INTRODUCTION
Cholangiocarcinoma (CCA) is a malignant tumor of the biliary epithelium associated with a high metastatic and mortality rate[1]. Incidence of this cancer has increased worldwide[2], and in Thailand the highest incidence is in the northeastern region, where Opisthorchis viverrini infection is also prevalent[3]. Although the exact molecular mechanisms of cholangiocarcinogenesis are still under investigation, alterations in important growth factor pathways, such as hepatocyte growth factor (HGF)/Met and ErbB2, have been suggested as being involved[4].
Overexpression and deregulation of Met, a receptor tyrosine kinase, have been reported in many types of cancers[5]. Met is activated via binding to its ligand, HGF, also known as scatter factor (SF), a soluble factor first identified as a growth factor for hepatocytes and a dissociation factor for epithelial cells[6]. Hitherto there have been a limited number of investigations into the role of Met in cholangiocarcinoma. Several reports have demonstrated a correlation between Met expression and CCA[7-10]. Immunohistochemical data indicate high expression of Met in well-differentiated CCA and hyperplastic bile ducts of nontumorous liver surrounding CCA, whereas Met expression is low in poorly differentiated tumor[7,8]. Met expression is increased in early developmental stages of CCA, suggesting a role in cholangiocarcinogenesis[9]. Moreover, there is a correlation between Met expression and CCA invasion through adjacent connective tissues[11]. HGF level has been shown also to correlate with CCA differentiation stages in both human and rat models[10,12].
HGF/Met activation induces a variety of biological processes, including cell scattering, invasion, proliferation and survival[13-15]. Among the various cellular responses induced by HGF, cell invasion and metastasis have been implicated strongly in numerous cancer types. HGF has been reported to promote the main requirements of tumor invasion, namely, disruption of cell-cell adhesion complex, cell adhesion to extracellular matrix (ECM), cell motility and production of matrix degrading enzymes, such as matrix metalloproteinases (MMPs) and urokinase plasminogen activator (uPA)[15-18]. Phosphoinositide 3-kinase (PI3K) and mitogen-activated protein kinases/extracellular signal-regulated kinases (MAPKs/ERKs) are the main intracellular signaling pathways implicated in HGF-induced invasion[19,20].
The present study focuses on the role of HGF/Met in CCA cell invasion and the mechanisms underlying cellular responses. Here, we demonstrate that Met is overexpressed in human CCA cell lines and that HGF stimulation induces CCA cell invasion, motility and E-cadherin translocation, but has no effect on MMPs or uPA activity. Use of inhibitors of MEK and PI3K indicate that HGF induces invasion in two different CCA cell lines via distinct signaling pathways.
MATERIALS AND METHODS
Cell culture
Human CCA cell lines HuCCA-1 and KKU-M213 were kindly provided by Professor S Sirisinha (Mahidol University, Bangkok, Thailand)[21,22] and Associate Professor B Sripa (Khon Kaen University, Khon Kaen, Thailand)[23,24], respectively. Cholangiocyte H69 cell line was kindly provided by Professor G Alpini (Texas A&M University, TX, USA) and Professor G Gores (Mayo Clinic, MN, USA). CCA cells were grown in HAM/F12 medium (Gibco Invitrogen Co., Auckland, NZ) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin G sodium, 100 μg/mL streptomycin sulfate, 0.25 μg/mL amphotericin B (Invitrogen Co., Auckland, NZ) and 15 mmol/L HEPES (USB Co., OH, USA) at 37°C under a humidified 50 mL/L CO2 atmosphere. H69 cells were cultured in DMEM/F12 and DMEM (1:1) (Gibco Invitrogen Co., Auckland, NZ) supplemented with hormones, epidermal growth factor and 10% FBS as previously described[25].
Western blotting analysis
Levels of Met, ERK1/2 and Akt and their phosphorylated forms, and E-cadherin, were determined by Western blotting. Cells (2 × 105) were cultured in 30-mm plates for two days, then incubated with 50 ng/mL recombinant NSO-produced human HGF (R&D Systems, Inc., MN, USA) in serum-free media for 15, 60 and 360 min in the presence or absence of LY294002 (Calbiochem, CA, USA) or U0126 (Tocris Bioscience, MO, USA). Cells were then lysed with 1 × SDS loading buffer (50 mmol/L Tris-HCl pH 6.8, 2% SDS, 10% glycerol and 100 mmol/L β-mercaptoethanol) and lysate proteins were separated by 8% SDS polyacrylamide gel-electrophoresis. Proteins were transferred to nitrocellulose membrane (Hybond ECL, GE healthcare, Buckinghamshire, UK), which was incubated with antibodies specific for Akt, ERK1/2 and their phospho-forms (Cell Signaling Technology, Danvers, MA) or with anti-Met, anti-E-Cadherin, anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) and anti-phospho-Met (Cell Signaling Technology, Danvers, MA) antibodies, followed by HRP-conjugated secondary antibodies. Signals were developed using Enhance Chemiluminescence kit (GE Healthcare, Buckinghamshire, UK) and detected with FluorChem SP (Alpha Innotech Corporation, San Leandro, CA). Band densities were quantitated using AlphaEaseFC software (Alpha Innotech Corporation, San Leandro, CA). The data were presented in the relative band density when compared to those at zero time points.
Invasion and motility assay
HGF-induced CCA cell invasiveness was determined by Matrigel Transwell in vitro invasion assay as described by Albini et al[26] with some modification. In brief, the upper chamber of a Transwell unit (6.5-mm diameter polycarbonate membrane with 8-μm pore size) (Corning Incorporated Life Science, Corning, NY), was coated with 30 μg of Matrigel (BD Biosciences, Bedford, MA). Cells (80% confluent) were harvested using TrypLE Express (Invitrogen, Co., Grand Island, NY) and resuspended in serum-free media in the presence or absence of 50 and 100 μmol/L LY294002 or 1 and 5 μmol/L U0126. A 200 μL aliquot of cell (105) suspension was added to the upper chamber. The lower chamber was filled with 600 μL of serum-free media containing 10, 50 or 100 ng/mL human HGF as chemoattractant. BSA (0.1% in serum-free medium) was used as negative control. After 6 h of incubation at 37°C under CO2 atmosphere, non-invading cells in the upper chamber were removed and cells that invaded the Matrigel and had attached to the lower surface of the Transwell membrane were fixed with 25% methanol for 30 min and stained with 0.5% crystal violet. Invaded cells were counted in 5 random fields under light microscope at 100 × magnification. The reported values represent mean ± SE of the results obtained from three independent experiments.
Motility assay was performed using the Transwell chamber in the same manner as in the invasion assay but Matrigel coating was omitted.
