Nonpharmacological Treatment Strategies for the Management of Canine Chronic Inflammatory Enteropathy—A Narrative Review
Abstract
:1. Introduction
2. Etiopathogenesis of CIE
2.1. Immune System
2.2. Intestinal Epithelial Barrier
2.3. Intestinal Microbiota and Main Postbiotics
3. Gut Microbiota Alterations in Dogs with CIE
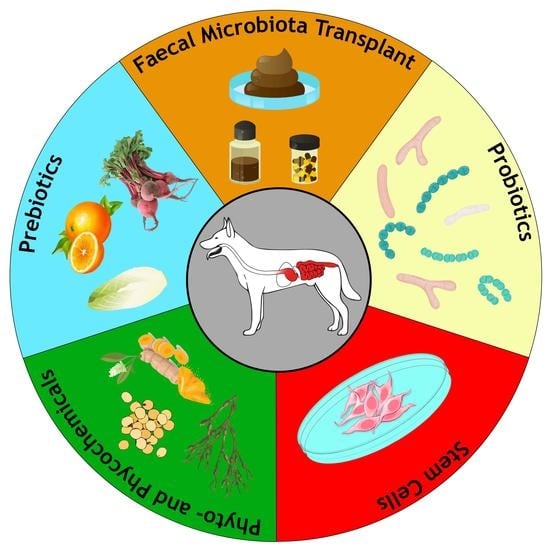
4. Main Nonpharmacological Therapies for CIE
4.1. Diet
4.1.1. Antigenicity, Digestibility and Nutrient-Responsiveness
4.1.2. Impact of Diet on Gut Microbiota Composition
4.2. Phytogenic Feed Additives
4.2.1. Prebiotics
4.2.2. Phyto- and Phycochemicals
4.3. Probiotics
4.4. Faecal Microbiota Transplantation
4.5. Stem Cell Therapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dandrieux, J.R.S. Inflammatory Bowel Disease versus Chronic Enteropathy in Dogs: Are They One and the Same? J. Small Anim. Pract. 2016, 57, 589–599. [Google Scholar] [CrossRef]
- Kathrani, A.; Werling, D.; Allenspach, K. Canine Breeds at High Risk of Developing Inflammatory Bowel Disease in the South-Eastern UK. Vet. Rec. 2011, 169, 635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchesi, M.C.; Timpano, C.C.; Busechian, S.; Pieramati, C.; Rueca, F. The Role of Diet in Managing Inflammatory Bowel Disease Affected Dogs: A Retrospective Cohort Study on 76 Cases. Vet. Ital. 2017, 53, 297–302. [Google Scholar] [CrossRef]
- Dandrieux, J.R.S.; Mansfield, C.S. Chronic Enteropathy In Canines: Prevalence, Impact And Management Strategies. Vet. Med. 2019, 10, 203–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, D.; Knuchel-Takano, A.; McCutchan, A.; Chang, Y.-M.; Downes, C.; Miller, S.; Stevens, K.; Verheyen, K.; Phillips, A.D.; Miah, S.; et al. A Comprehensive Pathological Survey of Duodenal Biopsies from Dogs with Diet-Responsive Chronic Enteropathy. J. Vet. Intern. Med. 2013, 27, 862–874. [Google Scholar] [CrossRef]
- Allenspach, K.; Wieland, B.; Gröne, A.; Gaschen, F. Chronic Enteropathies in Dogs: Evaluation of Risk Factors for Negative Outcome. J. