospheric oxygen. Exposure of samples to ambient oxygen, as well as complement and phagocytic cells from blood, will kill some of the bacteria before they can be cultured. Even under ideal conditions, H. pylori requires about 5 d to form visible colonies on solid media. By that time, any other bacteria or fungi present in the sample can overtake the culture.
Culture remains the gold standard for establishing the presence of viable H. pylori, but molecular methods have proven more sensitive in identifying alternative colonization sites. PCR is a sensitive method for detecting H. pylori in many locations, but does not prove whether the bacteria are alive or dead. In situ hybridization using probes that only bind mRNA can demonstrate viability. This method has been used to detect live H. pylori in the peritoneal cavities of pseudomyxoma peritonei cancer patients[61].
H. pylori DNA has been amplified from atherosclerotic plaques and the oral cavity for years, but the significance of H. pylori in these locations is still debated[62]. Many have reasonably questioned whether H. pylori truly colonizes those sites. For example, macrophages might have phagocytized H. pylori in the stomach and later traveled to atherosclerotic plaques. Similarly, oral H. pylori might be due to gastric reflux. Additional testing has been performed in laboratories worldwide, using a variety of techniques. H. pylori has been cultured from root canal samples, and occasionally from plaque[63,64], but is most often identified by PCR. The presence of H. pylori in the oral cavity correlates with its presence in the stomach[65-67], but oral H. pylori does not correlate with poor oral hygiene[68,69]. On the other hand, several studies suggest that untreated periodontal disease increases the risk of becoming re-infected after H. pylori eradication[70]. Similarly, reducing the number of oral H. pylori using antiseptic mouthwash and/or periodontal treatment improves the eradication rate following antibiotic therapy[71]. It is therefore possible that H. pylori gains a toehold in the mouth before colonizing the stomach and that the stomach can be reinfected by oral H. pylori.
Evidence of H. pylori colonization has also been found in the gallbladder, ears, nose, skin, and even eyes[72-75]. Aside from the gallbladder, H. pylori has only been found in the above locations when inflammatory or hyperproliferative pathology is present. These diseases will be discussed in more detail below.
There is ample evidence of other Helicobacter species in the stomach, intestine, and biliary tract. “H. heilmannii”, which is now known to comprise several species including H. bizzozeronii and H. salmonis, is occasionally present in the stomach and appears to cause the same diseases as H. pylori[76,77]. Other species that infect humans include H. cinaedi, H. pullorum, H. canis, H. canadensis, H. fennelliae, H. rappini, H. bilis, H. hepaticus, and H. suis[72,73]. Most of the aforementioned species are likely acquired from pets and livestock. H. cinaedi, and occasionally other species, can cause enterocolitis, cellulitis, and sepsis[77]. Some Helicobacter species have been postulated to cause inflammatory bowel disease[78,79], but non-pylori species have not been adequately studied and routine tests for those species do not exist.
Functional dyspepsia is defined as pain associated with the stomach or upper abdomen. Ulcers are one cause of dyspepsia, but many patients have no evidence of gastric damage. The role of H. pylori in non-ulcer dyspepsia has been somewhat controversial. The preponderance of evidence suggests that H. pylori infection contributes to some cases of dyspepsia. A randomized clinical trial involving 585 dyspepsia patients found that H. pylori eradication led to greater benefit in patients with epigastric pain syndrome than in postprandial distress syndrome[80]. These and other results suggest that the “test and treat” strategy is reasonable for non-ulcer dyspepsia patients[81], particularly those with epigastric pain. Dyspepsia symptoms do not always resolve following eradication therapy, but the risk of future ulcer and gastric cancer development are reduced.
Gastritis is universally present in H. pylori infected patients and is reproduced in various animal models. Acute gastritis, sometimes associated with dyspepsia or nausea, develops soon after initial infection. Acute gastritis affects the entire stomach and is accompanied by loss of acid secretion[82,83]. Neutrophils are recruited to the lamina propria and epithelium and damage results from reactive oxygen species and other neutrophil products. The acute phase gives way to chronic gastritis as lymphocytes replace neutrophils.
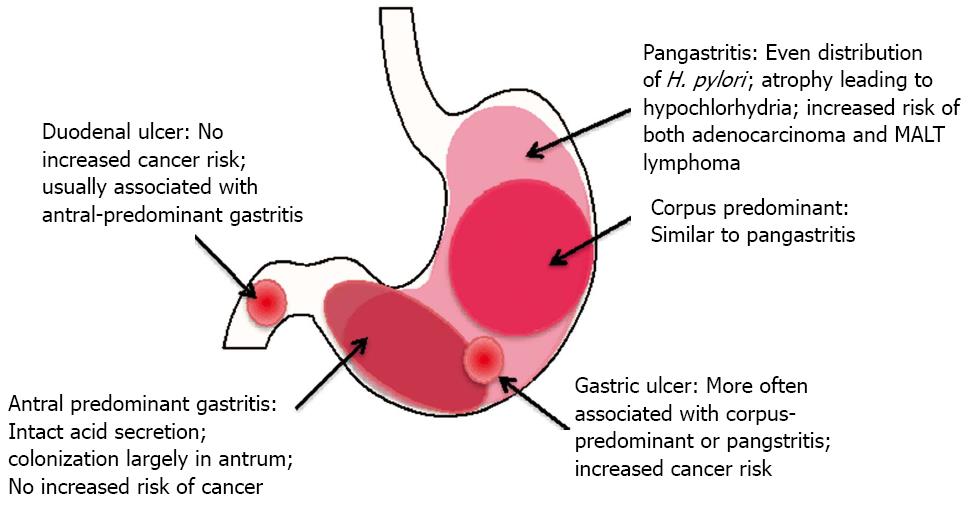
Chronic gastritis can be antrum-predominant, corpus-predominant, or diffuse (pangastritis or multifocal gastritis) (Figure 4). In antrum-predominant gastritis, acid secretion usually remains intact and H. pylori colonization is limited to the antrum[83]. Antral gastritis favors development of duodenal ulcers, while corpus-predominant gastritis favors gastric ulcer formation, sometimes progressing to metaplasia and adenocarcinoma[82,84]. Patients with diffuse gastritis typically have severely impaired acid secretion, which allows H. pylori to colonize the corpus. Chronic acid suppression mediated by proton pump inhibitors can lead to a switch from antral predominant to pangastritis[83]. Interestingly antrum-predominant gastritis and duodenal ulcers do not increase the risk of cancer development[85,86].
Historically, H. pylori has been responsible for 70%-85% of gastric ulcers and 90%-95% of duodenal ulcers[82,87]. The percentage of ulcers not associated with H. pylori is rising due to increased use of NSAIDs and decreasing prevalence of H. pylori infection; however H. pylori eradication is partially protective against ulcer development in NSAID users[87,88]. Also, the hypothesis that ulcers are caused by stress is reemerging. Several studies show that anxiety disorders and physical or emotional trauma correlate with ulcer development (reviewed in[89]). Studies testing the interaction between H. pylori and stress are lacking, so it is not clear whether those factors operate independently or whether there is an additive effect of infection and stress on ulcer formation.
H. pylori is an accepted cause of gastric adenocarcinoma and MALT lymphoma (official name: extranodal marginal zone B cell lymphoma of mucosa-associated lymphoid tissue). Gastric adenocarcinoma is divided into intestinal subtype and diffuse subtype. Intestinal type adenocarcinoma is more common and has been well studied. The sequence of pathological changes leading to intestinal type cancer starts with gastritis, followed by gastric atrophy (loss of glandular structure) and progressing to intestinal metaplasia, dysplasia, and finally carcinoma[14]. This progression occurs over many years; consequently, most patients are middle aged or older. Environmental factors, such as smoking and diet, increase the risk of developing gastric adenocarcinoma. H. pylori-associated adenocarcinoma most often occurs in the distal (non-cardia) stomach. H. pylori-mediated adenocarcinoma has been replicated in animal models, particularly in gerbils[85,90]. The presence of cagA, vacAs1m1, babA2, and oipA increase the risk of cancer[91-94]. Cancer risk is further elevated by the presence of certain EPIYA motifs in CagA[26,95]. A number of good reviews discuss the molecular mechanisms in detail[85,96,97].
Diffuse type adenocarcinoma is characterized by scattered tumor cells and lack of glandular organization. Diffuse-type gastric cancer tends to occur in younger individuals and may have a strong genetic component, although H. pylori also plays a role[98]. Environmental factors contribute little to this type of cancer. Pangastritis and rugal hyperplasia confer higher risks of diffuse-type adenocarcinoma[99,100]. The mechanisms underlying development of diffuse-type cancer are poorly understood, but abnormal DNA methylation is likely involved. H. pylori induces hypermethylation of the gene encoding E-cadherin, which is implicated in hereditary diffuse-type cancer[99,101].
The association between MALT lymphoma and H. pylori was first published in 1991[102] and has since been corroborated by numerous studies. H. pylori is responsible for 92%-98% of gastric MALT lymphoma[3]. The formation of lymphoid follicles is necessary, but not sufficient for development of MALT lymphoma. CagA and other virulence factors contribute little to the risk of developing MALT lymphoma[103]; however the presence of CagA within tumor-associated B cells correlated with earlier remission following H. pylori eradication compared with patients whose tumors did not contain CagA[104]. Most gastric MALT lymphomas are low grade when discovered and are dependent upon continued antigen stimulation. Lymphocytes are prone to developing genetic mutations, and prolonged antigen-driven proliferation of B lymphocytes eventually leads to a population that is antigen independent[105]. Interestingly, the tumor-associated B cells do not recognize H. pylori. Some have suggested that B cells recognize autoantigens, but an in-depth study of antigen specificity did not uphold previous results[105,106]. Thus it is not clear whether antigen specificity plays a role in MALT lymphoma development.
The first line of treatment for H. pylori-positive MALT lymphoma is eradication therapy. H. pylori eradication leads to lasting remission in about 80% of patients[107]. Some H. pylori-negative MALT lymphomas, including a recurrent parotid lymphoma, have also responded to eradication therapy, suggesting that these were either false negatives or perhaps caused by another Helicobacter species[108,109].
Gastric adenocarcinoma and MALT lymphoma were the first cancers associated with H. pylori, but they may not be the last. Several other cancers have been recently associated with H. pylori, though the evidence is not ironclad. The most common extragastric cancer potentially influenced by H. pylori is colorectal adenocarcinoma. Gastric H. pylori routinely passes through the intestinal tract, so it is not unreasonable to suspect that H. pylori could influence development of colorectal cancer. Several studies support this hypothesis. Three recent meta-analyses found a significantly increased risk of both colon polyps and colon cancer in H. pylori-infected patients[110-112]. One study examined the prevalence of gastric pathology in patients with hyperplastic polyps, colon adenoma, advanced adenoma, and colon cancer[113]. All patient groups had higher prevalences of H. pylori-positive gastritis and intestinal metaplasia. The odds ratios were largest in patients with colon cancer. Those patients had significantly increased risks of gastritis, intestinal metaplasia, gastric adenoma, gastric cancer, and gastric lymphoma. Grouping patients according to whether or not the cancer was advanced revealed that the infection rate was higher in advanced cases (86%) than on non-advanced cases (51%). Forty eight percent of control patients were infected[114]. Oddly, cagA positive strains were not more prevalent in cancer patients. In summary, it appears that H. pylori could contribute to the risk or aggressiveness of colorectal cancer, but much additional research is needed to confirm the association and determine the underlying mechanism.
Pseudomyxoma peritonei (PMP) is a rare peritoneal cancer that most often originates in the appendix[115]. Appendiceal rupture allows the cancer to invade the peritoneal cavity. Bacteria are able to enter the peritoneum at the time of rupture, but the viscous mucus prevents leakage of fecal material into the peritoneum and peritonitis does not ensue. Instead, a subset of bacteria present within the mucus persistently colonizes the peritoneal cavity. The appendiceal rupture site heals, trapping the tumor, mucus, and bacteria within the peritoneal cavity. Subsequent mucin accumulates in the peritoneal cavity causing compression of abdominal organs. Pseudomyxoma peritonei originating from the appendix is essentially similar to the mucinous subtype of colorectal adenocarcinoma. In fact, mucinous colonic polyps sometimes cause PMP[116]. Live H. pylori have been found within tumor and mucinous material[117]. Patients who were treated with standard H. pylori eradication therapy prior to surgery had reduced nuclear β-catenin, indicating cellular normalization, and tended to survive longer than patients given only standard care[61,118].
H. pylori could increase the risk of other lymphoma types. Diffuse large B cell lymphoma (DLBCL) is an aggressive cancer that can occur in the stomach and elsewhere. It is considered separate from gastric MALT lymphoma, although the former can sometimes transform into the latter[119]. This type of cancer is currently not believed to be H. pylori-associated; however, there have been a number of case reports in which H. pylori eradication alone led to complete remission[120]. Inaba et al[121] found that primary gastric diffuse large B cell lymphoma increases the risk of developing gastric adenocarcinoma, often within 5 years of treatment. The author proposes that chemotherapy and radiation may hasten the development of gastric adenocarcinoma in H. pylori-positive patients. Testing DLBCL patients for H. pylori may therefore be prudent.
As yet, there is only scant evidence that H. pylori influences extragastric lymphomas. One patient with rectal MALT lymphoma had a complete response to H. pylori eradication therapy and remained disease-free after 34 mo[122]. Ocular adnexal lymphoma (OAL) is a most often a MALT lymphoma presenting on the orbit or conjunctiva[123]. A comparison of patients suffering from ocular adnexal lymphoma, extragastric lymphoma in other body sites, and controls found that OAL patients were 32% more likely to be infected with H. pylori than controls. Furthermore, patients with lymphoma at other sites were 13% more likely to be infected. Both of these findings were highly significant[124]. H. pylori DNA has been found in one OAL tumor out of 6 tested[125]. To date, there are no reports of H. pylori eradication therapy being tested in OAL patients.
A number of studies report that H. pylori infection protects against Barrett’s esophagus and subsequent laryngeal adenocarcinoma development (see section on laryngeal disease); however, some studies suggest a positive association with laryngeal squamous cell carcinoma[126-128]. Burdick[129] examined 75 patients with laryngeal squamous cell cancer. All samples were positive for ureA, and 47%-49% of ureA positive samples were also cagA positive. The cagA gene was much more prevalent in lymph node positive tumors than in lympth node negative tumors. Additionally, cagA was far more prevalent in supraglottic tumors than in glottic or subglottic tumors. The survival rate was also lower in cagA positive patients. Guilemany et al[130] found a significant correlation between hypopharyngeal squamous cell carcinoma and H. pylori infection (P < 0.001). It should be noted that not all studies have found a positive correlation[131,132].
H. pylori is increasingly being associated with extragastric diseases. H. pylori is widely accepted as a cause of iron deficiency anemia and idiopathic thrombocytopenia, but the jury is still out on other diseases. In most cases, H. pylori is believed to be one of several causes, meaning that H. pylori eradication will only benefit a subset of patients. This section summarizes current epidemiological, clinical, and experimental evidence supporting a role for H. pylori in causing or exacerbating a range of diseases.
The association between H. pylori infection and iron deficiency anemia (IDA) is well established[133,134], but the mechanisms underlying this association are unclear. The most obvious mechanism for H. pylori to cause IDA is through competition for dietary iron. H. pylori requires higher concentrations of inorganic iron and zinc for in vitro growth than other pathogens[59], yet there is no evidence that H. pylori has more iron- or zinc-dependent enzymes than other bacteria. H. pylori may have less efficient iron acquisition symptoms than other organisms, but this would suggest that H. pylori competes poorly for dietary iron. H. pylori is able to acquire iron from host transferrin and lactoferrin, yet it binds the iron-free forms of these proteins more avidly than the iron-loaded proteins[60]. All of this points towards an evolutionary advantage for H. pylori strains that do not overly tax the host iron supply. Nonetheless, H. pylori genomes vary considerably from strain to strain, as do virulence properties. Yokota et al[135] found that certain polymorphisms within the H. pylori neutrophil-activating protein were more prevalent in strains from IDA patients than from non-IDA patients. Moreover, IDA strains harboring these polymorphisms internalized iron more rapidly than other strains. Inorganic iron dissolves best in highly acidic conditions. Loss of gastric acid secretion due to H. pylori infection could therefore reduce bioavailability of dietary iron. Excess lactoferrin in gastric tissue could impair iron uptake, since transferrin is the normal route of host iron uptake[136]. In male patients with refractory IDA, H. pylori infection was associated with higher serum TNFα levels, particularly in those infected with CagA+H. pylori[137]. This is relevant because TNFα and other pro-inflammatory cytokines can induce anemia.
Idiopathic thrombocytopenic purpura (ITP) is characterized by autoimmune destruction of platelets, which leads to bruising. Significant evidence points to H. pylori as a causative agent in some cases of ITP. For example, H. pylori eradication therapy increases platelet counts only in those who were infected with H. pylori, not those who were uninfected ruling out an off-target effect of antibiotics[138]. Among ITP patients treated for H. pylori infection, patients who had a lower serum pepsinogen I/II ratio responded better (i.e., greater improvement in platelet counts) than those with higher pepsinogen I/II ratios. This correlated with more severe atrophic gastritis in responders than in non-responders[139]. Host factors likely play a role as well. H. pylori-infected patients who lack a particular single nucleotide polymorphism in IL-β, the IL-β (-511) T allele, were more likely to develop ITP before age 50 years than uninfected ITP patients[140].