Determination of gelatinase and urokinase plasminogen activator activities
Gelatinase (MMP-2 and MMP-9) and uPA levels secreted into conditioned media were determined by gelatin and plasminogen gelatin zymography under non-reducing conditions. Cells (80% confluent) were incubated with serum-free media in the presence of HGF (0, 10, 50 and 100 ng/mL) for 6 h. For gelatinase activity assay, 20 × concentrated conditioned media was mixed with SDS loading buffer in the absence of sulfhydryl reducing agent and electrophoresed in 7.5% SDS-polyacrylamide gel containing 1 mg/mL gelatin. uPA zymography was performed in a similar manner except that 10 μg/mL plasminogen and 1 mg/mL gelatin were copolymerized with 10% SDS-polyacrylamide gel and conditioned media was not concentrated. Gels were washed twice with 2.5% TritonX-100 for 1 h to remove SDS, then incubated for 18 h in reaction buffer (for gelatinase: 50 mmol/L Tris-HCl pH 7.5, 10 mmol/L CaCl2, 1 μmol/L ZnCl2 and 1% TritonX-100; for uPA: 100 mmol/L Tris-HCl pH 7.8, 150 mmol/L NaCl and 1% Triton X-100). Gels were stained for 2 h with 0.25% Coomassie blue and destained with 45% methanol and 10% acetic acid. Unstained bands in gelatin gel with estimated molecular weight of 65 and 85 kDa corresponded to MMP-2 and MMP-9 respectively, and that of 45 kDa in plasminogen-gelatin gel corresponded to uPA.
Immunofluorescence analysis
CCA cells (3 × 105) were grown on sterile coverslips for two days. Then the monolayer cells were treated with 0-100 ng/mL HGF for 6 h. Cells were washed twice with PBS, fixed in solution containing 3% paraformaldehyde and 2% sucrose, permeabilized with 0.5% Triton X-100 and incubated with 10% FBS, 0.1% Triton X-100 in PBS. Cells were then incubated overnight at 4°C with mouse anti-E-cadherin monoclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), followed by fluorescent Alexa Fluor® 568-conjugated goat anti-mouse IgG secondary antibodies (Molecular Probes, Eugene, OR). After washing with PBS, the coverslips were mounted with 0.01% para-phenylenediamine dihydrochloride (Sigma Aldrich, Inc., St. Louis, MO) in 70% glycerol, and visualized under a confocal laser scanning microscope (Olympus FV1000; Olympus Co. Tokyo, Japan) equipped with Olympus FV10-ASW 1.7 software.
Statistical analysis
Invasion and motility results are expressed as mean ± SE. Multiple comparisons were performed using one-way analysis of variance (ANOVA) with P value < 0.05 considered statistically significant.
RESULTS
Met expression and phosphorylation in CCA cells
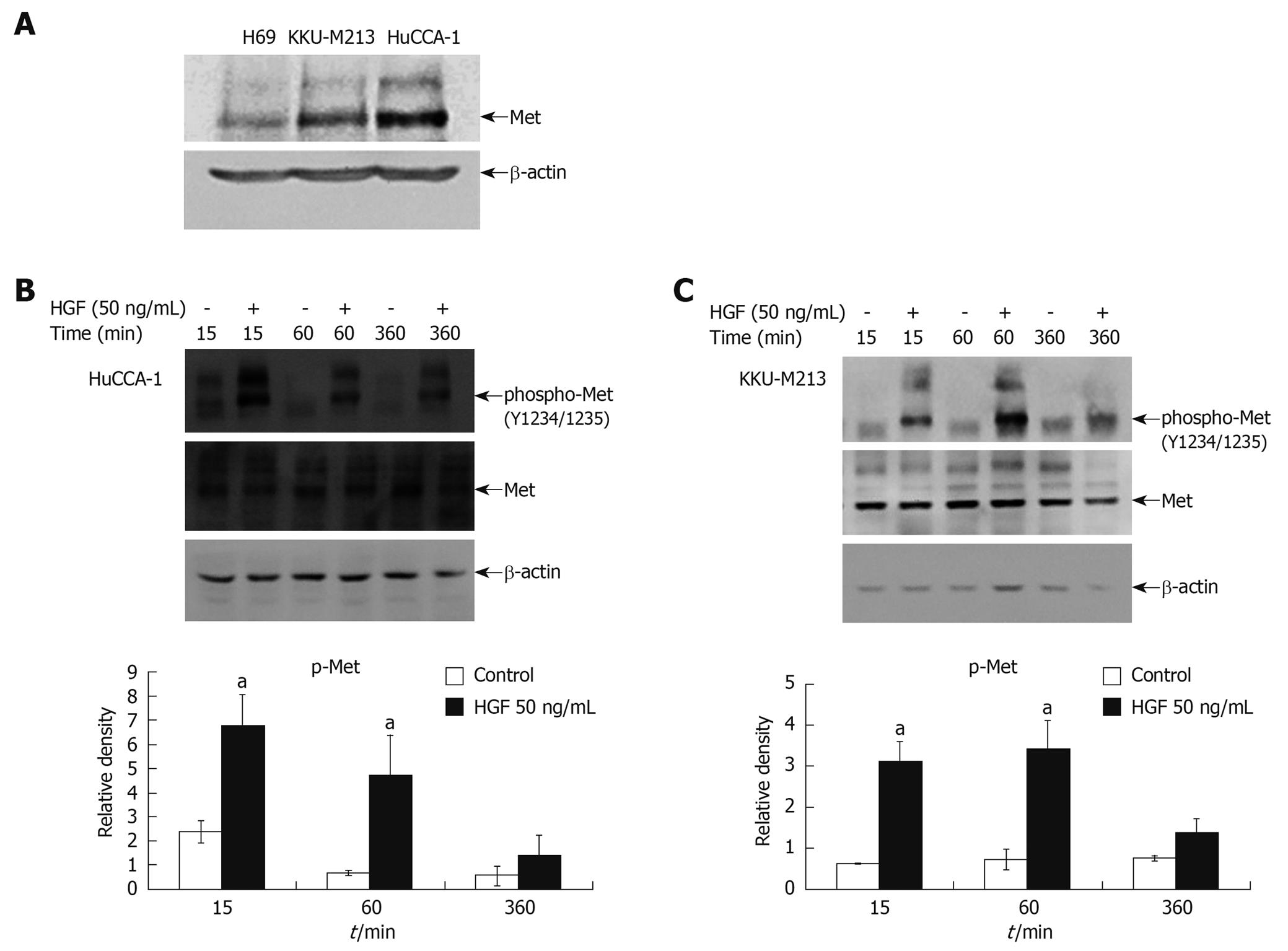
Western blotting analysis of both CCA cell lines (HuCCA-1 and KKU-M213) showed higher Met expression than in normal cholangiocytes (H69) (Figure 1A). Stimulation of cells by exogenous HGF resulted in induction of tyrosine phosphorylation at the critical autophosphorylation sites (pY1234/1235) in the catalytic domain of Met, but with a slight difference in the kinetics of Met activation between the two CCA cell lines; i.e. HGF stimulated a more rapid Met phosphorylation in HuCCA-1 cells (reaching a maximum at 15 min) than in KKU-M213 (maximum at about 15-60 min) (Figure 1B and C).
Figure 1 Steady state level of Met expression in cholangiocarcinoma cell lines and activation by hepatocyte growth factor (HGF).
Cell lysates from 80% confluent cells cultured in 10% fetal bovine serum (FBS) medium were examined for Met expression by Western blotting analysis (A). Lysates from HuCCA-1 (B) and KKU-M213 (C) cells treated with or without 50 ng/mL HGF for various times were analyzed by Western blotting for levels of Met and phospho-Met (pY1234/1235). The graphs show band densities of phospho-Met relative to those at zero time points. Data are presented as mean ± SE of results obtained from three independent experiments. aP < 0.05 vs untreated control.
Effects of HGF on CCA cell invasiveness and motility
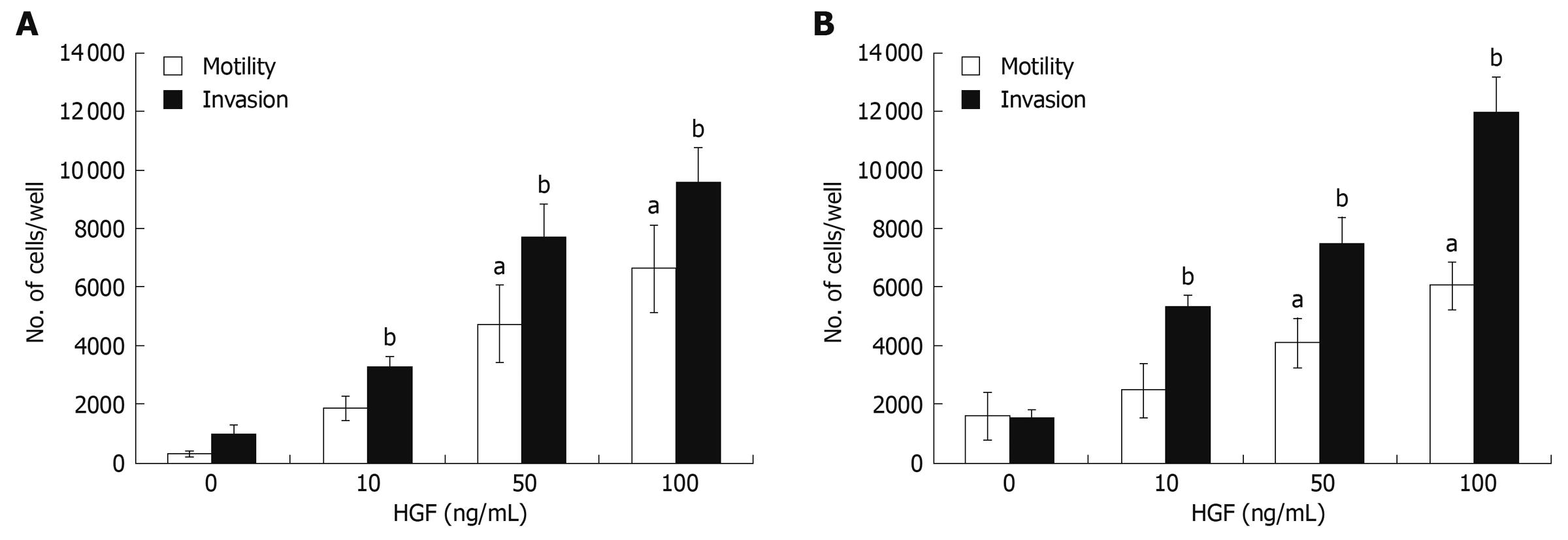
HGF has been reported as being able to induce invasion of several cancer cell types[27]. Here, CCA cell invasiveness and motility in response to HGF were investigated using a Transwell in vitro invasion/motility assay. In the absence of HGF, CCA cells showed abilities to migrate and invade, which were stimulated further by HGF in a dose-dependent manner over the concentration range of 10-100 ng/mL (Figure 2). Although basal migration and invasion abilities of HuCCA-1 were relatively low when compared to that of KKU-M213, they were dramatically stimulated by HGF to levels comparable to those of HGF-induced KKU-M213.
Figure 2 HGF induction of cholangiocarcinoma motility and invasiveness.
In vitro invasion and motility assays of HuCCA-1 (A) and KKU-M213 (B) cells were conducted in a Transwell unit coated with and without Matrigel. Cells (105) in serum-free medium were plated in the upper chamber of a Transwell unit and 0-100 ng/mL HGF added to the lower chamber. After 6 h of incubation, cells invading to the lower compartment of the Transwell unit were stained and counted. The numbers of invaded/motile cells are presented as mean ± SE of results obtained from three independent experiments. aP < 0.05, bP < 0.01, vs untreated control.
H69 cells, immortalized cholangiocytes, possessed very low invasive ability. Of 105 cells added to the upper compartment of the Transwell chamber, only 70 ± 21 cells invaded in the control and 335 ± 72 cells invaded upon HGF treatment. Although the HGF could induce H69 invasion, the level of invasion was marginal when compare to those of CCA cell lines.