H. pylori appears contribute to several inflammatory skin diseases. Rosacea is the most common skin disease potentially associated with H. pylori. In one study, H. pylori was present in 81% of rosacea patients who also had gastric complaints, and almost all of those patients harbored cagA+ strains[141]. A similar Egyptian study found that both cagA and the s1m1 allele of vacA were more prevalent in papular rosacea patients. The papular rosacea patients responded better to eradication therapy than the erythematous rosacea patients[142]. A limited study of ocular rosacea patients reports that the seven ocular rosacea patients responded better to H. pylori eradication therapy than did rosacea patients without ocular symptoms[143].
Chronic prurigo consists of intensely itchy nodules. There are several variants, including prurigo pigmentosa and prurigo chronica multiformis. These disease are most common in young women of Japanese or Turkish descent[144]. Limited studies suggest higher prevalence of H. pylori or clinical improvement following H. pylori eradication therapy[145]. H. pylori-associated gastric diseases are also more prevalent in these patients[145-147]. Immunohistochemical evidence of H. pylori in a prurigo pigmentosa patient strengthens the case for a link and suggests that H. pylori acts directly within the skin, rather than indirectly through inflammatory mediators[148]. These mechanisms are not mutually exclusive, but much more research is needed to clarify etiology and pathogenic mechanisms related to H. pylori.
Chronic idiopathic urticaria consists of itchy wheals that are present on most days with no known cause. A meta-analysis of 10 chronic urticaria studies completed prior to 2003 found that the overall remission rate following H. pylori eradication was 30.9%. This was statistically significant with an odds ratio of 2.9[149]. Chiu et al[150] found no difference in the H. pylori infection rates in chronic spontaneous urticaria (CSU) patients, but 63.6% of patients had complete remission after H. pylori eradication. Likewise, Campanati et al[151] found no difference in H. pylori prevalence, but found significant improvement following H. pylori eradication therapy. In the same study, the authors found an unusually high rate of small intestinal bacterial overgrowth in CSU patients. Those patients were treated with the recommended antibiotic, but their CSU symptoms did not improve. Yoshimasu and Furukawa[152] grouped patients according to anti-H. pylori titer. None of the patients with high titers was cured with antihistamine treatment alone. Eradication therapy raised the cure rate to 56%. Patients with low or absent anti-H. pylori titers were more likely to respond to antihistamine treatment alone. These studies point to the conclusion that H. pylori is one of several causes of CSU.
Case reports suggested an association between psoriasis and H. pylori[153,154], providing impetus for a larger study by Onsun et al[155]. Stratifying a group of 300 plaque psoriasis patients by symptom score revealed that 100% of patients with moderate or severe psoriasis were H. pylori positive, while only 37% of mild psoriasis patients were infected. H. pylori positive patients were treated with acitretin alone, H. pylori eradication therapy alone, or both. All treatment groups showed improvement after 8 wk, but improvement was more rapid when H. pylori eradication was included. H. pylori treatment alone was just as effective as both eradication therapy and acitretin[155].
H. pylori may contribute to diseases of pregnancy. Hyperemesis gravidarum consists of severe and prolonged vomiting that goes far beyond typical “morning sickness”. A meta-analysis of clinical studies found that the majority support the hypothesis that H. pylori contributes to hyperemesis gravidarum, although further studies are needed[156]. One study found that 80% of hyperemesis gravidarum patients were H. pylori-positive compared with 35% of controls[157]. Mansour and Nashaat[158] report improvements in H. pylori-positive hyperemesis gravidarum patients following eradication therapy[158].
A study comparing women with normal pregnancies to those with pre-eclampsia (PE) yielded striking results[159]. The correlation between H. pylori seropositivity and PE was highly significant, with an odds ratio of 9.22. The relationship was even stronger for pregnancies with both PE and fetal growth retardation (OR = 35.56). CagA+ strains were overrepresented in both PE and PE with fetal growth retardation, yielding odds ratios of 17.66 (PE) and 54.97 (PE with fetal growth retardation). In contrast, pregnancies with only fetal growth retardation were not associated with greater than expected H. pylori seropositivity. A more recent study found that antibodies against CagA cross-react with beta-actin of cytotrophoblast cells[160]. This may impair placental invasion.
Since H. pylori is frequently present in the oral cavity, perhaps it is not surprising that it is occasionally found in the ear, nose and throat. Aside from gastroesophageal reflux disease, too few studies have been done to determine whether H. pylori causes disease in those locations. Nonetheless, some of the data are tantalizing. In one study, H. pylori was cultured from 40% of adults with otitis media with effusion[161]. Other studies have used the Campylobacter-like organism test (CLO test) to identify H. pylori infections in the middle ears of patients with otitis medium or tympanosclerosis[162,163]. Melake et al[164] compared children undergoing myringotomy for chronic otitis media with children who were undergoing tonsillectomy/adenoidectomy, but did not have a history of otitis media. The study group was roughly three times more likely to have evidence of H. pylori in the stomach, adenoids, or tonsils than the control group. Almost all of the study patients with evidence of gastric H. pylori were also either culture- or PCR-positive for H. pylori in middle ear fluid. Interestingly, another study found that 48 of 49 biopsies taken from 37 children with adenotonsillar hypertrophy contained H. pylori[165], raising the possibility that the association between H. pylori and chronic otitis media could be stronger than it appeared to be in the Melake study. H. pylori worsened inflammation in a rabbit model of histamine-induced otitis media with effusion[166]. This suggests that H. pylori may not be the initial cause of otitis media, but these bacteria may exacerbate pre-existing infections.
Several studies have demonstrated the presence of H. pylori in nasal polyps[167-169]. One study failed to find a difference in gastric colonization between patients with nasal polyps vs patients with concha bullosa, but H. pylori was only found in nasal biopsies from nasal polyposis patients[170]. In a study of nasal polyps vs benign laryngeal disease, H. pylori was detected in all 60 patients with either nasal polyps or benign laryngeal disease. Interestingly, cagA-positive H. pylori was detected in 23.3% of patients with laryngeal disease, but in none of the nasal polyps[171]. CagA is often associated with more severe gastric disease, but this toxin may put H. pylori at a disadvantage in certain anatomical sites.
The relationship between H. pylori infection and esophageal diseases has been a controversial topic for years. According to the dogma, eradicating H. pylori increases gastric acid output. This leads to exacerbation of gastroesophageal acid reflux disease (GERD) and an increased risk for developing Barrett’s esophagus and esophageal adenocarcinoma. The decreasing prevalence of H. pylori infection is therefore predicted to increase the prevalence of esophageal diseases. Indeed, there has been an increase in the incidence of esophageal adenocarcinoma[172]. Some studies and meta-analyses have concluded that H. pylori eradication worsens gastroesophageal reflux disease, while others report no effect[173-176]. There is also an inverse correlation between H. pylori infection and Barrett’s esophagus[177-179]. A recent meta-analysis confirms the inverse correlation between H. pylori infection and esophageal adenocarcinoma in Eastern and Western populations. The analysis also revealed a significantly reduced risk of esophageal squamous cell carcinoma in Eastern populations, but not Western populations[173].
On the other hand, some studies suggest a more complex relationship between the incidence of esophageal disease and the prevalence of H. pylori. Studies of gastric pH and manometric values have not found a difference between H. pylori infected and uninfected patients or in patients before and after H. pylori eradication[175,180]. McColl reports that H. pylori-induced gastric atrophy reduces the risk of esophageal adenocarcinoma, but antral-predominant gastritis with increased acid production increases the risk[181]. In contrast, Derakshan et al[182] report that atrophy does not impact the risk of adenocarcinoma. While univariate analysis showed a protective effect of H. pylori infection on esophageal adenocarcinoma, this effect was lost upon multivariate analysis and only smoking and GERD symptoms > 2 times per week remained significant[182]. Obesity is one of the strongest risk factors for esophageal adenocarcinoma, and the worsening obesity epidemic could be partially responsible for the rise in esophageal adenocarcinoma cases[179].
If the dogma holds true, one would expect that regions of the world with lower H. pylori prevalence should have higher rates of esophageal adenocarcinoma and squamous cell carcinoma. Ethnic Malays in the Northeastern peninsular region of Malaysia have only a 4%-5% rate of H. pylori infection. The rates of esophageal adenocarcinoma and Barrett’s esophagus in this population were lower than in populations with greater H. pylori prevalence[183]. Therefore, lack of H. pylori alone does not explain the increasing prevalence of esophageal disease.
A few studies have implicated H. pylori and other Helicobacter species human liver and gallbladder disease. H. pylori has been detected in livers from hepatocellular carcinoma patients by PCR and histology[184]. Chronic cholecystitis patients were tested for the presence of H. pylori in the gallbladder mucosa. Patients with H. pylori positive gallbladders had increased levels of inducible nitric oxide synthase and higher incidences of adenomyomatosis and metaplasia[74]. Following the discovery of H. pylori, H. hepaticus was found in many colonies of research mice. This infection causes chronic active hepatitis and laboratory rodents are now routinely tested for Helicobacter infections[185]. While H. pylori predominantly colonizes the intestines, other Helicobacter species, such as H. hepaticus, H. bilis, and H. cinaedi infect the intestines and biliary tract in humans. H. bilis appears to be highly prevalent in patients with pancreaticobiliary maljunction[186]. The non-pylori helicobacters have been largely overlooked and more studies are needed to determine their prevalence and capacity to cause disease.
A handful of studies have linked H. pylori infection with bronchiectasis and chronic obstructive pulmonary disease (COPD)[187-189]. Tsang et al[188] compared the seroprevalence of H. pylori among bronchiectasis, tubercoulosis, and control groups. Bronchiectasis patients were 76.0% seropositive, while tuberculosis and control patients were 52.9% and 54.3% seropositive for H. pylori. Interestingly, one study aimed at determining whether H. pylori seroprevalence differs between inflammatory bowel disease patients and controls used COPD patients for comparison[190]. They reasoned that COPD patients would have similar degrees of antibiotic exposure to inflammatory bowel disease patients. Separate age-matched control groups were used for IBD and COPD patients because COPD patients tend to be older. Seroprevalence of H. pylori is clearly lower among IBD patients than among controls. On the other hand, H. pylori seroprevalence was higher in COPD patients than in that control group. They do not mention whether the difference reached statistical significance.
Conversely, one very large study failed to find an association between COPD risk and H. pylori infections in nonsmokers[191]. A small study of Turkish soldiers also failed to find a correlation between bronchiectasis and H. pylori[187]. Oddly, patients with a history of gastric or cardiovascular disease were excluded. Gastric and extragastric diseases related to H. pylori often coexist in patients and may indicate the presence of more virulent H. pylori strains and/or a genetic predisposition to disease. Thus, there is insufficient evidence to draw conclusions regarding the role of H. pylori and lung disease.
Credible evidence suggests a link between H. pylori infection and certain eye diseases, particularly open angle glaucoma. Kountouras et al[192] have been the primary proponents of this association, but studies have thus far been small; no study has involved patient groups larger than 100 subjects. In one study, the rate of H. pylori infection in glaucoma patients was almost double that of a control group. When these patients were followed for two years, intraocular pressure and visual field parameters were improved in patients who had undergone successful H. pylori eradication therapy, but not in patients who were H. pylori-negative or whose eradication therapy failed. Histological evidence has been found in eye tissue from 5 out of 43 H. pylori-positive patients, but in none of the control patients[75]. Cresyl violet staining was used to detect H. pylori. This stain reacts with Helicobacter and Campylobacter, but few other bacterial species. Therefore, the strength of this evidence is stronger than histology using less specific stains, but not as strong as that obtained from immunohistochemistry. Two studies, one in Canada and one in Israel, have failed to find a higher prevalence of H. pylori infection in glaucoma patients[193,194]. More studies are clearly warranted.
Central serous retinopathy (CSR) results when the retinal pigment epithelial barrier breaks down, causing fluid to leak into the subretinal space; this leads to visual impairment[195]. Permanent damage can result if the fluid is not reabsorbed quickly. To date, only a handful of clinical trials have been performed to explore the potential relationship between CSR and H. pylori; however, the results of these studies have been impressive. No published study has failed to find a correlation between CSR and H. pylori evidence. Three studies have measured the effects of H. pylori eradication on the course of the disease[195-197]. All three found some evidence of improvement. In the study by Rahbani-Nobar et al[196] one group of H. pylori-infected patients received eradication therapy, while another group did not. Reabsorbtion was significantly more rapid in the eradicated group than in the non-eradicated group.
There is tantalizing, but far from conclusive, evidence suggesting a link between H. pylori infection and development of Alzheimer’s and Parkinson’s diseases. In Parkinson’s disease, H. pylori clearly interacts with L-dopa. The bacteria can bind L-dopa and impact the absorption of L-dopa treatment[198-200]. Some studies indicate that H. pylori eradication reduces motor fluctuations, but one study found that motor fluctuations were lower in infected patients than in uninfected patients[199,201,202]. A large Danish study found an association between Parkinson’s disease diagnosis and H. pylori eradication treatment 5 or more years prior to Parkinson’s disease diagnosis[203]. This suggests that past H. pylori infection may be just as relevant to Parkinson’s disease as current infection. A double-blind, placebo-controlled trial found improvements in stride length, but worsening of rigidity, following eradication therapy in anti-nuclear antibody-negative patients[204]. Anti-nuclear antibody-positive patients did not benefit from eradication. Alarmingly, patients who remained H. pylori-positive following treatment (eradication failure) experienced rapid declines in motor functions. One interesting study examined the prevalence of H. suis, which frequently infects livestock, in Parkinson’s disease patients. H. suis colonization was found in 12% of patients, but only 0.6% of controls. Many of the H. suis-infected patients had previously undergone H. pylori eradication therapy[205]. They hypothesize that H. suis infection could explain the higher prevalence of Parkinson’s disease among farmers. At present, treatment of H. pylori in Parkinson’s disease patients is not recommended due to the potential for deterioration of motor function associated with eradication failure.
With respect to Alzheimer’s disease or general cognitive function, some studies have found correlations with H. pylori infection and some have not[206-208]. A very large, cross sectional study using data from the National Health and Nutrition Examination III, phase 1 found that H. pylori seropositivity was strongly associated with poorer cognition among adults aged 60-90 years[209]. A small study of Alzheimer’s patients and age-matched controls found that H. pylori eradication significantly improved cognitive status, as measured two years after treatment[210]. The five-year survival rate was improved in H. pylori-eradicated Alzheimer’s patients, but again, this was a small study[211]. An H. pylori histidine-rich, nickel binding protein, Hpn, forms amyloid structures in vitro and has been postulated to play a role in Alzheimer’s disease[212], but in vivo data are lacking.
The association between H. pylori infection and diabetes was only recently suggested[213], but the preponderance of studies have shown positive associations[214]. Metabolic syndrome consists of obesity, particularly central obesity combined with two or more other factors, such as insulin resistance, hypertension, dyslipidemia, or elevated fasting glucose. Metabolic syndrome is accepted as a precursor to diabetes[215]. A large, cross sectional study of Japanese patients revealed a significant relationship between H. pylori infection and metabolic syndrome (OR = 1.39, P < 0.001)[216]. Microalbinuria, neuropathy, and heart disease are common complications of diabetes. Patients infected with H. pylori, particularly cagA+H. pylori, were found to be at higher risk of microalbuminuria, which indicates endothelial leakage and is a risk factor for cardiovascular disease and diabetic nephropathy[217,218]. Studies also suggest at higher rate of diabetic complications, such as nephropathy, neuropathy, and retinopathy, in H. pylori-positive diabetics[219,220]. Coronary heart disease is also more prevalent in H. pylori-positive diabetics than H. pylori-negative diabetics[221].
The mechanisms underlying the association between H. pylori and diabetes are unclear. Chronic inflammation due to H. pylori infection is believed to contribute to many H. pylori-associated diseases, and diabetics have higher levels of inflammatory markers than non-diabetics[222]. Nonetheless, Jeon et al[223] did not find that the inflammatory markers IL-6 and C-reactive protein were elevated in H. pylori-positive diabetics compared to H. pylori-negative diabetics. Therefore, it appears that other causes of inflammation, perhaps including adiposity, trump H. pylori-mediated inflammation in diabetics.
Does H. pylori eradication benefit patients who have already developed diabetes? Very few studies have sought to answer this important question. CagA+H. pylori strains are strongly associated with poor glycemic control[218,224], so one would naturally think that these patients would benefit from eradication therapy. In a study by Akanuma et al[225] found that H. pylori eradication did not improve glycemic control. Experiments in mice actually suggest that H. pylori infection improves glucose homeostasis[226]. H. pylori eradication regimens are not 100% effective in any population, but type 2 diabetics are at higher risk for eradication failure[227].
The impact of H. pylori infection on ghrelin production could complicate the interpretation of diabetes studies. H. pylori is known to suppress gastric ghrelin, and ghrelin levels in the stomach rise following H. pylori eradication[228]. Ghrelin stimulates appetite, which could explain why some studies have found that patients gain weight following H. pylori eradication[225,229]. It is also possible that resolution of gastric symptoms leads to weight gain, but regardless of the mechanism, weight gain is not beneficial to diabetic patients. Clearly, more studies are needed to clarify the impact of H. pylori eradication on diabetic patients, but at this point in time, treatment of H. pylori infection in diabetic patients cannot be recommended.
An association between H. pylori and coronary artery disease was first suggested in 1994, twelve years after Barry Marshall and Robin Warren discovered H. pylori[230]. The methodologies and endpoints used in studies are diverse, making it difficult to compare studies. The presence of CagA seropositivity tends to strengthen associations with cardiac diseases including atherosclerosis, unstable angina, and cardiac X syndrome[231-237]. A meta-analysis found that cagA-positive H. pylori increases the risk of both ischemic stroke and coronary heart disease[62]. There is ample evidence demonstrating that H. pylori infection alters biomarkers that are associated with CVD. For example, several studies have linked H. pylori infection to alterations in lipid profiles[238-240]. Moreover, the amount of LDL cholesterol elevation correlated with the degree of gastric inflammation[241] and H. pylori eradication normalized lipid profiles[242,243]. H. pylori infection has also been found to correlate with increased carotid intimal thickness, reduced serum paraoxonase-1 activity, and increased risk of peripheral artery disease[244,245].
The role of H. pylori in promoting atherosclerosis or other manifestations of cardiovascular disease remains controversial because a number of studies have failed to find any association between H. pylori infection and the disease parameters studied[246,247]. Studies of healthy adults and children failed to find associations between C-reactive protein levels and H. pylori infection[247]. H. pylori infection was also not found to correlate with fibrinogen or von Willebrand factor levels in coronary angiography patients[248]. McDonagh et al[246] failed to find an increased odds ratio for coronary heart disease in H. pylori-infected patients. With so many conflicting studies, it is impossible to recommend testing for or treatment of H. pylori in any CVD patient population. Moreover, few in vivo studies have been carried out in animal models. Nonetheless, the number of studies positively correlating H. pylori infection with various aspects of CVD warrants continued research on the potential for H. pylori to influence various types of cardiovascular disease.
A recent meta-analysis of studies revealed that CagA seropositivity increased the risk of autoimmune thyroid disease by 2.24 fold (95%CI: 1.06-4.75). Infection with any H. pylori strain significantly increased the risk for Grave’s disease (OR = 4.35), but not for Hashimoto’s thyroiditis[249]. While the overall H. pylori infection rate was not elevated in Hashimoto’s thyroiditis, these patients did have a significantly higher rate of cagA+H. pylori infection than controls[250]. An exploration of both host genetics and H. pylori virulence factors revealed that CagA+ strains were more strongly associated with Grave’s disease than cagA- strains and certain HLA-DQA1 alleles altered the risk of developing Grave’s disease[251]. H. pylori infection, especially cagA+, was found to be more prevalent in patients with Grave’s disease than controls; CagA has some homology with thyroid peroxidase, which could trigger an autoimmune response[252]. Autoantibodies against thyroid peroxidase dropped significantly in all 5 H. pylori-positive patients who accepted eradication therapy, but did not drop in 5 who refused to be treated. Anti-thyroglobin titers also dropped in 4/5 treated patients[253]. H. pylori infection may also decrease the efficacy of thyroxine therapy in patients with hypothyroidism[254]. In support of the potential for H. pylori to cause thyroid disease, euthyroid patients with thyroid nodules were significantly more likely to be infected with H. pylori than patients without thyroid nodules (P = 0.002)[255].
Systemic sclerosis is an autoimmune disorder caused by autoantibodies Information regarding the potential for H. pylori to cause or worsen systemic sclerosis is scant, but intriguing. A few studies have found positive correlations between systemic sclerosis and H. pylori infection[256-258]. The presence of H. pylori infection is strongly associated with worse gastrointestinal, skin, and joint symptoms, as well as increased erythrocyte sedimentation rate in systemic sclerosis patients[259]. Danese et al[256] found that 90% of H. pylori strains infecting systemic sclerosis patients were cagA+, compared with only 37% in controls. Reinauer et al[260] demonstrated that H. pylori could be eradicated in systemic sclerosis patients, but did not follow up to determine whether symptom scores improved. Further studies are needed to confirm the association between H. pylori and systemic sclerosis and determine whether H. pylori eradication is beneficial.
In contrast to systemic sclerosis, several studies indicate a protective effect of H. pylori infection on multiple sclerosis[261,262], but the picture becomes more complicated when the presence of neuromyelitis optica (NMO) is included. NMO is an autoimmune disease in which antibodies against aquaporin lead to demyelination of the spinal cord and optic nerve, leading to paralysis and blindness. It has been considered to be a variant of multiple sclerosis, but is now proposed to be a separate disease[263]. Li et al[264] attempted to dissect the possible relationship between MS and H. pylori by correlating antibodies against HP-NAP with autoantibodies against aquaporin-4 (AQP4) and with circulating myeloperoxidase. Patients with MS and neuromyelitis optica were more likely than controls to be infected with H. pylori than those with conventional MS. There was a significant correlation between seropositivity for AQP4 and seropositivity for HP-NAP. HP-NAP seropositive patients also had higher myeloperoxidase levels and worse disability. H. pylori and Chlamydia pneumoniae seropositivity were found to be more prevalent in NMO patients who also have anti-AQP4 antibodies, but not in those lacking that autoimmune marker[265]. These data clearly indicate that MS and NMO patients must be studied separately with regard to the potential for H. pylori involvement. H. pylori antigens have been proposed as a treatment for multiple sclerosis[266], but the potential for causing or exacerbating other diseases makes this a risky proposition.
Campylobacter jejuni infection is known to be a risk factor for development of Guillain Barré syndrome, and H. pylori may contribute as well. Guillain Barré syndrome is an autoimmune attack on peripheral nerves that leads to paralysis, which is usually temporary. A few small studies have found higher H. pylori titers of H. pylori antibodies in serum or CSF in Guillain Barré patients than in controls[267-269]. Associations were particularly strong in patients with the acute inflammatory demyelinating polyradiculoneuropathy (AIDP) type of Guillain Barré syndrome. Another study found anti-VacA in all AIDP patients[270]. Homology between VacA and [Na(+) + K(+)]-ATPase is suggested as a potential cause.
The hygiene hypothesis proposes that inadequate exposure to certain bacteria during the first one or two years of life results in an immune system that is hypersensitive to environmental agents, leading to various allergic diseases. A Th2-skewed immune system favors allergies, while Th1 responses, which are induced by H. pylori, favor autoimmune and inflammatory diseases. The incidence of allergies has risen dramatically in recent decades, while the prevalence of H. pylori infection has fallen. This led investigators to search for a link between the two phenomena. Several studies suggested an inverse relationship between H. pylori infection and asthma. A meta-analysis confirmed this association[271]. Proof-of-principle has been established in a mouse model of asthma induced by ovalbumin or house dust mite allergen[272]. In accordance with the hygiene hypothesis, neonatal infection of mice proved most effective in preventing asthma. Protection correlated with infiltration of regulatory T cells into the lungs. Adoptive transfer of regulatory T cells from H. pylori-infected mice into allergic mice offered protection. Some evidence also suggests that H. pylori may be protective against food allergies, celiac disease, and allergic rhinitis as well[273-275]. CagA+ strains do not appear to be relevant to asthma, but purified HP-NAP reduced lung eosinophilia and Th2-associated cytokines in ovalbumin-induced asthma in mice[53]. Certainly, H. pylori is not the only organism implicated in allergy prevention. Actinobacteria, Bifidobacterium, and certain Clostridia and Bacteroides spp., are associated with a lower risk of allergic disease, as well as a higher overall diversity of organisms[276,277]. It is not known which organisms can most safely and effectively prevent or treat allergic disease.
Considering that H. pylori has numerous direct and indirect effects on host physiology, one should keep an open mind regarding the possibility that H. pylori contributes to a variety of extragastric diseases. H. pylori has now been detected by culture, PCR, or histology in several epithelial tissues, including the eyes, ears, nose, and skin. While it is not yet possible to draw firm conclusions regarding the role of H. pylori in most extragastric diseases, the evidence cannot be ignored. Studies with contradictory results could have several explanations. Regional variations in both human and H. pylori genotypes contribute to established H. pylori-associated diseases. Inconsistencies may also indicate that H. pylori is a minor contributor or serves to exacerbate, rather than cause, a particular disease. Just as H. pylori is not responsible for all cases of gastric cancer or ulcers, H. pylori is unlikely to be the sole cause of other disorders. Future studies should measure the presence of H. pylori virulence factors, in addition to overall seropositivity, since several diseases are associated with only some H. pylori genotypes. Studies designed to elucidate mechanisms through which H. pylori contributes to disease would also improve credibility.
Routine testing and treatment without endoscopy should be performed primarily in relatively young patients with evidence of dyspepsia, and without suspicion of severe pathology[278,279]. The test and treat strategy is considered less useful in regions where the prevalence of H. pylori is low (20% or less). Patients who present with alarm symptoms of vomiting, gastrointestinal bleeding, or weight loss in patients or are over 45 years of age should proceed with an endoscopic evaluation[278].
Preemptive H. pylori eradication can reduce gastric cancer risk and is considered appropriate for (1) first degree relatives of gastric cancer patients, (2) patients previously treated for gastric neoplasia; (3) patients with severe pan-gastritis, corpus-predominant gastritis, or atrophy; and (4) patients with other risk factors such as heavy smoking or exposure to coal dust[279].
Testing should be considered for certain other populations. The combination of long-term NSAID use and H. pylori infection increases the risk of ulcer development. Therefore, testing for H. pylori is recommended prior to starting long-term NSAID therapy[280,281]. Individuals with unexplained iron deficiency anemia have been shown in several series to have an improvement in anemia with treatment of H. pylori in select patients[133,134,282]. H. pylori testing may be considered in patients with chronic idiopathic thrombocytopenia as eradication may result in improvement in platelet function[283]. There is not sufficient evidence to warrant H. pylori testing for most extragastric diseases at this time; however, many patients with potentially H. pylori-associated diseases also have gastric symptoms. Non-invasive tests may be considered for these patients.
A number of testing methods are available for detection of H. pylori. Invasive methods use endoscopy as the vehicle to obtain tissue for histology, rapid urease testing, polymerase chain reaction, or culture[278]. Culture is no longer considered necessary for confirmation of H. pylori infection, but cultured organisms can be tested for antibiotic resistance. Biopsies are also required for PCR analysis.
The urea breath test or fecal antigen testing are the preferred methods for establishment of active infection[279]; however these recommendations come with caveats. The urea breath test consists of drinking carbon-13 labeled or carbon-14 labeled urea. The ingested radiolabeled carbon is converted to carbon dioxide and ammonia by the urease created by the H. pylori. The carbon dioxide is absorbed in the bloodstream and a sample of expired air is analyzed for the presence of the labeled carbon. The test also has the advantage of identifying active infection, which cannot be categorized by serology. The sensitivity and specificity for the urea breath test is 99% and 98%, respectively[284]. Since the urea breath test requires specialized equipment, it is not always feasible in small clinics.
Stool also may be submitted for analysis for H. pylori antigens using monoclonal or polyclonal antibodies. The test initially used an ELISA process, but has been modified into an immunocard, making it suitable for small clinics and field-testing in third world countries. The sensitivity and specificity for the test is 94.1% and 91.8%, respectively. Stool testing also is more economical than endoscopy for confirmation of eradication. Patient submission of stool samples may be a limitation of this test for some patients, but it is highly suitable for infants and children[285,286].
Proton pump inhibitors and H2 receptor antagonists need to be withdrawn for several days prior to testing using the urea breath test or stool antigen test due to the potential for false negatives. In addition, antibiotics taken during the four weeks prior to testing can suppress the infection and lead to a false negative. Some studies suggest that waiting until 8 wk post-treatment improves test accuracy[285].
Serology testing for H. pylori is widely available and relatively inexpensive. Microtiter plates coated with H. pylori antigens, combined with a secondary antibody, and are used to detect H. pylori-specific IgG. Serology results are not affected by acid suppression therapy or recent antibiotic use; however, seropositivity is not a confirmation of current H. pylori infection. Likewise, serology cannot be used to confirm cure following an anti-microbial regimen. These antibodies persist for an extended period of time, often greater than half a year. Due to the often-patchy distribution of H. pylori, serum samples are more consistent than other tissue samples. The sensitivity and specificity of serology for detection of initial infection compares to the gold standard of histology with sensitivity of 85% and specificity of 79%[287].
Endoscopy is required to obtain biopsy material and to assess the degree of pathology in the stomach. Giemsa, Warthin-Starry, and Diff-Quik stains in addition to standard hematoxylin and eosin staining can be used to identify H. pylori. Stains such as Giemsa provide identification of the bacteria, assessment of inflammation, and also provide evidence for intestinal metaplasia. No other tests allow for the identification of intestinal metaplasia. Multiple biopsies are needed for increased diagnostic yield targeting at least three areas including the angularis, the greater curvature of the corpus, and from the greater curvature of the antrum. The high cost of infrastructure, variability in pathologist review, and training of personnel are limitations that prevent this method from being considered as the gold standard for diagnosis[278,288]. Biopsies from the corpus, in addition to the antrum, increases the diagnostic yield by 10%[289].
Rapid Urease testing involves utilization of the H. pylori urease to identify active organisms. Samples of gastric tissue are placed on a reactive strip or agar gel. A pH sensitive color indicator, buffer, and urea are added to the sample. Urea is converted to ammonia causing a pH increase and subsequent color change in the sample area of the test device. Readings can be taken between 1 h and 24 h based on the specific commercial test kit used. Sensitivity greater than 93% and specificity of 98% is reported for rapid urease testing[290].
Culture provides a high specificity for the diagnosis of H. pylori; however, it often lacks the sensitivity seen with other invasive methods. From a practical standpoint, culture techniques are technically challenging and costly. H. pylori is a fastidious, microaerophilic organism requiring 5-7 d to form visible colonies on solid media. Laboratory personnel must culture specimens promptly and avoid prolonged exposure to ambient oxygen levels to maximize the organism’s survival. Culture options for detection are primarily recommended for situations requiring specific antimicrobial sensitivity testing. The tissue may be placed directly on culture media, dragged across the agar surface, or gently homogenized and plated.
PCR techniques can be used to detect H. pylori DNA most reliably in biopsy samples, but saliva and feces have also been used. PCR sensitivity nearing 100% and specificity of 100% can be obtained[291]. Contamination from improperly cleaned endoscopes may create false positive results. False negative results may occur due to PCR inhibitors within gastric tissue or feces. Wide acceptance of PCR techniques in the clinical setting is limited and PCR is primarily used in research settings[292]. Fluorescence in situ hybridization (FISH) permits sensitive and specific detection of H. pylori in gastric biopsies without the need for DNA extraction. A fluorescently labeled DNA probe hybridizes with the complementary sequence within the bacterial chromosome. FISH can detect coccoid H. pylori and is more sensitive than standard staining procedures[293]. The use of different fluorescent dyes can permit simultaneous detection of H. pylori 16S rRNA and gene sequences associated with antibiotic resistance[294]. A commercially available kit has proven to be reliable and far more rapid than culture-based methods for simultaneous detection of H. pylori and clarithromycin resistance[295,296].
Optimal treatment for Helicobacter pylori has yet to be defined for all patients. Furthermore, rates of antibiotic resistance vary by region, and local resistance data should be used to guide treatment where available. Suggested regimens have included quadruple therapy, triple therapy, and sequential therapy as summarized in guidelines published by the American College of Gastroenterology and Maastricht Conference (Table 1). Quadruple therapy utilizes a combination of a proton pump inhibitor (PPI), bismuth product, and antibiotics containing metronidazole and tetracycline for 10 to 14 d. Bismuth salts are not available in all areas of the world. Patients who receive triple therapy are usually administered a regimen containing a PPI, amoxicillin, and clarithromycin for 10-14 d[297,298]. Sequential therapy begins with amoxicillin plus a PPI for the first five days and finishes with triple therapy including a PPI, clarithromycin, and tinidazole[299]. The Maastricht Conference Guidelines suggest selection of H. pylori treatment regimen without clarithromycin if the resistance rate exceeds 20%[279].
| Therapy | Treatment |
| Triple therapy | Duration: 7-14 d |
| PPI twice a day at higher dose or esomeprazole 40 mg by mouth daily | |
| Amoxicillin 1 g by mouth bid | |
| Clarithromycin 500 mg by mouth twice daily | |
| Quadruple therapy | Duration: 10-14 d |
| PPI twice a day at higher dose or esomeprazole 40 mg by mouth daily | |
| Tetracycline 500 mg by mouth four times daily | |
| Bismuth 120 mg four times daily | |
| Metronidazole 250 mg four times daily | |
| Sequential therapy | Duration: 10 d |
| Day 1-5 | |
| PPI twice a day at higher dose or esomeprazole 40 mg by mouth daily | |
| Amoxicillin 1 g by mouth bid | |
| Day 6-10 | |
| PPI twice a day at higher dose or esomeprazole 40 mg by mouth daily | |
| Clarithromycin 500 mg by mouth twice daily | |
| Tinidazole 500 mg by mouth twice daily |
Resistance of H. pylori to commonly used antibiotics is on the rise worldwide. Overall, H. pylori resistance to metronidazole is prevalent, while resistance to amoxicillin and tetracycline are low; however the picture is far more complex at the regional level. For example, amoxicillin resistance is below 3% in America and Europe, but over 60% in Africa[300]. Africa also has the highest rates of resistance to metronidazole (92.4%) and tetracycline (43.9%)[300]. Metronidazole resistance is above 50% in much of the world but there are indications that metronidazole resistance may be dropping in northern Europe[301]. Within Europe, resistance patterns vary by country and even within a country. For example, the reported clarithromycin resistance rate is 1.5% in Sweden, but 7.5% in Germany and clarithromycin resistance in Italy is lower in the north than in the south[301]. Increasing resistance to clarithromycin and levofloxacin has been attributed to widespread use of these antibiotics for respiratory tract and urinary tract infections, respectively[300]. In the (Helicobacter pylori Antimicrobial Resistance Monitoring Program), a resistance pattern showed 29.1% of United States isolates were resistant to one antimicrobial agent and 5% were resistant to two or more antimicrobial agents[302]. Multidrug resistance remains low worldwide[300], offering hope that rescue therapy will work in most patients. Previous treatment for H. pylori is the single largest risk factor for drug resistance[302]. Success rates of antimicrobial therapy do not always mirror in vitro susceptibility data. This could be partially due to variability in antibiotic resistance testing protocols and on poor patient compliance[301]. In summary, local antimicrobial resistance data should be consulted to increase the chances of eradication success following the first treatment.