Effects of HGF on E-cadherin expression and localization and matrix metalloproteinase and uPA secretion
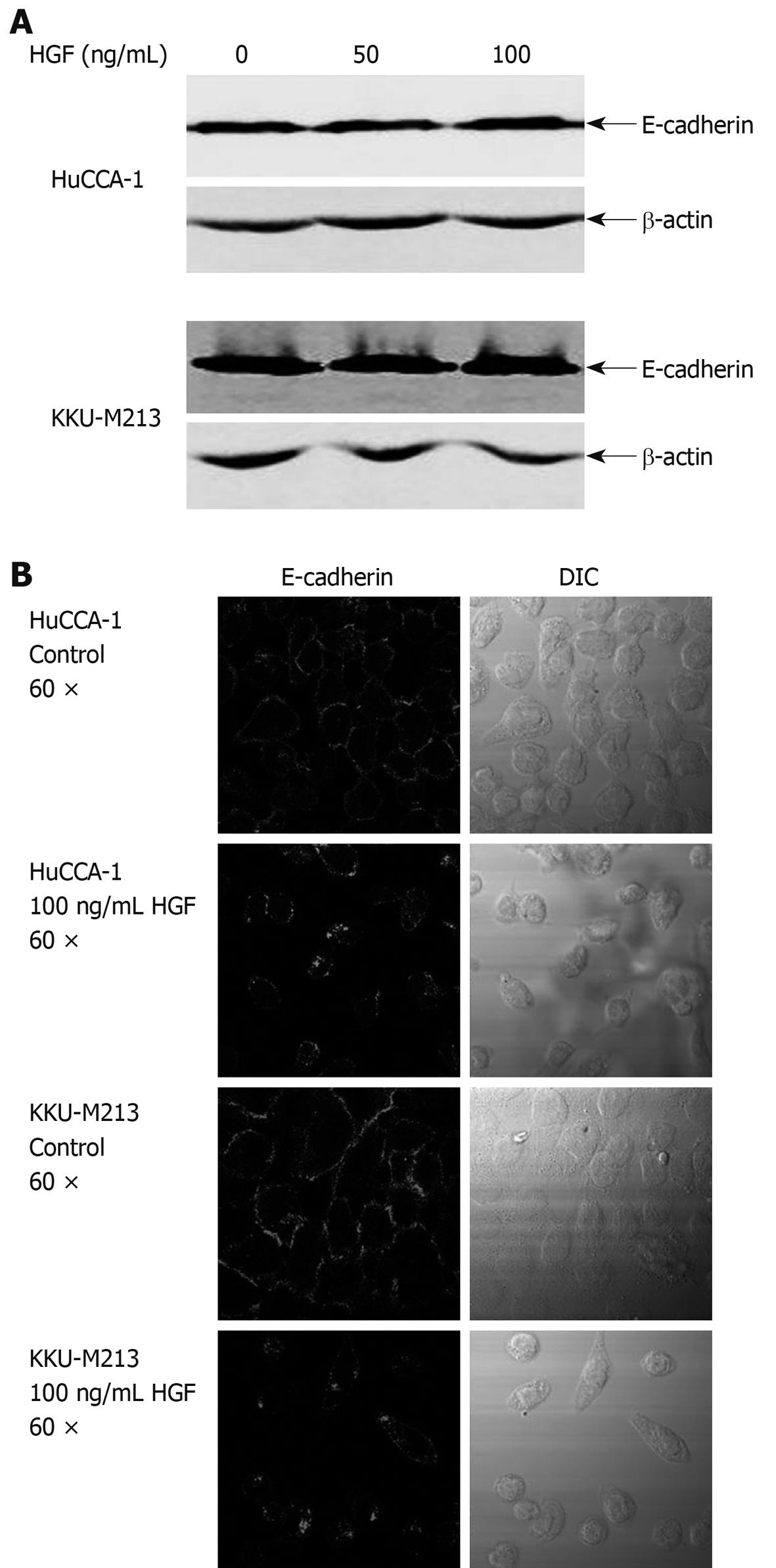
HGF is able to induce changes in expression and localization of E-cadherin resulting in cell movement in several type of cancers[28-30]. To investigate the possibility of an involvement of E-cadherin in HGF-induced CCA cell migration, we determined the effects of HGF on E-cadherin expression by Western blotting and on localization by immunofluorescence staining. E-cadherin protein level did not change within 6 h of HGF treatment (Figure 3A). However, immunofluoresence demonstrated that HGF altered E-cadherin localization from the cell boundary to the cytoplasmic compartment (Figure 3B and C).
Figure 3 Effects of HGF on E-cadherin expression and localization.
A: cholangiocarcinoma (CCA) cells were treated with HGF for 6 h, then cell lysate was analyzed by Western blotting with anti-E-cadherin and -β-actin monoclonal antibodies; B: After treatment with 0 and 100 ng/mL HGF for 6 h, cells were analyzed by immunofluorescence using anti-E-cadherin antibody and visualized under confocal laser scanning microscopy (60 × objective magnification plus 2 × digital magnification).
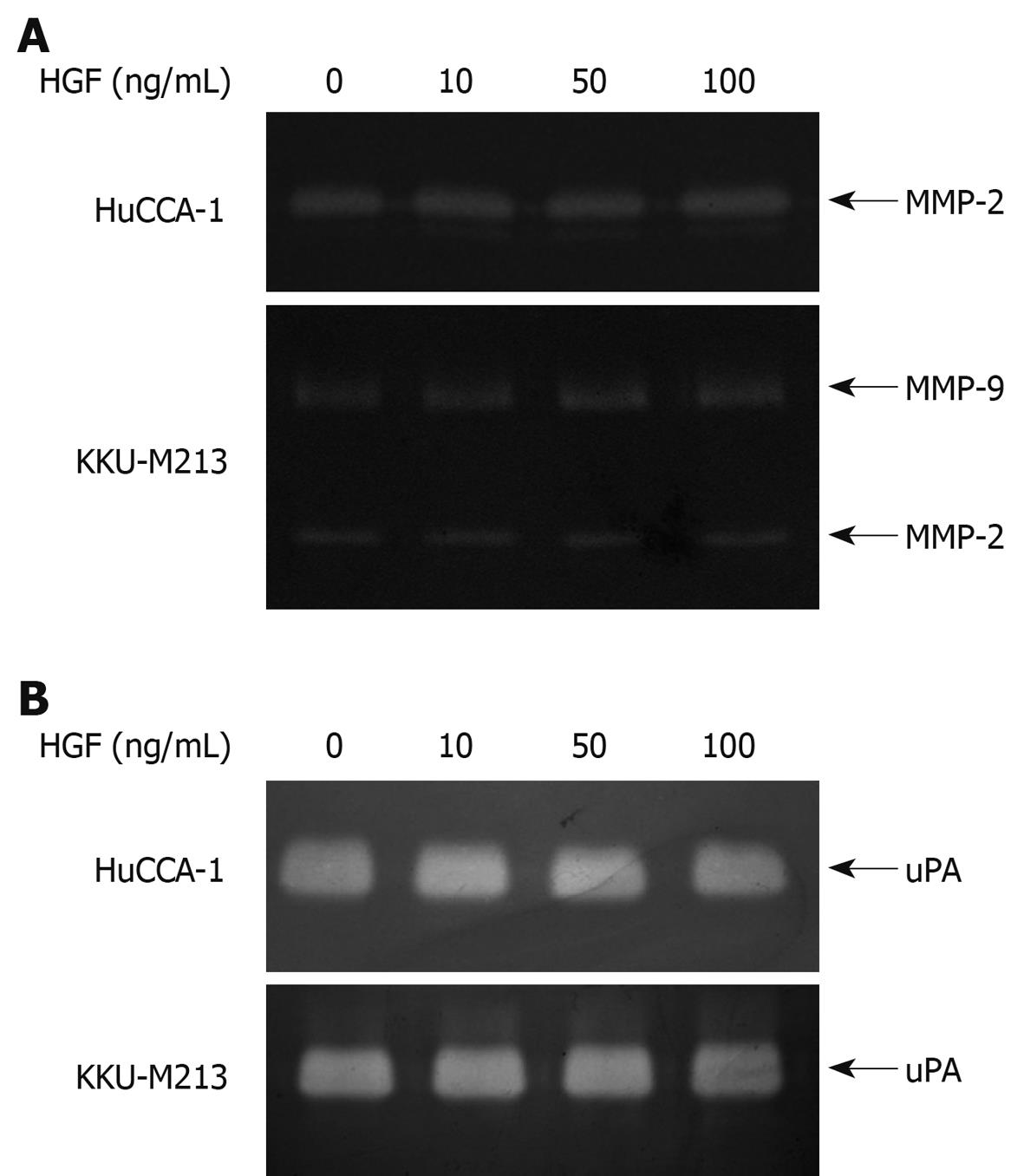
The effect of HGF on secretion of matrix degrading enzymes, a major factor contributing to cell invasiveness, was investigated by gelatin zymography. Zymograms from conditioned media of HuCCA-1 cells showed a clear band indicating MMP-2 activity, while those of KKU-M213 cells revealed both MMP-2 and MMP-9 activities (Figure 4A), demonstrating that the two CCA cell lines constitutively expressed high amounts of MMP-2 and/or MMP-9 at basal levels. However, these enzyme activities were not increased following HGF treatment (Figure 3A). Similarly, high basal activity of uPA was found in both CCA cell lines, which was not affected by the presence of HGF (Figure 4B).