Specific H. pylori mutations responsible for antibiotic resistance have been identified. Inactivation of the rdxA gene is well known to contribute to metronidazole resistance, though other mechanisms likely exist[303]. The point mutations A2143G, A2142G, and A2142C are responsible for clarithromycin resistance in H. pylori. Although the test is not yet available in the clinical setting, the A2143G mutation was associated with a lower eradication rate. In a clinical trial, sequential therapy was found to be more successful in eradication than triple therapy[300]. Certain gyrA mutations are associated with fluoroquinolone resistance[304]. For individuals who have failed eradication with the primary regimen, use of alternative antibiotics must be considered. In the future, it may be feasible to determine likely antimicrobial resistance patterns using H. pylori DNA isolated from stool without the need for endoscopy[305].
Recently, the concept of sequential therapy has been proposed with a 10-14 d regimen of a PPI twice daily and amoxicillin twice daily for five days, followed by a PPI plus clarithromycin and tinidazole twice daily[306]. Zullo et al[307] reported in a 2007 pooled-data analysis, an eradication rate greater than 90% with an intention to treat analysis thus revealing a higher eradication rate than standard therapy. A recent meta-analysis revealed an odds ratio (OR) for eradication of 2.99 (95%CI: 2.7-3.62), yielding a number needed to treat (NNT) of 6 in favor of sequential therapy compared to triple therapy. Additionally, the OR for eradication with sequential therapy compared with 10 d of triple therapy was 2.92 (95%CI: 1.95-4.38) with a NNT of 8 positive for sequential therapy. Factoring for clarithromycin resistance, the OR for eradication with sequential therapy was 10.21 (95%CI: 2.01-34.58) compared with triple therapy; however, the number of patients was small[299]. Another large meta-analysis found superiority compared to triple therapy with 93.5% eradication (95%CI: 91.3 to 95.5) compared to 76.9% (95%CI: 71.0-82.8) for standard triple therapy[308]. Sequential therapy appeared to be superior in subgroup analyses involving trial quality, smoking status, ulcer disease or non-ulcer dyspepsia, clarithromycin or imidazole resistance or both, duration of therapy and diagnostic method[308].
A novel regimen for treatment of H. pylori was proposed in an open label prospective trial of 653 patients using levofloxacin, omeprazole, nitazoxanide, and doxycycline (LOAD) for 7 or 10 d compared to triple therapy with lansoprazole, amoxicillin, and clarithromycin (LAC). In this intention-to treat analysis, the rate of eradication for LOAD-7 d regimen was 90% and 88.9% for LOAD-10 d regimen and combination eradication rate of 89.4% vs 73.3% eradication rate with LAC. No adverse event differences were noted in the two groups[309]. The pill burden was limited to twice daily with both regimens, however, the current cost of the LOAD regimen, in particular nitazoxanide, may limit its widespread usage. Additionally, more comprehensive multi-center trials will be needed to confirm these results.
In a pooled analysis of several refereed studies and abstracts, retreatment with triple and quadruple therapies were found to achieve eradication rates in the range of 70%-76%. Additionally, the substitution of two antibiotics compared to one antibiotic in the re-treatment regimen was found to be superior for eradication[310]. Attempts should be made to avoid antibiotics previously utilized for H. pylori eradication. As clarithromycin is utilized for most triple therapy regimens, quadruple therapy is often utilized as the salvage therapy. A levofloxacin 500 mg daily, PPI, and amoxicillin 1 g twice daily regimen is suggested in the American College of Gastroenterology guidelines with an eradication rate possibly higher than quadruple therapy[278]. In a multicenter, prospective trial, rifabutin-based rescue therapy with amoxicillin and PPI for ten days after three previous treatment failures was found to have an approximate 50% eradication rate providing an additional strategy for refractory infections[311]. After the second attempt at treatment and confirmation of failure of eradication, antibiotic sensitivity testing should be implemented with tissue acquisition and culture for guidance in selecting the appropriate antimicrobial regimen.
A number of studies have investigated the roles of probiotics in improving eradication rates and decreasing antibiotic side effects, but there is not yet a consensus regarding their utility. A recent meta-analysis evaluating probiotics including both Lactobacillus and Bifidobacterium species reports increased eradication in adults, but no effect in children. Total side effects were decreased with the supplementation of probiotics. Individual side effects were not assessed in this meta-analysis; however, the effects of confounders could not be assessed due to the small trial sizes[312]. Another recent meta-analysis differentiated between studies using Lactobacillus alone vs combinations containing other genera[313]. They found that the eradication rates were raised significantly by 17% when Lactobacillus was used alone, compared with only 2.8% in patients treated with a combination of species. Contrary to the findings of the aforementioned study, they did not detect a reduction of overall side effects. Diarrhea may be reduced with the usage of Saccharomyces boulardii, but other adverse effects (epigastric pain, taste disturbances, nausea, and gas/bloating) were not affected. Trial numbers are limited with this organism[314]. A few trials have evaluated lactoferrin as adjuvant therapy. A meta-analysis in 2009 found benefit, but small sizes of the trials and lack of robust data necessitate additional clinical trials[315]. A recent study compared the effects of probiotics or a combination of probiotics and lactoferrin on eradication and severity of antibiotic-related side effects[316]. Neither treatment improved eradication, but both reduced side effects and increased patient compliance. The addition of lactoferrin to probiotics did not confer any additional benefit compared to probiotics alone. In summary, there is some evidence that probiotics improve eradication rates, but evidence that probiotics can reduce the side effects of eradication therapy is not conclusive. Given the excellent safety profile of probiotics, it may be reasonable to suggest that patients eat yogurt or take an over-the-counter supplement containing Lactobacillus and/or other probiotic species.
Simvastatin as a supplement to clarithromycin, amoxicillin, and omeprazole has been shown to increase the eradication rate of H. pylori when used as an adjuvant in a randomized controlled trial. No differences were noted in compliance or side effects. The proposed mechanism for augmented eradication is the anti-inflammatory effects of Simvastatin, which are unrelated to its cholesterol-lowering activity (Nseir, 22646167). Further studies are needed for confirmation before advocating this strategy.
Generally, bismuth compounds, tetracycline, and flouroquinolones regimens should be avoided in pregnancy due to potential teratogenic effects. Metallic taste is a common side effect with metronidazole and clarithromycin. Photosensitivity with doxycycline and tetracycline is possible. Additionally, metronidazole may cause an adverse reaction with alcohol and with prolonged usage may create a peripheral neuropathy. Other less common side effects are noted with each of these medications.
Based on Maastricht IV/Florence Consensus Report, eradication of H. pylori should be confirmed with a urea breath test or a laboratory-based, validated monoclonal stool test 4 wk after the conclusion of treatment. In cases of a gastric ulcer or gastric MALT lymphoma, follow-up is suggested with an esophagogastroduodenoscopy and biopsy of gastric tissue[279]. The ACG Guidelines also suggests testing with individuals with persistent dyspepsia symptoms following resection with an early gastric cancer[278].
H. pylori has proven to be a more complex pathogen than early research indicated. Multiple surface carbohydrate structures, outer membrane proteins, and toxins interact to modify host cell signaling and the immune response. While CagA remains the dominant virulence factor, HP-NAP and others significantly alter host physiology. The reach of H. pylori extends beyond the stomach. H. pylori can occasionally colonize the mouth, nose, ears, and skin. Whether H. pylori is a primary cause of disease in these locations or colonizes only after other agents initiate disease remains to be determined. H. pylori causes or is suspected to cause several autoimmune diseases. It is not known whether H. pylori must be present outside the stomach to initiate autoimmune disease. As of yet, routine testing for H. pylori is recommended only for patients with gastric symptoms, iron deficiency anemia, and idiopathic thrombocytopenic purpura. Recommendations will likely change as more is learned about H. pylori’s potential to cause other diseases. In addition to the urea breath test, the fecal antigen test has proven reliable and useful for detecting current H. pylori infection. The fecal antigen test does not require specialized equipment. Increasing antibiotic resistance is reducing the utility of metronidazole and clarithromycin, necessitating the use of alternative treatment regimens. Sequential therapy has proven to be a more effective than standard triple therapy for eradication of H. pylori. Thirty years after its discovery, H. pylori remains an enigmatic pathogen with many secrets yet to be revealed.
P- Reviewer: Choe BH, Smith SI S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Linz B, Balloux F, Moodley Y, Manica A, Liu H, Roumagnac P, Falush D, Stamer C, Prugnolle F, van der Merwe SW. An African origin for the intimate association between humans and Helicobacter pylori. Nature. 2007;445:915-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 682] [Cited by in F6Publishing: 607] [Article Influence: 35.7] [Reference Citation Analysis (3)] |
| 2. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3302] [Cited by in F6Publishing: 3116] [Article Influence: 77.9] [Reference Citation Analysis (0)] |
| 3. | Mbulaiteye SM, Hisada M, El-Omar EM. Helicobacter Pylori associated global gastric cancer burden. Front Biosci (Landmark Ed). 2009;14:1490-1504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Gao W, Cheng H, Hu F, Li J, Wang L, Yang G, Xu L, Zheng X. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter. 2010;15:460-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Bytzer P, O’Morain C. Treatment of Helicobacter pylori. Helicobacter. 2005;10 Suppl 1:40-46. [PubMed] [Cited in This Article: ] |
| 6. | Suzuki R, Shiota S, Yamaoka Y. Molecular epidemiology, population genetics, and pathogenic role of Helicobacter pylori. Infect Genet Evol. 2012;12:203-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Mishra S. Is Helicobacter pylori good or bad? Eur J Clin Microbiol Infect Dis. 2013;32:301-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Lertsethtakarn P, Ottemann KM, Hendrixson DR. Motility and chemotaxis in Campylobacter and Helicobacter. Annu Rev Microbiol. 2011;65:389-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 218] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 9. | McGee DJ, George AE, Trainor EA, Horton KE, Hildebrandt E, Testerman TL. Cholesterol enhances Helicobacter pylori resistance to antibiotics and LL-37. Antimicrob Agents Chemother. 2011;55:2897-2904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Wang HJ, Cheng WC, Cheng HH, Lai CH, Wang WC. Helicobacter pylori cholesteryl glucosides interfere with host membrane phase and affect type IV secretion system function during infection in AGS cells. Mol Microbiol. 2012;83:67-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Beigier-Bompadre M, Moos V, Belogolova E, Allers K, Schneider T, Churin Y, Ignatius R, Meyer TF, Aebischer T. Modulation of the CD4+ T-cell response by Helicobacter pylori depends on known virulence factors and bacterial cholesterol and cholesterol α-glucoside content. J Infect Dis. 2011;204:1339-1348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Backert S, Clyne M. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2011;16 Suppl 1:19-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Olofsson A, Vallström A, Petzold K, Tegtmeyer N, Schleucher J, Carlsson S, Haas R, Backert S, Wai SN, Gröbner G. Biochemical and functional characterization of Helicobacter pylori vesicles. Mol Microbiol. 2010;77:1539-1555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 143] [Cited by in F6Publishing: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Kim SS, Ruiz VE, Carroll JD, Moss SF. Helicobacter pylori in the pathogenesis of gastric cancer and gastric lymphoma. Cancer Lett. 2011;305:228-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Tegtmeyer N, Hartig R, Delahay RM, Rohde M, Brandt S, Conradi J, Takahashi S, Smolka AJ, Sewald N, Backert S. A small fibronectin-mimicking protein from bacteria induces cell spreading and focal adhesion formation. J Biol Chem. 2010;285:23515-23526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 16. | Tegtmeyer N, Wessler S, Backert S. Role of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori pathogenesis. FEBS J. 2011;278:1190-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 193] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 17. | Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat Rev Gastroenterol Hepatol. 2010;7:629-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 420] [Cited by in F6Publishing: 428] [Article Influence: 30.6] [Reference Citation Analysis (1)] |
| 18. | Higashi H, Tsutsumi R, Muto S, Sugiyama T, Azuma T, Asaka M, Hatakeyama M. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 755] [Cited by in F6Publishing: 758] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 19. | Bourzac KM, Guillemin K. Helicobacter pylori-host cell interactions mediated by type IV secretion. Cell Microbiol. 2005;7:911-919. [PubMed] [Cited in This Article: ] |
| 20. | Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430-1434. [PubMed] [Cited in This Article: ] |
| 21. | Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 379] [Cited by in F6Publishing: 370] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 22. | Fischer W, Prassl S, Haas R. Virulence mechanisms and persistence strategies of the human gastric pathogen Helicobacter pylori. Curr Top Microbiol Immunol. 2009;337:129-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 23. | Cover TL, Blaser MJ. Helicobacter pylori in health and disease. Gastroenterology. 2009;136:1863-1873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 474] [Cited by in F6Publishing: 459] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 24. | Tanaka H, Yoshida M, Nishiumi S, Ohnishi N, Kobayashi K, Yamamoto K, Fujita T, Hatakeyama M, Azuma T. The CagA protein of Helicobacter pylori suppresses the functions of dendritic cell in mice. Arch Biochem Biophys. 2010;498:35-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Stein M, Bagnoli F, Halenbeck R, Rappuoli R, Fantl WJ, Covacci A. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol Microbiol. 2002;43:971-980. [PubMed] [Cited in This Article: ] |
| 26. | Jones KR, Joo YM, Jang S, Yoo YJ, Lee HS, Chung IS, Olsen CH, Whitmire JM, Merrell DS, Cha JH. Polymorphism in the CagA EPIYA motif impacts development of gastric cancer. J Clin Microbiol. 2009;47:959-968. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Lee IO, Kim JH, Choi YJ, Pillinger MH, Kim SY, Blaser MJ, Lee YC. Helicobacter pylori CagA phosphorylation status determines the gp130-activated SHP2/ERK and JAK/STAT signal transduction pathways in gastric epithelial cells. J Biol Chem. 2010;285:16042-16050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 28. | Kim IJ, Blanke SR. Remodeling the host environment: modulation of the gastric epithelium by the Helicobacter pylori vacuolating toxin (VacA). Front Cell Infect Microbiol. 2012;2:37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Sewald X, Fischer W, Haas R. Sticky socks: Helicobacter pylori VacA takes shape. Trends Microbiol. 2008;16:89-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Cover TL, Blanke SR. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat Rev Microbiol. 2005;3:320-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 372] [Cited by in F6Publishing: 380] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 31. | Palframan SL, Kwok T, Gabriel K. Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis. Front Cell Infect Microbiol. 2012;2:92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 175] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 32. | Papini E, Satin B, Norais N, de Bernard M, Telford JL, Rappuoli R, Montecucco C. Selective increase of the permeability of polarized epithelial cell monolayers by Helicobacter pylori vacuolating toxin. J Clin Invest. 1998;102:813-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 182] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Chung C, Olivares A, Torres E, Yilmaz O, Cohen H, Perez-Perez G. Diversity of VacA intermediate region among Helicobacter pylori strains from several regions of the world. J Clin Microbiol. 2010;48:690-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Lu H, Yamaoka Y, Graham DY. Helicobacter pylori virulence factors: facts and fantasies. Curr Opin Gastroenterol. 2005;21:653-659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Toller IM, Neelsen KJ, Steger M, Hartung ML, Hottiger MO, Stucki M, Kalali B, Gerhard M, Sartori AA, Lopes M. Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proc Natl Acad Sci USA. 2011;108:14944-14949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 230] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 36. | Ishijima N, Suzuki M, Ashida H, Ichikawa Y, Kanegae Y, Saito I, Borén T, Haas R, Sasakawa C, Mimuro H. BabA-mediated adherence is a potentiator of the Helicobacter pylori type IV secretion system activity. J Biol Chem. 2011;286:25256-25264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 37. | Borén T, Falk P, Roth KA, Larson G, Normark S. Attachment of Helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262:1892-1895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 840] [Cited by in F6Publishing: 777] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 38. | Mahdavi J, Sondén B, Hurtig M, Olfat FO, Forsberg L, Roche N, Angstrom J, Larsson T, Teneberg S, Karlsson KA. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science. 2002;297:573-578. [PubMed] [Cited in This Article: ] |
| 39. | Yamaoka Y. Increasing evidence of the role of Helicobacter pylori SabA in the pathogenesis of gastroduodenal disease. J Infect Dev Ctries. 2008;2:174-181. [PubMed] [Cited in This Article: ] |
| 40. | Senkovich OA, Yin J, Ekshyyan V, Conant C, Traylor J, Adegboyega P, McGee DJ, Rhoads RE, Slepenkov S, Testerman TL. Helicobacter pylori AlpA and AlpB bind host laminin and influence gastric inflammation in gerbils. Infect Immun. 2011;79:3106-3116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Yamaoka Y, Kwon DH, Graham DY. A M(r) 34,000 proinflammatory outer membrane protein (oipA) of Helicobacter pylori. Proc Natl Acad Sci USA. 2000;97:7533-7538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 292] [Cited by in F6Publishing: 305] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 42. | Backert S, Clyne M, Tegtmeyer N. Molecular mechanisms of gastric epithelial cell adhesion and injection of CagA by Helicobacter pylori. Cell Commun Signal. 2011;9:28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 113] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 43. | Franco AT, Johnston E, Krishna U, Yamaoka Y, Israel DA, Nagy TA, Wroblewski LE, Piazuelo MB, Correa P, Peek RM. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379-387. [PubMed] [Cited in This Article: ] |
| 44. | Wu WK, Cho CH, Lee CW, Fan D, Wu K, Yu J, Sung JJ. Dysregulation of cellular signaling in gastric cancer. Cancer Lett. 2010;295:144-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 45. | Evans DJ, Evans DG, Takemura T, Nakano H, Lampert HC, Graham DY, Granger DN, Kvietys PR. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63:2213-2220. [PubMed] [Cited in This Article: ] |
| 46. | Smith JL. The physiological role of ferritin-like compounds in bacteria. Crit Rev Microbiol. 2004;30:173-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 47. | de Bernard M, D’Elios MM. The immune modulating activity of the Helicobacter pylori HP-NAP: Friend or foe? Toxicon. 2010;56:1186-1192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 48. | Amedei A, Cappon A, Codolo G, Cabrelle A, Polenghi A, Benagiano M, Tasca E, Azzurri A, D’Elios MM, Del Prete G. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest. 2006;116:1092-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 214] [Cited by in F6Publishing: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 49. | Montemurro P, Barbuti G, Dundon WG, Del Giudice G, Rappuoli R, Colucci M, De Rinaldis P, Montecucco C, Semeraro N, Papini E. Helicobacter pylori neutrophil-activating protein stimulates tissue factor and plasminogen activator inhibitor-2 production by human blood mononuclear cells. J Infect Dis. 2001;183:1055-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Ramachandran M, Yu D, Wanders A, Essand M, Eriksson F. An infection-enhanced oncolytic adenovirus secreting H. pylori neutrophil-activating protein with therapeutic effects on neuroendocrine tumors. Mol Ther. 2013;21:2008-2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Codolo G, Fassan M, Munari F, Volpe A, Bassi P, Rugge M, Pagano F, D’Elios MM, de Bernard M. HP-NAP inhibits the growth of bladder cancer in mice by activating a cytotoxic Th1 response. Cancer Immunol Immunother. 2012;61:31-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 52. | Amedei A, Codolo G, Del Prete G, de Bernard M, D’Elios MM. The effect of Helicobacter pylori on asthma and allergy. J Asthma Allergy. 2010;3:139-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Codolo G, Mazzi P, Amedei A, Del Prete G, Berton G, D’Elios MM, de Bernard M. The neutrophil-activating protein of Helicobacter pylori down-modulates Th2 inflammation in ovalbumin-induced allergic asthma. Cell Microbiol. 2008;10:2355-2363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Kountouras J, Zavos C, Gavalas E, Deretzi G, Tsona A, Katsinelos P, Grigoriadis S, Pilpilidis I, Tzilves D, Vardaka E. A rebuttal to the potential anti-tumour benefit of Helicobacter pylori-induced neutrophil-activating protein. Cancer Immunol Immunother. 2012;61:445-46; discussion 445-46;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 55. | Chevalier C, Thiberge JM, Ferrero RL, Labigne A. Essential role of Helicobacter pylori gamma-glutamyltranspeptidase for the colonization of the gastric mucosa of mice. Mol Microbiol. 1999;31:1359-1372. [PubMed] [Cited in This Article: ] |
| 56. | Gong M, Ling SS, Lui SY, Yeoh KG, Ho B. Helicobacter pylori gamma-glutamyl transpeptidase is a pathogenic factor in the development of peptic ulcer disease. Gastroenterology. 2010;139:564-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Oertli M, Noben M, Engler DB, Semper RP, Reuter S, Maxeiner J, Gerhard M, Taube C, Müller A. Helicobacter pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc Natl Acad Sci USA. 2013;110:3047-3052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 58. | Flahou B, Haesebrouck F, Chiers K, Van Deun K, De Smet L, Devreese B, Vandenberghe I, Favoreel H, Smet A, Pasmans F. Gastric epithelial cell death caused by Helicobacter suis and Helicobacter pylori γ-glutamyl transpeptidase is mainly glutathione degradation-dependent. Cell Microbiol. 2011;13:1933-1955. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 59. | Testerman TL, Conn PB, Mobley HL, McGee DJ. Nutritional requirements and antibiotic resistance patterns of Helicobacter species in chemically defined media. J Clin Microbiol. 2006;44:1650-1658. [PubMed] [Cited in This Article: ] |
| 60. | Senkovich O, Ceaser S, McGee DJ, Testerman TL. Unique host iron utilization mechanisms of Helicobacter pylori revealed with iron-deficient chemically defined media. Infect Immun. 2010;78:1841-1849. [PubMed] [Cited in This Article: ] |
| 61. | Semino-Mora C, Testerman TL, Liu H, Whitmire JM, Studeman K, Jia Y, McAvoy TJ, Francis J, Nieroda C, Sardi A. Antibiotic treatment decreases microbial burden associated with pseudomyxoma peritonei and affects β-catenin distribution. Clin Cancer Res. 2013;19:3966-3976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Zhang S, Guo Y, Ma Y, Teng Y. Cytotoxin-associated gene-A-seropositive virulent strains of Helicobacter pylori and atherosclerotic diseases: a systematic review. Chin Med J (Engl). 2008;121:946-951. [PubMed] [Cited in This Article: ] |
| 63. | Hirsch C, Tegtmeyer N, Rohde M, Rowland M, Oyarzabal OA, Backert S. Live Helicobacter pylori in the root canal of endodontic-infected deciduous teeth. J Gastroenterol. 2012;47:936-940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Sudhakar U, Anusuya CN, Ramakrishnan T, Vijayalakshmi R. Isolation of Helicobacter pylori from dental plaque: A microbiological study. J Indian Soc Periodontol. 2008;12:67-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Lukeš P, Pavlík E, Potužníková B, Plzák J, Nártová E, Doseděl J, Katra R, Sterzl I, Betka J, Astl J. Comparison of Helicobacter pylori genotypes obtained from the oropharynx and stomach of the same individuals - a pilot study. Prague Med Rep. 2012;113:231-239. [PubMed] [Cited in This Article: ] |
| 66. | Morales-Espinosa R, Fernandez-Presas A, Gonzalez-Valencia G, Flores-Hernandez S, Delgado-Sapien G, Mendez-Sanchez JL, Sanchez-Quezada E, Muñoz-Pérez L, Leon-Aguilar R, Hernandez-Guerrero J. Helicobacter pylori in the oral cavity is associated with gastroesophageal disease. Oral Microbiol Immunol. 2009;24:464-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Liu Y, Yue H, Li A, Wang J, Jiang B, Zhang Y, Bai Y. An epidemiologic study on the correlation between oral Helicobacter pylori and gastric H. pylori. Curr Microbiol. 2009;58:449-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Chaudhry S, Khan AA, Butt AK, Idrees M, Izhar M, Iqbal HA. Helicobacter pylori in dental plaque; is it related to brushing frequency, plaque load and oral health status? J Coll Physicians Surg Pak. 2011;21:589-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 69. | Namiot DB, Leszczyńska K, Namiot Z, Chilewicz M, Bucki R, Kemona A. The occurrence of Helicobacter pylori antigens in dental plaque; an association with oral health status and oral hygiene practices. Adv Med Sci. 2010;55:167-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Bouziane A, Ahid S, Abouqal R, Ennibi O. Effect of periodontal therapy on prevention of gastric Helicobacter pylori recurrence: a systematic review and meta-analysis. J Clin Periodontol. 2012;39:1166-1173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 71. | Song HY, Li Y. Can eradication rate of gastric Helicobacter pylori be improved by killing oral Helicobacter pylori? World J Gastroenterol. 2013;19:6645-6650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 27] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | O’Rourke JL, Grehan M, Lee A. Non-pylori Helicobacter species in humans. Gut. 2001;49:601-606. [PubMed] [Cited in This Article: ] |
| 73. | Flahou B, Haesebrouck F, Smet A, Yonezawa H, Osaki T, Kamiya S. Gastric and enterohepatic non-Helicobacter pylori Helicobacters. Helicobacter. 2013;18 Suppl 1:66-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Zhou D, Guan WB, Wang JD, Zhang Y, Gong W, Quan ZW. A comparative study of clinicopathological features between chronic cholecystitis patients with and without Helicobacter pylori infection in gallbladder mucosa. PLoS One. 2013;8:e70265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Zavos C, Kountouras J, Sakkias G, Venizelos I, Deretzi G, Arapoglou S. Histological presence of Helicobacter pylori bacteria in the trabeculum and iris of patients with primary open-angle glaucoma. Ophthalmic Res. 2012;47:150-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 76. | Joo M, Kwak JE, Chang SH, Kim H, Chi JG, Kim KA, Yang JH, Lee JS, Moon YS, Kim KM. Helicobacter heilmannii-associated gastritis: clinicopathologic findings and comparison with Helicobacter pylori-associated gastritis. J Korean Med Sci. 2007;22:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 77. | Solnick JV. Clinical significance of Helicobacter species other than Helicobacter pylori. Clin Infect Dis. 2003;36:349-354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Thomson JM, Hansen R, Berry SH, Hope ME, Murray GI, Mukhopadhya I, McLean MH, Shen Z, Fox JG, El-Omar E. Enterohepatic helicobacter in ulcerative colitis: potential pathogenic entities? PLoS One. 2011;6:e17184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 79. | Hansen R, Thomson JM, Fox JG, El-Omar EM, Hold GL. Could Helicobacter organisms cause inflammatory bowel disease? FEMS Immunol Med Microbiol. 2011;61:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 80. | Xu S, Wan X, Zheng X, Zhou Y, Song Z, Cheng M, Du Y, Hou X. Symptom improvement after helicobacter pylori eradication in patients with functional dyspepsia-A multicenter, randomized, prospective cohort study. Int J Clin Exp Med. 2013;6:747-756. [PubMed] [Cited in This Article: ] |
| 81. | Selgrad M, Kandulski A, Malfertheiner P. Dyspepsia and Helicobacter pylori. Dig Dis. 2008;26:210-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 82. | Kusters JG, van Vliet AH, Kuipers EJ. Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev. 2006;19:449-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1446] [Cited by in F6Publishing: 1410] [Article Influence: 78.3] [Reference Citation Analysis (1)] |
| 83. | McColl KE, el-Omar E, Gillen D. Interactions between H. pylori infection, gastric acid secretion and anti-secretory therapy. Br Med Bull. 1998;54:121-138. [PubMed] [Cited in This Article: ] |
| 84. | Lochhead P, El-Omar EM. Helicobacter pylori infection and gastric cancer. Best Pract Res Clin Gastroenterol. 2007;21:281-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 75] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 85. | Konturek PC, Konturek SJ, Brzozowski T. Helicobacter pylori infection in gastric cancerogenesis. J Physiol Pharmacol. 2009;60:3-21. [PubMed] [Cited in This Article: ] |
| 86. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3126] [Cited by in F6Publishing: 3021] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 87. | Musumba C, Jorgensen A, Sutton L, Van Eker D, Moorcroft J, Hopkins M, Pritchard DM, Pirmohamed M. The relative contribution of NSAIDs and Helicobacter pylori to the aetiology of endoscopically-diagnosed peptic ulcer disease: observations from a tertiary referral hospital in the UK between 2005 and 2010. Aliment Pharmacol Ther. 2012;36:48-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 88. | Tang CL, Ye F, Liu W, Pan XL, Qian J, Zhang GX. Eradication of Helicobacter pylori infection reduces the incidence of peptic ulcer disease in patients using nonsteroidal anti-inflammatory drugs: a meta-analysis. Helicobacter. 2012;17:286-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 89. | Overmier JB, Murison R. Restoring psychology’s role in peptic ulcer. Appl Psychol Health Well Being. 2013;5:5-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 90. | Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, Neish AS, Collier-Hyams L, Perez-Perez GI, Hatakeyama M. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci USA. 2005;102:10646-10651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 392] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 91. | Zagari RM, Bazzoli F. Gastric cancer: who is at risk? Dig Dis. 2004;22:302-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 92. | Zambon CF, Navaglia F, Basso D, Rugge M, Plebani M. Helicobacter pylori babA2, cagA, and s1 vacA genes work synergistically in causing intestinal metaplasia. J Clin Pathol. 2003;56:287-291. [PubMed] [Cited in This Article: ] |
| 93. | Gerhard M, Lehn N, Neumayer N, Borén T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci USA. 1999;96:12778-12783. [PubMed] [Cited in This Article: ] |
| 94. | Yamaoka Y, Ojo O, Fujimoto S, Odenbreit S, Haas R, Gutierrez O, El-Zimaity HM, Reddy R, Arnqvist A, Graham DY. Helicobacter pylori outer membrane proteins and gastroduodenal disease. Gut. 2006;55:775-781. [PubMed] [Cited in This Article: ] |
| 95. | Batista SA, Rocha GA, Rocha AM, Saraiva IE, Cabral MM, Oliveira RC, Queiroz DM. Higher number of Helicobacter pylori CagA EPIYA C phosphorylation sites increases the risk of gastric cancer, but not duodenal ulcer. BMC Microbiol. 2011;11:61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 96. | Wroblewski LE, Peek RM. Helicobacter pylori in gastric carcinogenesis: mechanisms. Gastroenterol Clin North Am. 2013;42:285-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 97. | Peek RM, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 417] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 98. | Piazuelo MB, Epplein M, Correa P. Gastric cancer: an infectious disease. Infect Dis Clin North Am. 2010;24:853-69, vii. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 99. | Yamamoto E, Suzuki H, Takamaru H, Yamamoto H, Toyota M, Shinomura Y. Role of DNA methylation in the development of diffuse-type gastric cancer. Digestion. 2011;83:241-249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 100. | Oguri A, Ohmiya N, Taguchi A, Itoh A, Hirooka Y, Niwa Y, Maeda O, Ando T, Goto H. Rugal hyperplastic gastritis increases the risk of gastric carcinoma, especially diffuse and p53-independent subtypes. Eur J Gastroenterol Hepatol. 2007;19:561-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 101. | Nasri S, More H, Graziano F, Ruzzo A, Wilson E, Dunbier A, McKinney C, Merriman T, Guilford P, Magnani M. A novel diffuse gastric cancer susceptibility variant in E-cadherin (CDH1) intron 2: a case control study in an Italian population. BMC Cancer. 2008;8:138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 102. | Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175-1176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1293] [Cited by in F6Publishing: 1177] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 103. | Lehours P, Ménard A, Dupouy S, Bergey B, Richy F, Zerbib F, Ruskoné-Fourmestraux A, Delchier JC, Mégraud F. Evaluation of the association of nine Helicobacter pylori virulence factors with strains involved in low-grade gastric mucosa-associated lymphoid tissue lymphoma. Infect Immun. 2004;72:880-888. [PubMed] [Cited in This Article: ] |