Figure 4 Effect of HGF on levels of secreted matrix degrading enzymes from cholangiocarcinoma HuCCA-1 and KKU-M213 cell lines.
Cells were treated with various concentrations of HGF (0-100 ng/mL) in serum-free medium for 6 h. Conditioned media were then analyzed for MMP-2 (approximate 65 kDa) and MMP-9 (approximate 85 kDa) gelatinolytic activity by gelatin zymography (A) and for uPA by plasminogen-gelatin zymography (B).
Involvement of ERK1/2 and PI3K signaling pathways in HGF-induced CCA cell invasiveness
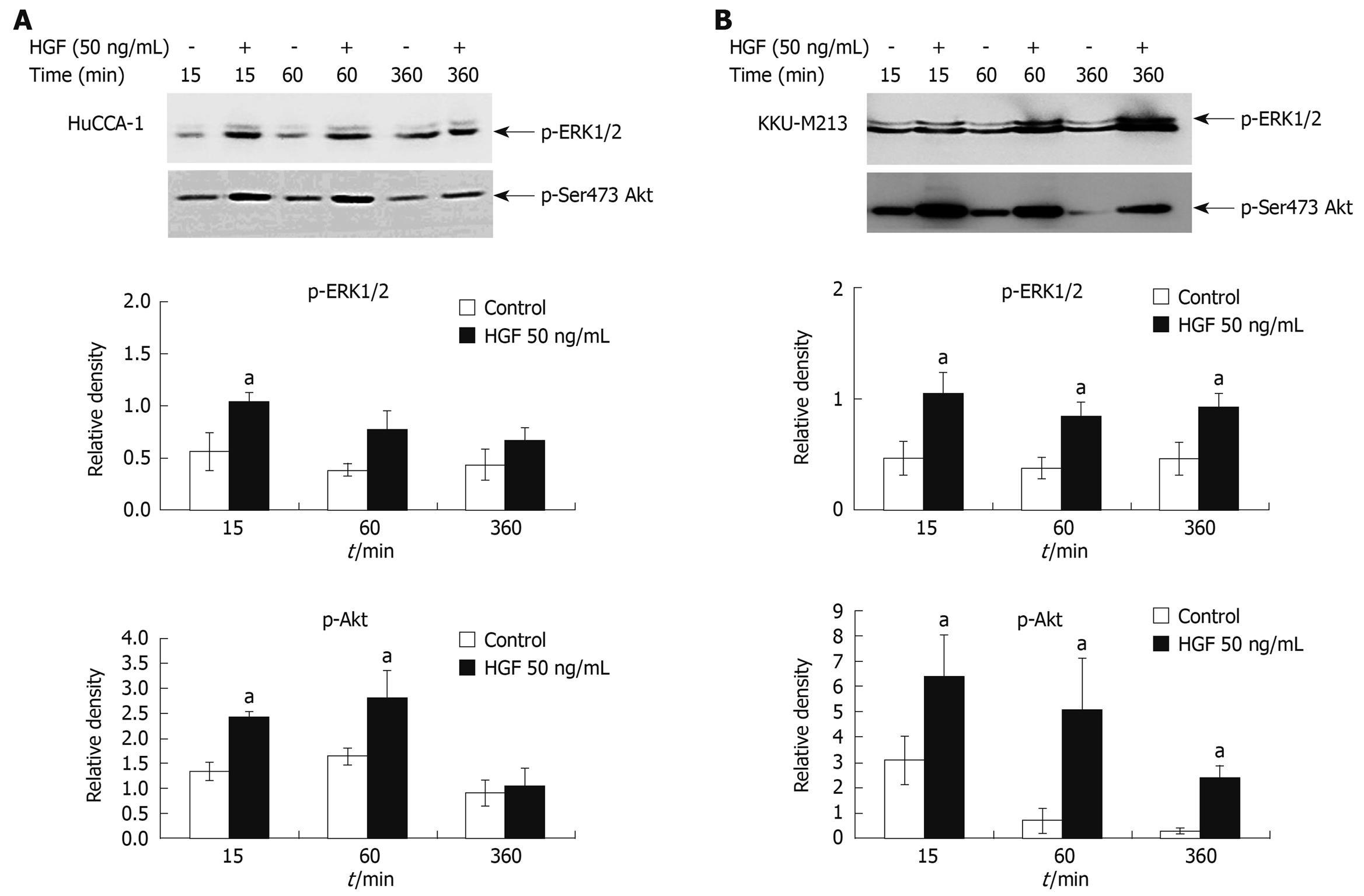
The mechanism responsible for HGF-induced invasiveness of CCA cell lines was investigated by examining the signaling pathways of ERK1/2 and PI3K. HGF (50 ng/mL) stimulated both HuCCA-1 and KKU-M213 phosphorylation of ERK1/2 and Akt, with the latter being the major downstream effector of PI3K (Figure 5A and B). However, different time response profiles were observed between these two cell lines in HGF-induced ERK1/2 and Akt activation. In KKU-M213 cells, HGF significantly induced activation of ERK1/2 and Akt at up to 360 min, whereas in HuCCA-1 cells, after 360 min of induction, activation decreased to nearly those of unstimulated levels.