| 104. | Kuo SH, Chen LT, Lin CW, Wu MS, Hsu PN, Tsai HJ, Chu CY, Tzeng YS, Wang HP, Yeh KH. Detection of the Helicobacter pylori CagA protein in gastric mucosa-associated lymphoid tissue lymphoma cells: clinical and biological significance. Blood Cancer J. 2013;3:e125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 105. | Suarez F, Lortholary O, Hermine O, Lecuit M. Infection-associated lymphomas derived from marginal zone B cells: a model of antigen-driven lymphoproliferation. Blood. 2006;107:3034-3044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 293] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 106. | Lenze D, Berg E, Volkmer-Engert R, Weiser AA, Greiner A, Knörr-Wittmann C, Anagnostopoulos I, Stein H, Hummel M. Influence of antigen on the development of MALT lymphoma. Blood. 2006;107:1141-1148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 107. | Zullo A, Hassan C, Cristofari F, Andriani A, De Francesco V, Ierardi E, Tomao S, Stolte M, Morini S, Vaira D. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2010;8:105-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 181] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 108. | Raderer M, Streubel B, Wöhrer S, Häfner M, Chott A. Successful antibiotic treatment of Helicobacter pylori negative gastric mucosa associated lymphoid tissue lymphomas. Gut. 2006;55:616-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 109. | Iwai H, Nakamichi N, Nakae K, Konishi M, Inaba M, Hoshino S, Baba S, Amakawa R. Parotid mucosa-associated lymphoid tissue lymphoma regression after Helicobacter pylori eradication. Laryngoscope. 2009;119:1491-1494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 110. | Hong SN, Lee SM, Kim JH, Lee TY, Kim JH, Choe WH, Lee SY, Cheon YK, Sung IK, Park HS. Helicobacter pylori infection increases the risk of colorectal adenomas: cross-sectional study and meta-analysis. Dig Dis Sci. 2012;57:2184-2194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 111. | Wang F, Sun MY, Shi SL, Lv ZS. Helicobacter pylori infection and normal colorectal mucosa-adenomatous polyp-adenocarcinoma sequence: a meta-analysis of 27 case-control studies. Colorectal Dis. 2014;16:246-252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 112. | Rokkas T, Sechopoulos P, Pistiolas D, Kothonas F, Margantinis G, Koukoulis G. The relationship of Helicobacter pylori infection and colon neoplasia, on the basis of meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:1286-1294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 113. | Sonnenberg A, Genta RM. Helicobacter pylori is a risk factor for colonic neoplasms. Am J Gastroenterol. 2013;108:208-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 114. | Shmuely H, Melzer E, Braverman M, Domniz N, Yahav J. Helicobacter pylori infection is associated with advanced colorectal neoplasia. Scand J Gastroenterol. 2014;49:35-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 115. | Smeenk RM, Bruin SC, van Velthuysen ML, Verwaal VJ. Pseudomyxoma peritonei. Curr Probl Surg. 2008;45:527-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 116. | Goldstein PJ, Cabanas J, da Silva RG, Sugarbaker PH. Pseudomyxoma peritonei arising from colonic polyps. Eur J Surg Oncol. 2006;32:764-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 117. | Semino-Mora C, Liu H, McAvoy T, Nieroda C, Studeman K, Sardi A, Dubois A. Pseudomyxoma peritonei: is disease progression related to microbial agents? A study of bacteria, MUC2 AND MUC5AC expression in disseminated peritoneal adenomucinosis and peritoneal mucinous carcinomatosis. Ann Surg Oncol. 2008;15:1414-1423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 118. | Gilbreath JJ, Semino-Mora C, Friedline CJ, Liu H, Bodi KL, McAvoy TJ, Francis J, Nieroda C, Sardi A, Dubois A. A core microbiome associated with the peritoneal tumors of pseudomyxoma peritonei. Orphanet J Rare Dis. 2013;8:105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 119. | Deutsch AJ, Steinbauer E, Hofmann NA, Strunk D, Gerlza T, Beham-Schmid C, Schaider H, Neumeister P. Chemokine receptors in gastric MALT lymphoma: loss of CXCR4 and upregulation of CXCR7 is associated with progression to diffuse large B-cell lymphoma. Mod Pathol. 2013;26:182-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 120. | Cavanna L, Pagani R, Seghini P, Zangrandi A, Paties C. High grade B-cell gastric lymphoma with complete pathologic remission after eradication of Helicobacter pylori infection: report of a case and review of the literature. World J Surg Oncol. 2008;6:35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 121. | Inaba K, Kushima R, Murakami N, Kuroda Y, Harada K, Kitaguchi M, Yoshio K, Sekii S, Takahashi K, Morota M. Increased risk of gastric adenocarcinoma after treatment of primary gastric diffuse large B-cell lymphoma. BMC Cancer. 2013;13:499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 122. | De Sanctis V, Marignani M, Angeletti S, Assisi D, Armosini V, Valeriani M, Minniti G, Cox MC, Ruco L, Enrici RM. Anti-Helicobacter pylori therapy in primary MALT lymphoma of rectum. Tumori. 2012;98:e105-e110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 123. | Stefanovic A, Lossos IS. Extranodal marginal zone lymphoma of the ocular adnexa. Blood. 2009;114:501-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 124. | Decaudin D, Ferroni A, Vincent-Salomon A, Beldjord K, Validire P, de Cremoux P, Validire P, Plancher C, Mathiot C, Macintyre E. Ocular adnexal lymphoma and Helicobacter pylori gastric infection. Am J Hematol. 2010;85:645-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 125. | Chan CC, Shen D, Mochizuki M, Gonzales JA, Yuen HK, Guex-Crosier Y, Lehoang P. Detection of Helicobacter pylori and Chlamydia pneumoniae genes in primary orbital lymphoma. Trans Am Ophthalmol Soc. 2006;104:62-70. [PubMed] [Cited in This Article: ] |
| 126. | Aygenc E, Selcuk A, Celikkanat S, Ozbek C, Ozdem C. The role of Helicobacter pylori infection in the cause of squamous cell carcinoma of the larynx. Otolaryngol Head Neck Surg. 2001;125:520-521. [PubMed] [Cited in This Article: ] |
| 127. | Gong H, Shi Y, Zhou L, Tao L, Shi Y, Cao W, Cheng L. Helicobacter pylori infection of the larynx may be an emerging risk factor for laryngeal squamous cell carcinoma. Clin Transl Oncol. 2012;14:905-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 128. | Shi Y, Gong H, Zhou L, Tao L, Shi Y, Cao W, Cheng L. Association between Helicobacter pylori infection and laryngeal squamous cell carcinoma in a Chinese male population. ORL J Otorhinolaryngol Relat Spec. 2011;73:295-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 129. | Burduk PK. Association between infection of virulence cagA gene Helicobacter pylori and laryngeal squamous cell carcinoma. Med Sci Monit. 2013;19:584-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 130. | Guilemany JM, Langdon C, Ballesteros F, Blanch JL. Prognostic significance and association of Helicobacter pylori infection in pharyngolaryngeal cancer. Eur Arch Otorhinolaryngol. 2014;271:2539-2543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 131. | Masoud N, Manouchehr K, Najmeh D, Monireh H. Lack of association between Helicobacter pylori and laryngeal carcinoma. Asian Pac J Cancer Prev. 2008;9:81-82. [PubMed] [Cited in This Article: ] |
| 132. | Kizilay A, Saydam L, Aydin A, Kalcioglu MT, Ozturan O, Aydin NE. Histopathologic examination for Helicobacter pylori as a possible etiopathogenic factor in laryngeal carcinoma. Chemotherapy. 2006;52:80-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 133. | Huang X, Qu X, Yan W, Huang Y, Cai M, Hu B, Wu L, Lin H, Chen Z, Zhu C. Iron deficiency anaemia can be improved after eradication of Helicobacter pylori. Postgrad Med J. 2010;86:272-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 134. | Yuan W, Li Yumin D, Yang L. Iron deficiency anemia in Helicobacter pylori infection: meta-analysis of randomized controlled trials. Scand J Gastroenterol. 2010;45:665-676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 135. | Yokota S, Toita N, Yamamoto S, Fujii N, Konno M. Positive relationship between a polymorphism in Helicobacter pylori neutrophil-activating protein a gene and iron-deficiency anemia. Helicobacter. 2013;18:112-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 136. | Choe YH, Oh YJ, Lee NG, Imoto I, Adachi Y, Toyoda N, Gabazza EC. Lactoferrin sequestration and its contribution to iron-deficiency anemia in Helicobacter pylori-infected gastric mucosa. J Gastroenterol Hepatol. 2003;18:980-985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 137. | Afifi MT, Abd El-Aziz HK, Hamed NA, Barghash NA, Abdo A, Gamal M. Role of Helicobacter pylori in refractory iron deficiency anaemia. Br J Biomed Sci. 2009;66:133-136. [PubMed] [Cited in This Article: ] |
| 138. | Asahi A, Nishimoto T, Okazaki Y, Suzuki H, Masaoka T, Kawakami Y, Ikeda Y, Kuwana M. Helicobacter pylori eradication shifts monocyte Fcgamma receptor balance toward inhibitory FcgammaRIIB in immune thrombocytopenic purpura patients. J Clin Invest. 2008;118:2939-2949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 139. | Sato R, Murakami K, Okimoto T, Watanabe K, Kodama M, Fujioka T. Development of corpus atrophic gastritis may be associated with Helicobacter pylori-related idiopathic thrombocytopenic purpura. J Gastroenterol. 2011;46:991-997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 140. | Satoh T, Pandey JP, Okazaki Y, Asahi A, Kawakami Y, Ikeda Y, Kuwana M. Single nucleotide polymorphism of interleukin-1beta associated with Helicobacter pylori infection in immune thrombocytopenic purpura. Tissue Antigens. 2009;73:353-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 141. | Argenziano G, Donnarumma G, Iovene MR, Arnese P, Baldassarre MA, Baroni A. Incidence of anti-Helicobacter pylori and anti-CagA antibodies in rosacea patients. Int J Dermatol. 2003;42:601-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 142. | El-Khalawany M, Mahmoud A, Mosbeh AS, A B D Alsalam F, Ghonaim N, Abou-Bakr A. Role of Helicobacter pylori in common rosacea subtypes: a genotypic comparative study of Egyptian patients. J Dermatol. 2012;39:989-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 143. | Daković Z, Vesić S, Vuković J, Milenković S, Janković-Terzić K, Dukić S, Pavlović MD. Ocular rosacea and treatment of symptomatic Helicobacter pylori infection: a case series. Acta Dermatovenerol Alp Pannonica Adriat. 2007;16:83-86. [PubMed] [Cited in This Article: ] |
| 144. | Baykal C, Buyukbabani N, Akinturk S, Saglik E. Prurigo pigmentosa: not an uncommon disease in the Turkish population. Int J Dermatol. 2006;45:1164-1168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 145. | Akashi R, Ishiguro N, Shimizu S, Kawashima M. Clinical study of the relationship between Helicobacter pylori and chronic urticaria and prurigo chronica multiformis: effectiveness of eradication therapy for Helicobacter pylori. J Dermatol. 2011;38:761-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 146. | Shiotani A, Sakurane M, Furukawa F. Helicobacter pylori-positive patients with pruritic skin diseases are at increased risk for gastric cancer. Aliment Pharmacol Ther. 2004;20 Suppl 1:80-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 147. | Shiotani A, Okada K, Yanaoka K, Itoh H, Nishioka S, Sakurane M, Matsunaka M. Beneficial effect of Helicobacter pylori eradication in dermatologic diseases. Helicobacter. 2001;6:60-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 148. | Missall TA, Pruden S, Nelson C, Fohn L, Vidal CI, Hurley MY. Identification of Helicobacter pylori in skin biopsy of prurigo pigmentosa. Am J Dermatopathol. 2012;34:446-448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 149. | Federman DG, Kirsner RS, Moriarty JP, Concato J. The effect of antibiotic therapy for patients infected with Helicobacter pylori who have chronic urticaria. J Am Acad Dermatol. 2003;49:861-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 17] [Reference Citation Analysis (0)] |
| 150. | Chiu YC, Tai WC, Chuah SK, Hsu PI, Wu DC, Wu KL, Huang CC, Ho JC, Ring J, Chen WC. The Clinical Correlations of Helicobacter pylori Virulence Factors and Chronic Spontaneous Urticaria. Gastroenterol Res Pract. 2013;2013:436727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 151. | Campanati A, Gesuita R, Giannoni M, Piraccini F, Sandroni L, Martina E, Conocchiari L, Bendia E, Di Sario A, Offidani A. Role of small intestinal bacterial overgrowth and Helicobacter pylori infection in chronic spontaneous urticaria: a prospective analysis. Acta Derm Venereol. 2013;93:161-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 152. | Yoshimasu T, Furukawa F. Eradication therapy for urticaria with high titers of anti H. pylori IgG antibody. Allergol Int. 2014;63:37-40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 153. | Ali M, Whitehead M. Clearance of chronic psoriasis after eradication therapy for Helicobacter pylori infection. J Eur Acad Dermatol Venereol. 2008;22:753-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 20] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 154. | Martin Hübner A, Tenbaum SP. Complete remission of palmoplantar psoriasis through Helicobacter pylori eradication: a case report. Clin Exp Dermatol. 2008;33:339-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 155. | Onsun N, Arda Ulusal H, Su O, Beycan I, Biyik Ozkaya D, Senocak M. Impact of Helicobacter pylori infection on severity of psoriasis and response to treatment. Eur J Dermatol. 2012;22:117-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 156. | Golberg D, Szilagyi A, Graves L. Hyperemesis gravidarum and Helicobacter pylori infection: a systematic review. Obstet Gynecol. 2007;110:695-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 157. | Guven MA, Ertas IE, Coskun A, Ciragil P. Serologic and stool antigen assay of Helicobacter pylori infection in hyperemesis gravidarum: which test is useful during early pregnancy? Taiwan J Obstet Gynecol. 2011;50:37-41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 158. | Mansour GM, Nashaat EH. Role of Helicobacter pylori in the pathogenesis of hyperemesis gravidarum. Arch Gynecol Obstet. 2011;284:843-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 159. | Cardaropoli S, Rolfo A, Piazzese A, Ponzetto A, Todros T. Helicobacter pylori’s virulence and infection persistence define pre-eclampsia complicated by fetal growth retardation. World J Gastroenterol. 2011;17:5156-5165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 43] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 160. | Franceschi F, Di Simone N, D’Ippolito S, Castellani R, Di Nicuolo F, Gasbarrini G, Yamaoka Y, Todros T, Scambia G, Gasbarrini A. Antibodies anti-CagA cross-react with trophoblast cells: a risk factor for pre-eclampsia? Helicobacter. 2012;17:426-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 161. | Bai X, Wang D, Fan Z, Han Y, Xu L, Zhang G, Lu S, Liu W, Li J, Wang H. Helicobacter pylori may cause otitis media with effusion: a pilot study. B-ENT. 2012;8:261-264. [PubMed] [Cited in This Article: ] |
| 162. | Iriz A, Eryilmaz A, Gocer C, Acar A, Boynuegri S, Dursun E. Could Helicobacter pylori play a role in the aetiopathogenesis of tympanosclerosis? J Laryngol Otol. 2011;125:1121-1124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 163. | Park CW, Chung JH, Min HJ, Kim KR, Tae K, Cho SH, Lee SH. Helicobacter pylori in middle ear of children with otitis media with effusion. Chin Med J (Engl). 2011;124:4275-4278. [PubMed] [Cited in This Article: ] |
| 164. | Melake NA, Shaker GH, Salama MA. Incidence of Helicobacter pylori infection and their clarithromycin-resistant strains in otitis media with effusion regarding phenotypic and genotypic studies. Saudi Pharm J. 2012;20:345-353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 165. | Kraus J, Nártová E, Pavlík E, Katra R, Sterzl I, Astl J. Prevalence of Helicobacter pylori in adenotonsillar hypertrophy in children. Acta Otolaryngol. 2014;134:88-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 166. | Aycicek A, Çetinkaya Z, Kıyıcı H, Bukulmez A, Yucedag F. Can Helicobacter pylori cause inflammation in the middle ear? Int J Pediatr Otorhinolaryngol. 2012;76:1087-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 167. | Cvorovic L, Brajovic D, Strbac M, Milutinovic Z, Cvorovic V. Detection of Helicobacter pylori in nasal polyps: preliminary report. J Otolaryngol Head Neck Surg. 2008;37:192-195. [PubMed] [Cited in This Article: ] |
| 168. | Včeva A, Danić D, Včev A, Birtić D, Mihalj H, Zubčić Z, Kotromanović Z, Danić Hadžibegović A. The significance of Helicobacter pylori in patients with nasal polyposis. Med Glas (Zenica). 2012;9:281-286. [PubMed] [Cited in This Article: ] |
| 169. | Jelavic B, Grgić M, Cupić H, Kordić M, Vasilj M, Baudoin T. Prognostic value of Helicobacter pylori sinonasal colonization for efficacy of endoscopic sinus surgery. Eur Arch Otorhinolaryngol. 2012;269:2197-2202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 170. | Koc C, Arikan OK, Atasoy P, Aksoy A. Prevalence of Helicobacter pylori in patients with nasal polyps: a preliminary report. Laryngoscope. 2004;114:1941-1944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 171. | Burduk PK, Kaczmarek A, Budzynska A, Kazmierczak W, Gospodarek E. Detection of Helicobacter pylori and cagA gene in nasal polyps and benign laryngeal diseases. Arch Med Res. 2011;42:686-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 172. | Hur C, Miller M, Kong CY, Dowling EC, Nattinger KJ, Dunn M, Feuer EJ. Trends in esophageal adenocarcinoma incidence and mortality. Cancer. 2013;119:1149-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 374] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 173. | Xie FJ, Zhang YP, Zheng QQ, Jin HC, Wang FL, Chen M, Shao L, Zou DH, Yu XM, Mao WM. Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World J Gastroenterol. 2013;19:6098-6107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 108] [Cited by in F6Publishing: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 174. | Minatsuki C, Yamamichi N, Shimamoto T, Kakimoto H, Takahashi Y, Fujishiro M, Sakaguchi Y, Nakayama C, Konno-Shimizu M, Matsuda R. Background factors of reflux esophagitis and non-erosive reflux disease: a cross-sectional study of 10,837 subjects in Japan. PLoS One. 2013;8:e69891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 175. | Xinias I, Maris T, Mavroudi A, Panteliadis C, Vandenplas Y. Helicobacter pylori infection has no impact on manometric and pH-metric findings in adolescents and young adults with gastroesophageal reflux and antral gastritis: eradication results to no significant clinical improvement. Pediatr Rep. 2013;5:e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 176. | Rodrigues Jr L, Faria CM, Geocze S, Chehter L. Helicobacter pylori eradication does not influence gastroesophageal reflux disease: a prospective, parallel, randomized, open-label, controlled trial. Arq Gastroenterol. 2012;49:56-63. [PubMed] [Cited in This Article: ] |
| 177. | Rokkas T, Pistiolas D, Sechopoulos P, Robotis I, Margantinis G. Relationship between Helicobacter pylori infection and esophageal neoplasia: a meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1413-147, 1413-147. [PubMed] [Cited in This Article: ] |
| 178. | Rubenstein JH, Inadomi JM, Scheiman J, Schoenfeld P, Appelman H, Zhang M, Metko V, Kao JY. Association between Helicobacter pylori and Barrett’s esophagus, erosive esophagitis, and gastroesophageal reflux symptoms. Clin Gastroenterol Hepatol. 2014;12:239-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 179. | Thrift AP, Pandeya N, Whiteman DC. Current status and future perspectives on the etiology of esophageal adenocarcinoma. Front Oncol. 2012;2:11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 180. | Zullo A, Hassan C, Repici A, Bruzzese V. Helicobacter pylori eradication and reflux disease onset: did gastric acid get “crazy”? World J Gastroenterol. 2013;19:786-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 181. | McColl KE. Helicobacter pylori and oesophageal cancer--not always protective. Gut. 2007;56:457-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 182. | Derakhshan MH, Malekzadeh R, Watabe H, Yazdanbod A, Fyfe V, Kazemi A, Rakhshani N, Didevar R, Sotoudeh M, Zolfeghari AA. Combination of gastric atrophy, reflux symptoms and histological subtype indicates two distinct aetiologies of gastric cardia cancer. Gut. 2008;57:298-305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 183. | Lee YY, Mahendra Raj S, Graham DY. Helicobacter pylori infection--a boon or a bane: lessons from studies in a low-prevalence population. Helicobacter. 2013;18:338-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 184. | Tian XF, Fan XG, Zhang Y, Huang Y, Dai H, Ying RS. Procuration and identification of bacteria in paraffin-embedded liver tissues of hepatocellular carcinoma by laser-assisted microdissection technique. APMIS. 2008;116:10-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 185. | Ward JM, Anver MR, Haines DC, Benveniste RE. Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am J Pathol. 1994;145:959-968. [PubMed] [Cited in This Article: ] |
| 186. | Kosaka T, Tajima Y, Kuroki T, Mishima T, Adachi T, Tsuneoka N, Fukuda K, Kanematsu T. Helicobacter bilis colonization of the biliary system in patients with pancreaticobiliary maljunction. Br J Surg. 2010;97:544-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 187. | Ilvan A, Ozturkeri H, Capraz F, Cermik H, Kunter E. Investigation of Helicobacter pylori in bronchoscopic lung specimens of young male patients with bronchiectasis but without gastrointestinal symptoms. Clin Microbiol Infect. 2004;10:257-260. [PubMed] [Cited in This Article: ] |
| 188. | Tsang KW, Lam SK, Lam WK, Karlberg J, Wong BC, Hu WH, Yew WW, Ip MS. High seroprevalence of Helicobacter pylori in active bronchiectasis. Am J Respir Crit Care Med. 1998;158:1047-1051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 189. | Roussos A, Philippou N, Krietsepi V, Anastasakou E, Alepopoulou D, Koursarakos P, Iliopoulos I, Gourgoulianis K. Helicobacter pylori seroprevalence in patients with chronic obstructive pulmonary disease. Respir Med. 2005;99:279-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 190. | Prónai L, Schandl L, Orosz Z, Magyar P, Tulassay Z. Lower prevalence of Helicobacter pylori infection in patients with inflammatory bowel disease but not with chronic obstructive pulmonary disease - antibiotic use in the history does not play a significant role. Helicobacter. 2004;9:278-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 191. | Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest. 2005;128:1239-1244. [PubMed] [Cited in This Article: ] |
| 192. | Kountouras J, Mylopoulos N, Chatzopoulos D, Zavos C, Boura P, Konstas AG, Venizelos J. Eradication of Helicobacter pylori may be beneficial in the management of chronic open-angle glaucoma. Arch Intern Med. 2002;162:1237-1244. [PubMed] [Cited in This Article: ] |
| 193. | Galloway PH, Warner SJ, Morshed MG, Mikelberg FS. Helicobacter pylori infection and the risk for open-angle glaucoma. Ophthalmology. 2003;110:922-925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 194. | Kurtz S, Regenbogen M, Goldiner I, Horowitz N, Moshkowitz M. No association between Helicobacter pylori infection or CagA-bearing strains and glaucoma. J Glaucoma. 2008;17:223-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 195. | Casella AM, Berbel RF, Bressanim GL, Malaguido MR, Cardillo JA. Helicobacter pylori as a potential target for the treatment of central serous chorioretinopathy. Clinics (Sao Paulo). 2012;67:1047-1052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 196. | Rahbani-Nobar MB, Javadzadeh A, Ghojazadeh L, Rafeey M, Ghorbanihaghjo A. The effect of Helicobacter pylori treatment on remission of idiopathic central serous chorioretinopathy. Mol Vis. 2011;17:99-103. [PubMed] [Cited in This Article: ] |
| 197. | Dang Y, Mu Y, Zhao M, Li L, Guo Y, Zhu Y. The effect of eradicating Helicobacter pylori on idiopathic central serous chorioretinopathy patients. Ther Clin Risk Manag. 2013;9:355-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 198. | Niehues M, Hensel A. In-vitro interaction of L-dopa with bacterial adhesins of Helicobacter pylori: an explanation for clinicial differences in bioavailability? J Pharm Pharmacol. 2009;61:1303-1307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 199. | Pierantozzi M, Pietroiusti A, Brusa L, Galati S, Stefani A, Lunardi G, Fedele E, Sancesario G, Bernardi G, Bergamaschi A. Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology. 2006;66:1824-1829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 200. | Lahner E, Annibale B, Delle Fave G. Systematic review: Helicobacter pylori infection and impaired drug absorption. Aliment Pharmacol Ther. 2009;29:379-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 201. | Lee WY, Yoon WT, Shin HY, Jeon SH, Rhee PL. Helicobacter pylori infection and motor fluctuations in patients with Parkinson’s disease. Mov Disord. 2008;23:1696-1700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 202. | Rahne KE, Tagesson C, Nyholm D. Motor fluctuations and Helicobacter pylori in Parkinson’s disease. J Neurol. 2013;260:2974-2980. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 203. | Nielsen HH, Qiu J, Friis S, Wermuth L, Ritz B. Treatment for Helicobacter pylori infection and risk of Parkinson’s disease in Denmark. Eur J Neurol. 2012;19:864-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 204. | Dobbs SM, Dobbs RJ, Weller C, Charlett A, Bjarnason IT, Lawson AJ, Letley D, Harbin L, Price AB, Ibrahim MA. Differential effect of Helicobacter pylori eradication on time-trends in brady/hypokinesia and rigidity in idiopathic parkinsonism. Helicobacter. 2010;15:279-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 205. | Blaecher C, Smet A, Flahou B, Pasmans F, Ducatelle R, Taylor D, Weller C, Bjarnason I, Charlett A, Lawson AJ. Significantly higher frequency of Helicobacter suis in patients with idiopathic parkinsonism than in control patients. Aliment Pharmacol Ther. 2013;38:1347-1353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 206. | Kountouras J, Boziki M, Gavalas E, Zavos C, Deretzi G, Grigoriadis N, Tsolaki M, Chatzopoulos D, Katsinelos P, Tzilves D. Increased cerebrospinal fluid Helicobacter pylori antibody in Alzheimer’s disease. Int J Neurosci. 2009;119:765-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 207. | Malaguarnera M, Bella R, Alagona G, Ferri R, Carnemolla A, Pennisi G. Helicobacter pylori and Alzheimer’s disease: a possible link. Eur J Intern Med. 2004;15:381-386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 208. | Shiota S, Murakami K, Yoshiiwa A, Yamamoto K, Ohno S, Kuroda A, Mizukami K, Hanada K, Okimoto T, Kodama M. The relationship between Helicobacter pylori infection and Alzheimer’s disease in Japan. J Neurol. 2011;258:1460-1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 209. | Beydoun MA, Beydoun HA, Shroff MR, Kitner-Triolo MH, Zonderman AB. Helicobacter pylori seropositivity and cognitive performance among US adults: evidence from a large national survey. Psychosom Med. 2013;75:486-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 210. | Kountouras J, Boziki M, Gavalas E, Zavos C, Grigoriadis N, Deretzi G, Tzilves D, Katsinelos P, Tsolaki M, Chatzopoulos D. Eradication of Helicobacter pylori may be beneficial in the management of Alzheimer’s disease. J Neurol. 2009;256:758-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 211. | Kountouras J, Boziki M, Gavalas E, Zavos C, Deretzi G, Chatzigeorgiou S, Katsinelos P, Grigoriadis N, Giartza-Taxidou E, Venizelos I. Five-year survival after Helicobacter pylori eradication in Alzheimer disease patients. Cogn Behav Neurol. 2010;23:199-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 212. | Ge R, Sun X, Wang D, Zhou Q, Sun H. Histidine-rich protein Hpn from Helicobacter pylori forms amyloid-like fibrils in vitro and inhibits the proliferation of gastric epithelial AGS cells. Biochim Biophys Acta. 2011;1813:1422-1427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 213. | Oldenburg B, Diepersloot RJ, Hoekstra JB. High seroprevalence of Helicobacter pylori in diabetes mellitus patients. Dig Dis Sci. 1996;41:458-461. [PubMed] [Cited in This Article: ] |
| 214. | Zhou X, Zhang C, Wu J, Zhang G. Association between Helicobacter pylori infection and diabetes mellitus: a meta-analysis of observational studies. Diabetes Res Clin Pract. 2013;99:200-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 215. | Sathyaprakash R, Henry RR. Preventing diabetes by treating aspects of the metabolic syndrome. Curr Diab Rep. 2002;2:416-422. [PubMed] [Cited in This Article: ] |
| 216. | Gunji T, Matsuhashi N, Sato H, Fujibayashi K, Okumura M, Sasabe N, Urabe A. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. Am J Gastroenterol. 2008;103:3005-3010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 217. | Chung GE, Heo NJ, Park MJ, Chung SJ, Kang HY, Kang SJ. Helicobacter pylori seropositivity in diabetic patients is associated with microalbuminuria. World J Gastroenterol. 2013;19:97-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 218. | Ibrahim A, Zaher T, Ghonemy TA, El-Azim SA, El-Azim MA, Ramadan A. Impact of cytotoxin-associated gene A of Helicobacter pylori strains on microalbuminuria in type 2 diabetes. Saudi J Kidney Dis Transpl. 2010;21:694-700. [PubMed] [Cited in This Article: ] |
| 219. | Agrawal RP, Sharma R, Garg D, Pokharna R, Kochar DK, Kothari RP. Role of Helicobacter pylori in causation of diabetic gastropathies and non-gastrointestinal complications in type 2 diabetes. J Indian Med Assoc. 2010;108:140-143. [PubMed] [Cited in This Article: ] |
| 220. | Wang F, Fu Y, Lv Z. Association of Helicobacter pylori infection with diabetic complications: a meta-analysis. Endocr Res. 2014;39:7-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 221. | El-Eshmawy MM, El-Hawary AK, Abdel Gawad SS, El-Baiomy AA. Helicobacter pylori infection might be responsible for the interconnection between type 1 diabetes and autoimmune thyroiditis. Diabetol Metab Syndr. 2011;3:28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 222. | Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, Xiao X, Shan ZL, Zhang Y, Yao P. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36:166-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 494] [Cited by in F6Publishing: 532] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 223. | Jeon CY, Haan MN, Cheng C, Clayton ER, Mayeda ER, Miller JW, Aiello AE. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care. 2012;35:520-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 224. | Chen Y, Blaser MJ. Association between gastric Helicobacter pylori colonization and glycated hemoglobin levels. J Infect Dis. 2012;205:1195-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 225. | Akanuma M, Yanai A, Sakamoto K, Hirata Y, Yamaji Y, Kawazu S, Maeda S. Influence of Helicobacter pylori eradication on the management of type 2 diabetes. Hepatogastroenterology. 2012;59:641-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 226. | Bassaganya-Riera J, Dominguez-Bello MG, Kronsteiner B, Carbo A, Lu P, Viladomiu M, Pedragosa M, Zhang X, Sobral BW, Mane SP. Helicobacter pylori colonization ameliorates glucose homeostasis in mice through a PPAR γ-dependent mechanism. PLoS One. 2012;7:e50069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 227. | Ataseven H, Demir M, Gen R. Effect of sequential treatment as a first-line therapy for Helicobacter pylori eradication in patients with diabetes mellitus. South Med J. 2010;103:988-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 228. | Boltin D, Niv Y. Ghrelin, Helicobacter pylori and body mass: is there an association? Isr Med Assoc J. 2012;14:130-132. [PubMed] [Cited in This Article: ] |
| 229. | Lane JA, Murray LJ, Harvey IM, Donovan JL, Nair P, Harvey RF. Randomised clinical trial: Helicobacter pylori eradication is associated with a significantly increased body mass index in a placebo-controlled study. Aliment Pharmacol Ther. 2011;33:922-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 230. | Mendall MA, Goggin PM, Molineaux N, Levy J, Toosy T, Strachan D, Camm AJ, Northfield TC. Relation of Helicobacter pylori infection and coronary heart disease. Br Heart J. 1994;71:437-439. [PubMed] [Cited in This Article: ] |
| 231. | Khodaii Z, Vakili H, Ghaderian SM, Najar RA, Panah AS. Association of Helicobacter pylori infection with acute myocardial infarction. Coron Artery Dis. 2011;22:6-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 232. | Mayr M, Kiechl S, Mendall MA, Willeit J, Wick G, Xu Q. Increased risk of atherosclerosis is confined to CagA-positive Helicobacter pylori strains: prospective results from the Bruneck study. Stroke. 2003;34:610-615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 233. | Pasceri V, Cammarota G, Patti G, Cuoco L, Gasbarrini A, Grillo RL, Fedeli G, Gasbarrini G, Maseri A. Association of virulent Helicobacter pylori strains with ischemic heart disease. Circulation. 1998;97:1675-1679. [PubMed] [Cited in This Article: ] |
| 234. | Pieniazek P, Karczewska E, Duda A, Tracz W, Pasowicz M, Konturek SJ. Association of Helicobacter pylori infection with coronary heart disease. J Physiol Pharmacol. 1999;50:743-751. [PubMed] [Cited in This Article: ] |
| 235. | Niccoli G, Franceschi F, Cosentino N, Giupponi B, De Marco G, Merra G, Conte M, Montone RA, Ferrante G, Bacà M. Coronary atherosclerotic burden in patients with infection by CagA-positive strains of Helicobacter pylori. Coron Artery Dis. 2010;21:217-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 236. | Franceschi F, Niccoli G, Ferrante G, Gasbarrini A, Baldi A, Candelli M, Feroce F, Saulnier N, Conte M, Roccarina D. CagA antigen of Helicobacter pylori and coronary instability: insight from a clinico-pathological study and a meta-analysis of 4241 cases. Atherosclerosis. 2009;202:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 237. | Rasmi Y, Raeisi S, Seyyed Mohammadzad MH. Association of inflammation and cytotoxin-associated gene a positive strains of helicobacter pylori in cardiac syndrome x. Helicobacter. 2012;17:116-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 238. | Laurila A, Bloigu A, Näyhä S, Hassi J, Leinonen M, Saikku P. Association of Helicobacter pylori infection with elevated serum lipids. Atherosclerosis. 1999;142:207-210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 239. | Chimienti G, Russo F, Lamanuzzi BL, Nardulli M, Messa C, Di Leo A, Correale M, Giannuzzi V, Pepe G. Helicobacter pylori is associated with modified lipid profile: impact on Lipoprotein(a). Clin Biochem. 2003;36:359-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 240. | Satoh H, Saijo Y, Yoshioka E, Tsutsui H. Helicobacter Pylori infection is a significant risk for modified lipid profile in Japanese male subjects. J Atheroscler Thromb. 2010;17:1041-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 241. | Kucukazman M, Yavuz B, Sacikara M, Asilturk Z, Ata N, Ertugrul DT, Yalcin AA, Yenigun EC, Kizilca G, Okten H. The relationship between updated Sydney System score and LDL cholesterol levels in patients infected with Helicobacter pylori. Dig Dis Sci. 2009;54:604-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 242. | Gen R, Demir M, Ataseven H. Effect of Helicobacter pylori eradication on insulin resistance, serum lipids and low-grade inflammation. South Med J. 2010;103:190-196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 243. | Kanbay M, Gür G, Yücel M, Yilmaz U, Boyacioğlu S. Does eradication of Helicobacter pylori infection help normalize serum lipid and CRP levels? Dig Dis Sci. 2005;50:1228-1231. [PubMed] [Cited in This Article: ] |