Figure 5 HGF induction of ERK1/2 and Akt phosphorylation in cholangiocarcinoma HuCCA-1 and KKU-M213 cell lines.
About 80% confluent cells were treated with 50 ng/mL HGF in serum-free medium for 15, 60, 360 min. Lysates from HuCCA-1 (A) and KKU-M213 (B) cells were assessed for total and phosphorylated forms of ERK1/2 and Akt by Western blotting assay. The graphs showed band densities of phospho-ERK1/2 and phospho-Akt relative to those at zero time points. Data are presented as mean ± SE of results obtained from three independent experiments. aP < 0.05 vs untreated control.
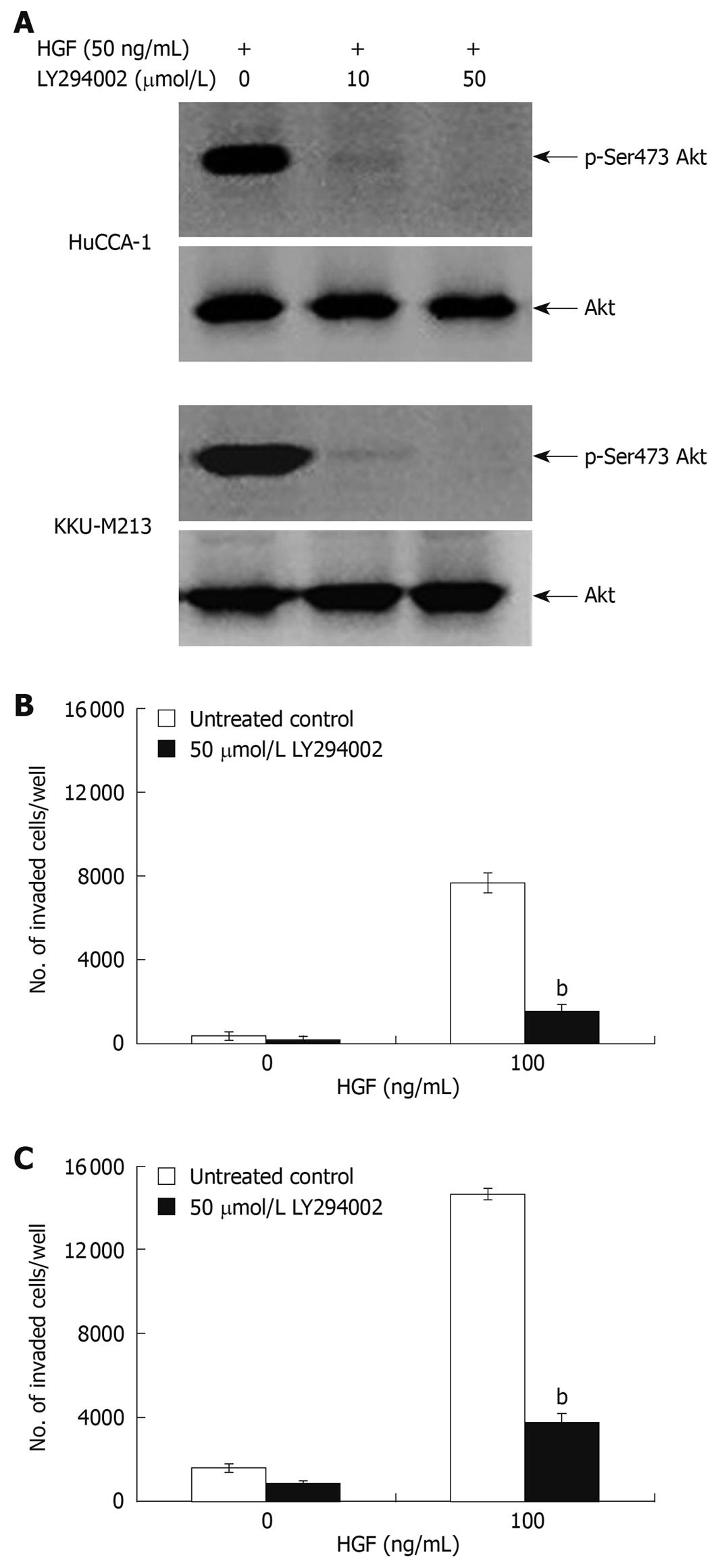
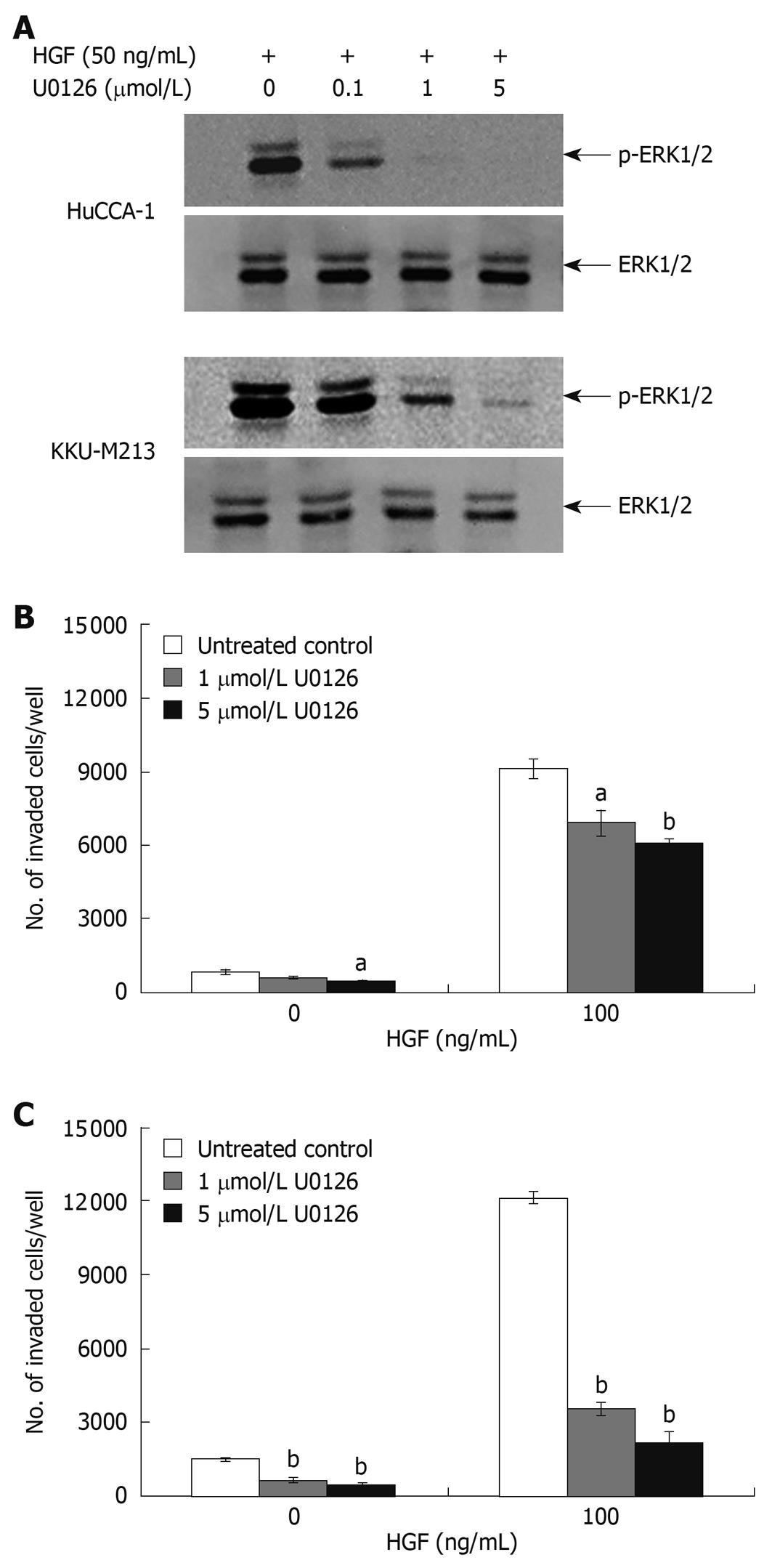
To confirm the roles of these two signaling pathways in response to HGF stimulation, we tested the antagonistic effect of U0126 and LY294002; a MEK1 and a PI3K inhibitor, respectively. LY294002 (50 μmol/L) inhibited HGF-stimulated phosphorylation of Akt in both CCA cell lines to an undetectable level (Figure 6A) and markedly inhibited HGF-induced cell invasion, but did not have any significant effect on the invasion of non HGF-stimulated cells (Figure 6B and C). U0126 (1 and 5 μmol/L) reduced HGF-induced invasion of KKU-M213 cells (to 29% and 18% of untreated control, respectively) (Figure 7C). However, U0126 only had a marginal inhibitory effect on HGF-induced invasion of the HuCCA-1 cell line (Figure 7B). Nevertheless, U0126 completely inhibited ERK1/2 phosphorylation of HuCCA-1 cells, whereas phospho-ERK1/2 was still detectable in KKU-M213 cells even at the highest U0126 concentration used (Figure 7A).
Figure 6 Suppression of HGF-induced cholangiocarcinoma cell invasiveness by PI3-kinase inhibitor, LY294002.
HuCCA-1 and KKU-M213 cells were treated with 50 ng/mL HGF in the absence (control) or presence of 10 and 50 μmol/L LY294002 for 6 h, and subsequently Akt phosphorylation was determined by Western blotting (A). In vitro invasion of HuCCA-1 (B) and KKU-M213 (C) cells was evaluated in the absence or presence of HGF with or without 50 μmol/L LY294002. Numbers of invaded cells are presented as mean ± SE of results obtained from three independent experiments. bP < 0.01 vs control.
Figure 7 Suppression of HGF-induced cholangiocarcinoma cell invasiveness by MEK1 inhibitor, U0126.
HuCCA-1 and KKU-M213 cells were treated with 50 ng/mL HGF in the absence (control) or presence of 0.1, 1 and 5 μmol/L U0126 for 6 h, and subsequently ERK1/2 phosphorylation was determined by Western blotting (A). In vitro invasion of HuCCA-1 (B) and KKU-M213 (C) cells was evaluated in the absence or presence of HGF with or without 1 and 5 μmol/L U0126. Numbers of invaded cells are presented as mean ± SE of results obtained from three independent experiments. aP < 0.05 and bP < 0.01 vs control.
DISCUSSION