| 244. | Akbas HS, Basyigit S, Suleymanlar I, Kemaloglu D, Koc S, Davran F, Demir I, Suleymanlar G. The assessment of carotid intima media thickness and serum paraoxonase-1 activity in Helicobacter pylori positive subjects. Lipids Health Dis. 2010;9:92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 245. | Sawayama Y, Hamada M, Otaguro S, Maeda S, Ohnishi H, Fujimoto Y, Taira Y, Hayashi J. Chronic Helicobacter pylori infection is associated with peripheral arterial disease. J Infect Chemother. 2008;14:250-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 246. | McDonagh TA, Woodward M, Morrison CE, McMurray JJ, Tunstall-Pedoe H, Lowe GD, McColl KE, Dargie HJ. Helicobacter pylori infection and coronary heart disease in the North Glasgow MONICA population. Eur Heart J. 1997;18:1257-1260. [PubMed] [Cited in This Article: ] |
| 247. | Manolakis A, Kapsoritakis AN, Potamianos SP. A review of the postulated mechanisms concerning the association of Helicobacter pylori with ischemic heart disease. Helicobacter. 2007;12:287-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 248. | Carter AM, Moayyedi P, Catto A, Heppell RM, Axon AT, Grant PJ. The influence of Helicobacter pylori status on circulating levels of the coagulation factors fibrinogen, von Willebrand factor, factor VII, and factor VIII. Helicobacter. 1996;1:65-69. [PubMed] [Cited in This Article: ] |
| 249. | Shi WJ, Liu W, Zhou XY, Ye F, Zhang GX. Associations of Helicobacter pylori infection and cytotoxin-associated gene A status with autoimmune thyroid diseases: a meta-analysis. Thyroid. 2013;23:1294-1300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 250. | Bassi V, Marino G, Iengo A, Fattoruso O, Santinelli C. Autoimmune thyroid diseases and Helicobacter pylori: the correlation is present only in Graves’s disease. World J Gastroenterol. 2012;18:1093-1097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 29] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 251. | Wang Y, Zhu S, Xu Y, Wang X, Zhu Y. Interaction between gene A-positive Helicobacter pylori and human leukocyte antigen II alleles increase the risk of Graves disease in Chinese Han population: an association study. Gene. 2013;531:84-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 252. | Bassi V, Santinelli C, Iengo A, Romano C. Identification of a correlation between Helicobacter pylori infection and Graves’ disease. Helicobacter. 2010;15:558-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 253. | Bertalot G, Montresor G, Tampieri M, Spasiano A, Pedroni M, Milanesi B, Favret M, Manca N, Negrini R. Decrease in thyroid autoantibodies after eradication of Helicobacter pylori infection. Clin Endocrinol (Oxf). 2004;61:650-652. [PubMed] [Cited in This Article: ] |
| 254. | Bugdaci MS, Zuhur SS, Sokmen M, Toksoy B, Bayraktar B, Altuntas Y. The role of Helicobacter pylori in patients with hypothyroidism in whom could not be achieved normal thyrotropin levels despite treatment with high doses of thyroxine. Helicobacter. 2011;16:124-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 255. | Shen Z, Qin Y, Liu Y, Lu Y, Munker S, Chen L, Yu C, Chen P, Li Y. Helicobacter pylori infection is associated with the presence of thyroid nodules in the euthyroid population. PLoS One. 2013;8:e80042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 256. | Danese S, Zoli A, Cremonini F, Gasbarrini A. High prevalence of Helicobacter pylori type I virulent strains in patients with systemic sclerosis. J Rheumatol. 2000;27:1568-1569. [PubMed] [Cited in This Article: ] |
| 257. | Farina G, Rosato E, Francia C, Proietti M, Donato G, Ammendolea C, Pisarri S, Salsano F. High incidence of Helicobacter pylori infection in patients with systemic sclerosis: association with Sicca Syndrome. Int J Immunopathol Pharmacol. 2001;14:81-85. [PubMed] [Cited in This Article: ] |
| 258. | Yazawa N, Fujimoto M, Kikuchi K, Kubo M, Ihn H, Sato S, Tamaki T, Tamaki K. High seroprevalence of Helicobacter pylori infection in patients with systemic sclerosis: association with esophageal involvement. J Rheumatol. 1998;25:650-653. [PubMed] [Cited in This Article: ] |
| 259. | Radić M, Kaliterna DM, Bonacin D, Vergles JM, Radić J, Fabijanić D, Kovačić V. Is Helicobacter pylori infection a risk factor for disease severity in systemic sclerosis? Rheumatol Int. 2013;33:2943-2948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 260. | Reinauer S, Goerz G, Ruzicka T, Susanto F, Humfeld S, Reinauer H. Helicobacter pylori in patients with systemic sclerosis: detection with the 13C-urea breath test and eradication. Acta Derm Venereol. 1994;74:361-363. [PubMed] [Cited in This Article: ] |
| 261. | Mohebi N, Mamarabadi M, Moghaddasi M. Relation of helicobacter pylori infection and multiple sclerosis in Iranian patients. Neurol Int. 2013;5:31-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 262. | Li W, Minohara M, Su JJ, Matsuoka T, Osoegawa M, Ishizu T, Kira J. Helicobacter pylori infection is a potential protective factor against conventional multiple sclerosis in the Japanese population. J Neuroimmunol. 2007;184:227-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 263. | Barnett MH, Sutton I. Neuromyelitis optica: not a multiple sclerosis variant. Curr Opin Neurol. 2012;25:215-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 264. | Li W, Minohara M, Piao H, Matsushita T, Masaki K, Matsuoka T, Isobe N, Su JJ, Ohyagi Y, Kira J. Association of anti-Helicobacter pylori neutrophil-activating protein antibody response with anti-aquaporin-4 autoimmunity in Japanese patients with multiple sclerosis and neuromyelitis optica. Mult Scler. 2009;15:1411-1421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 265. | Yoshimura S, Isobe N, Matsushita T, Yonekawa T, Masaki K, Sato S, Kawano Y, Kira J. Distinct genetic and infectious profiles in Japanese neuromyelitis optica patients according to anti-aquaporin 4 antibody status. J Neurol Neurosurg Psychiatry. 2013;84:29-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 266. | Pezeshki MZ, Zarrintan S, Zarrintan MH. Helicobacter pylori nanoparticles as a potential treatment of conventional multiple sclerosis. Med Hypotheses. 2008;70:1223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 267. | Chiba S, Sugiyama T, Matsumoto H, Hisano K, Awakawa T, Hiura K, Saitoh M, Imai K. Antibodies against Helicobacter pylori were detected in the cerebrospinal fluid obtained from patients with Guillain-Barré syndrome. Ann Neurol. 1998;44:686-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 268. | Ghabaee M, Ghanbarian D, Brujeni GN, Bokaei S, Siavoshi F, Gharibzadeh S. Could Helicobacter pylori play an important role in axonal type of Guillain-Barré syndrome pathogenesis? Clin Neurol Neurosurg. 2010;112:193-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 269. | Kountouras J, Deretzi G, Zavos C, Karatzoglou P, Touloumis L, Nicolaides T, Chatzopoulos D, Venizelos I. Association between Helicobacter pylori infection and acute inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. 2005;12:139-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 270. | Chiba S, Sugiyama T, Yonekura K, Tanaka S, Matsumoto H, Fujii N, Ebisu S, Sekiguchi K. An antibody to VacA of Helicobacter pylori in cerebrospinal fluid from patients with Guillain-Barre syndrome. J Neurol Neurosurg Psychiatry. 2002;73:76-78. [PubMed] [Cited in This Article: ] |
| 271. | Zhou X, Wu J, Zhang G. Association between Helicobacter pylori and asthma: a meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:460-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 272. | Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, Müller A. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest. 2011;121:3088-3093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 326] [Cited by in F6Publishing: 334] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 273. | Konturek PC, Rienecker H, Hahn EG, Raithel M. Helicobacter pylori as a protective factor against food allergy. Med Sci Monit. 2008;14:CR452-CR458. [PubMed] [Cited in This Article: ] |
| 274. | Lebwohl B, Blaser MJ, Ludvigsson JF, Green PH, Rundle A, Sonnenberg A, Genta RM. Decreased risk of celiac disease in patients with Helicobacter pylori colonization. Am J Epidemiol. 2013;178:1721-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 275. | Imamura S, Sugimoto M, Kanemasa K, Sumida Y, Okanoue T, Yoshikawa T, Yamaoka Y. Inverse association between Helicobacter pylori infection and allergic rhinitis in young Japanese. J Gastroenterol Hepatol. 2010;25:1244-1249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 276. | Garn H, Neves JF, Blumberg RS, Renz H. Effect of barrier microbes on organ-based inflammation. J Allergy Clin Immunol. 2013;131:1465-1478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 277. | Kaplan JL, Shi HN, Walker WA. The role of microbes in developmental immunologic programming. Pediatr Res. 2011;69:465-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 278. | Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 819] [Cited by in F6Publishing: 792] [Article Influence: 46.6] [Reference Citation Analysis (1)] |
| 279. | Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1541] [Cited by in F6Publishing: 1494] [Article Influence: 124.5] [Reference Citation Analysis (3)] |
| 280. | Huang JQ, Sridhar S, Hunt RH. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: a meta-analysis. Lancet. 2002;359:14-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 560] [Cited by in F6Publishing: 498] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 281. | Chan FK, Sung JJ, Chung SC, To KF, Yung MY, Leung VK, Lee YT, Chan CS, Li EK, Woo J. Randomised trial of eradication of Helicobacter pylori before non-steroidal anti-inflammatory drug therapy to prevent peptic ulcers. Lancet. 1997;350:975-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 308] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 282. | DuBois S, Kearney DJ. Iron-deficiency anemia and Helicobacter pylori infection: a review of the evidence. Am J Gastroenterol. 2005;100:453-459. [PubMed] [Cited in This Article: ] |
| 283. | Arnold DM, Bernotas A, Nazi I, Stasi R, Kuwana M, Liu Y, Kelton JG, Crowther MA. Platelet count response to H. pylori treatment in patients with immune thrombocytopenic purpura with and without H. pylori infection: a systematic review. Haematologica. 2009;94:850-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 284. | Gisbert JP, Pajares JM. Review article: 13C-urea breath test in the diagnosis of Helicobacter pylori infection -- a critical review. Aliment Pharmacol Ther. 2004;20:1001-1017. [PubMed] [Cited in This Article: ] |
| 285. | Gisbert JP, Pajares JM. Stool antigen test for the diagnosis of Helicobacter pylori infection: a systematic review. Helicobacter. 2004;9:347-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 286. | Vaira D, Malfertheiner P, Mégraud F, Axon AT, Deltenre M, Hirschl AM, Gasbarrini G, O’Morain C, Garcia JM, Quina M. Diagnosis of Helicobacter pylori infection with a new non-invasive antigen-based assay. HpSA European study group. Lancet. 1999;354:30-33. [PubMed] [Cited in This Article: ] |
| 287. | Loy CT, Irwig LM, Katelaris PH, Talley NJ. Do commercial serological kits for Helicobacter pylori infection differ in accuracy? A meta-analysis. Am J Gastroenterol. 1996;91:1138-1144. [PubMed] [Cited in This Article: ] |
| 288. | el-Zimaity HM. Accurate diagnosis of Helicobacter pylori with biopsy. Gastroenterol Clin North Am. 2000;29:863-869. [PubMed] [Cited in This Article: ] |
| 289. | van IJzendoorn MC, Laheij RJ, de Boer WA, Jansen JB. The importance of corpus biopsies for the determination of Helicobacter pylori infection. Neth J Med. 2005;63:141-145. [PubMed] [Cited in This Article: ] |
| 290. | Midolo P, Marshall BJ. Accurate diagnosis of Helicobacter pylori. Urease tests. Gastroenterol Clin North Am. 2000;29:871-878. [PubMed] [Cited in This Article: ] |
| 291. | Li C, Ha T, Ferguson DA, Chi DS, Zhao R, Patel NR, Krishnaswamy G, Thomas E. A newly developed PCR assay of H. pylori in gastric biopsy, saliva, and feces. Evidence of high prevalence of H. pylori in saliva supports oral transmission. Dig Dis Sci. 1996;41:2142-2149. [PubMed] [Cited in This Article: ] |
| 292. | Makristathis A, Hirschl AM, Lehours P, Mégraud F. Diagnosis of Helicobacter pylori infection. Helicobacter. 2004;9 Suppl 1:7-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 293. | Samarbaf-Zadeh AR, Tajbakhsh S, Moosavian SM, Sadeghi-Zadeh M, Azmi M, Hashemi J, Masjedi-Zadeh A. Application of fluorescent in situ hybridization (FISH) for the detection of Helicobacter pylori. Med Sci Monit. 2006;12:CR426-CR430. [PubMed] [Cited in This Article: ] |
| 294. | Yilmaz O, Demiray E. Clinical role and importance of fluorescence in situ hybridization method in diagnosis of H pylori infection and determination of clarithromycin resistance in H pylori eradication therapy. World J Gastroenterol. 2007;13:671-675. [PubMed] [Cited in This Article: ] |
| 295. | Morris JM, Reasonover AL, Bruce MG, Bruden DL, McMahon BJ, Sacco FD, Berg DE, Parkinson AJ. Evaluation of seaFAST, a rapid fluorescent in situ hybridization test, for detection of Helicobacter pylori and resistance to clarithromycin in paraffin-embedded biopsy sections. J Clin Microbiol. 2005;43:3494-3496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 296. | Aktepe OC, Ciftçi IH, Safak B, Uslan I, Dilek FH. Five methods for detection of Helicobacter pylori in the Turkish population. World J Gastroenterol. 2011;17:5172-5176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 297. | Miehlke S, Bayerdörffer E, Graham DY. Treatment of Helicobacter pylori infection. Semin Gastrointest Dis. 2001;12:167-179. [PubMed] [Cited in This Article: ] |
| 298. | Laheij RJ, Rossum LG, Jansen JB, Straatman H, Verbeek AL. Evaluation of treatment regimens to cure Helicobacter pylori infection--a meta-analysis. Aliment Pharmacol Ther. 1999;13:857-864. [PubMed] [Cited in This Article: ] |
| 299. | Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009;104:3069-379; quiz 1080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 300. | De Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, Ierardi E, Zullo A. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis. 2010;19:409-414. [PubMed] [Cited in This Article: ] |
| 301. | Ierardi E, Giorgio F, Losurdo G, Di Leo A, Principi M. How antibiotic resistances could change Helicobacter pylori treatment: A matter of geography? World J Gastroenterol. 2013;19:8168-8180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 70] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 302. | Duck WM, Sobel J, Pruckler JM, Song Q, Swerdlow D, Friedman C, Sulka A, Swaminathan B, Taylor T, Hoekstra M. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis. 2004;10:1088-1094. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 303. | Kim SY, Joo YM, Lee HS, Chung IS, Yoo YJ, Merrell DS, Cha JH. Genetic analysis of Helicobacter pylori clinical isolates suggests resistance to metronidazole can occur without the loss of functional rdxA. J Antibiot (Tokyo). 2009;62:43-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 304. | Cattoir V, Nectoux J, Lascols C, Deforges L, Delchier JC, Megraud F, Soussy CJ, Cambau E. Update on fluoroquinolone resistance in Helicobacter pylori: new mutations leading to resistance and first description of a gyrA polymorphism associated with hypersusceptibility. Int J Antimicrob Agents. 2007;29:389-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 305. | Xing JZ, Clarke C, Zhu L, Gabos S. Development of a microelectronic chip array for high-throughput genotyping of Helicobacter species and screening for antimicrobial resistance. J Biomol Screen. 2005;10:235-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 306. | De Francesco V, Margiotta M, Zullo A, Hassan C, Troiani L, Burattini O, Stella F, Di Leo A, Russo F, Marangi S. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med. 2006;144:94-100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 307. | Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut. 2007;56:1353-1357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 308. | Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 309. | Basu PP, Rayapudi K, Pacana T, Shah NJ, Krishnaswamy N, Flynn M. A randomized study comparing levofloxacin, omeprazole, nitazoxanide, and doxycycline versus triple therapy for the eradication of Helicobacter pylori. Am J Gastroenterol. 2011;106:1970-1975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 310. | Hojo M, Miwa H, Nagahara A, Sato N. Pooled analysis on the efficacy of the second-line treatment regimens for Helicobacter pylori infection. Scand J Gastroenterol. 2001;36:690-700. [PubMed] [Cited in This Article: ] |
| 311. | Gisbert JP, Castro-Fernandez M, Perez-Aisa A, Cosme A, Molina-Infante J, Rodrigo L, Modolell I, Cabriada JL, Gisbert JL, Lamas E. Fourth-line rescue therapy with rifabutin in patients with three Helicobacter pylori eradication failures. Aliment Pharmacol Ther. 2012;35:941-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 312. | Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 313. | Zheng X, Lyu L, Mei Z. Lactobacillus-containing probiotic supplementation increases Helicobacter pylori eradication rate: evidence from a meta-analysis. Rev Esp Enferm Dig. 2013;105:445-453. [PubMed] [Cited in This Article: ] |
| 314. | Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 153] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 315. | Sachdeva A, Nagpal J. Meta-analysis: efficacy of bovine lactoferrin in Helicobacter pylori eradication. Aliment Pharmacol Ther. 2009;29:720-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 316. | de Bortoli N, Leonardi G, Ciancia E, Merlo A, Bellini M, Costa F, Mumolo MG, Ricchiuti A, Cristiani F, Santi S. Helicobacter pylori eradication: a randomized prospective study of triple therapy versus triple therapy plus lactoferrin and probiotics. Am J Gastroenterol. 2007;102:951-956. [PubMed] [Cited in This Article: ] |