Overexpression of Met has been reported in CCA and is correlated with progression and invasion of this type of cancer[9,11]. In this study, we demonstrated that HGF induced cell invasion, motility and change in E-cadherin localization in two human CCA cell lines, HuCCA-1 and KKU-M213, both of which overexpress Met; but without affecting secretion of the matrix degrading enzymes, MMP-2, MMP-9 and uPA. However, the signaling pathways underlying HGF-induced invasiveness of the two cell lines were different, with ERK1/2 activation being more important for HGF-induced KKU-M213 cell invasion than for HuCCA-1 cell invasion.
Two major factors contributing to an increase in cancer cell invasiveness are enhancement of extracellular matrix degradation and activation of cell motility. The effects of HGF on induction of these phenomena vary with different cell types. For instance, HGF enhances cell motility but not MMP-9 or uPA activities in breast cancer MDA-MB-231 cell line[16], while it induces both motility and matrix degrading enzyme expression in colon cancer Caco-2, prostate cancer PC-3 and DU-145 cells[31,32]. In our study, HGF induced invasion of both CCA cell lines by increasing motility but not MMP-2, MMP-9 or uPA levels. As the expression of the basal levels of these matrix degrading enzymes was already high in both CCA cell lines, this may be sufficient for providing cellular transmigration. Therefore, induction of cell motility alone by HGF, without augmenting extracellular matrix degrading enzyme levels, appears to be sufficient for cell invasiveness. Alterations of only some process(es) required for cell invasion have been reported as being able to alter cell invasiveness. For instance, inhibitors of ERK1/2[33] and myosin light chain kinase[34] suppress prostate cancer cell invasion by decreasing cell motility but not matrix degrading enzyme activity.
E-cadherin is the key mediator of cell-cell adhesion. Cell scattering induced by HGF results from disruption of E-cadherin function, either by reducing expression or changing its cellular localization[29]. In this study, we found that HGF caused E-cadherin to move from membrane to cytoplasm but had no effect on amount. These results are consistent with previous studies in a keratinocyte cell line, in which HGF reduced E-cadherin at cell-cell boundaries without changing its protein level[35,36]. Although we did not investigate the mechanism of HGF-disrupted E-cadherin function, previous reports have implicated the involvement of Ras-RIN2-Rab5 and β-catenin in this process. Kimura et al[37] demonstrated in a cell free system that HGF activates Ras which binds and activates RIN2, a Rab5-GEF (guanine nucleotide exchange factor of Rab5), leading to Rab5 activation. This active Rab5, a small G protein regulating endocytosis, in turn promotes E-cadherin endocytosis. In addition, Shibamoto et al[36] showed that HGF promotes tyrosine phosphorylation of β-catenin and decreases E-cadherin at the cell-cell boundaries resulting in the reduction of cell-cell adhesion mediated by E-cadherin.
Basement membrane normally acts as a barrier for tumor cell invasion; therefore, it is generally expected that the rate of invasion at which a cell degrades this barrier should be slower than or equal to the rate of cell migration. However, with the HuCCA-1 cell line, the basal cell invasion rate (with no HGF stimulation) was higher than that of migration. This suggests that some component(s) in Matrigel may have a role in inducing HuCCA-1 cell invasion. In support of this notion, Chintala et al[38] have shown that Matrigel and components of ECM (namely, type IV collagen and fibronectin) induce migration and invasion of many glioma cell lines.
In the KKU-M213 cell line, HGF was better at inducing invasion than migration, and this was not related to the stimulation of secretion of matrix degrading enzymes. A possible explanation is the existence of a synergism between HGF and extracellular matrix component(s) in the Matrigel. A combination of HGF and Matrigel induced higher motility than HGF alone (data not shown). Cooperation between HGF and ECM component(s) to promote cell migration could occur by enhancing the function of integrins[16,39], adhesion molecules regulating a variety of cellular properties including adhesion and migration by binding to ECM components. HGF induces cell scattering and migration by increasing integrin α2 expression in MDCK cells[39] and also promotes breast cancer MDA-MB-231 cell invasion and adhesion by inducing integrin aggregation at lamellipodia, thereby enhancing avidity of integrins to their ligands in ECM and increasing association of integrin to actin, which may participate in cell migration[16].
A variety of signaling pathways are involved in HGF-induced cell invasiveness, including PI3K, ERK1/2 and Src[40]. In CCA, Src, FAK[41] and ERK1/2[42] are involved in HGF-induced HuCCA-1 cell invasion. Here, we showed that HGF induced Met activation concomitant with the promotion of both ERK1/2 and Akt phosphorylation in these two CCA cell lines. To reveal the involvement of ERK and PI3K pathways in HGF-induced invasion, inhibitors of specific signaling transduction pathways were used. PI3K inhibitor (LY294002) significantly inhibited both HuCCA-1 and KKU-M213 cell invasion stimulated by HGF, while basal invasion was marginally affected. As for the ERK pathway, U0126, a specific inhibitor of MEK1, drastically reduced HGF-promoted KKU-M213 cell invasion, while slightly reducing HGF-induced HuCCA-1 invasion, even though it inhibited ERK1/2 phosphorylation of the latter cell line to a greater extent than in the former. The insensitivity of HGF-stimulated HuCCA-1 invasion to U0126 treatment suggests a reduced dependence of this CCA cell line on the ERK signaling pathway, whereas HGF-induced KKU-M213 invasion is dependent on both PI3K and ERK1/2 activation.
ERK1/2 activation is known to regulate a variety of cellular functions, such as proliferation, differentiation, migration, and invasion in response to diverse extracellular stimuli[43]. Duration of ERK1/2 activation is one of the factors determining a particular cellular response[39,44]. McCawley et al[45] showed that EGF and HGF have the ability to induce SCC-12F keratinocyte migration. These two growth factors induce sustained ERK1/2 activation, which is associated with enhanced MMP-9 expression and SCC cell migration[45,46]. In MDCK cells, HGF induces sustained ERK1/2 activation, promoting cell scattering and migration via the enhancement of integrin-α2 expression, whereas EGF induces transient ERK1/2 activation, which has no effect on cell scattering[39]. Our data indicated that prolonged ERK1/2 activation was crucial for HGF-induced invasion of KKU-M213 cells, but was not necessary for HuCCA-1 cells in which HGF rapidly and transiently activated ERK. Thus, sustained ERK activation provides a possible explanation for the difference in downstream signaling pathways observed in HGF-induced invasion of the two CCA cell lines. Moreover, this sustained ERK1/2 activation may be responsible for a synergism between HGF and Matrigel in KKU-M213 cells by inducing integrin expression, as in MDCK cells[39].
In summary, this study provides evidence for the contribution of a HGF signaling pathway to the induction of CCA cell invasion. HGF promoted invasion via stimulation of cell motility, but not MMP or uPA secretion. HGF regulated invasiveness of two independent CCA cell lines by different signaling pathways, with PI3K being a common pathway underlying HGF-induced invasiveness in both cell lines, whereas the importance of ERK1/2 was determined by the duration of ERK1/2 activation. However, the mechanisms regulating temporal ERK1/2 activation and possible synergism between HGF and matrix in inducing invasion remains to be elucidated. Understanding the signaling mechanism responsible for CCA invasiveness will be valuable to help identify better targets for cancer therapy, such as that associated with a common rather than a cell specific pathway.
COMMENTS
Background
Cholangiocarcinoma (CCA) is a malignant tumor of the biliary epithelium associated with a high metastatic and mortality rate. Incidence of this cancer has increased worldwide, and the highest incidence occurs in northeast Thailand. Overexpression of Met has been reported in CCA and is correlated with progression and invasion of this type of cancer.
Research frontiers
Hepatocyte growth factor (HGF)/Met activation induces a variety of biological processes, including cell scattering, invasion, proliferation and survival. Although several reports have demonstrated a correlation between Met expression and CCA, hitherto there have been only a limited number of detailed investigations into the role of Met in cholangiocarcinoma.
Innovations and breakthroughs
HGF induced cell invasion and motility and altered E-cadherin localization in two human CCA cell lines overexpressing Met, without affecting the matrix degrading enzymes, matrix metalloproteinase (MMP)-2, MMP-9 and urokinase plasminogen activator (uPA). This is the first report of a difference in the signaling pathways responsible for the HGF-induced invasiveness of the two human CCA cell lines, in that extracellular signal-regulated kinase (ERK)1/2 activation is more important for HGF-induced invasion of one cell line than of the other.
Applications
Understanding the role of HGF/Met in CCA invasiveness and the molecular mechanisms underlying this process provides valuable information to help identify targets for future treatment of CCA patients.
Terminology
Phosphoinositide 3-kinase (PI3K) and ERK are signaling molecules downstream of many receptor tyrosine kinases including Met. These proteins have been shown to play an important role in cell invasion, a crucial factor of cancer metastasis. In this study HGF is shown to stimulate cell invasion and motility of CCA cell lines through PI3K and/or ERK pathways.
Peer review
CCA is a common malignant tumor with a high metastatic and mortality rate. Investigation into its molecular mechanism is important for understanding the pathogenesis of CCA. This study focused on the role of HGF/Met in CCA cell invasion and the mechanisms underlying cellular responses. Although a number of papers on this field have been published, this study still adds some new information into the knowledge already documented.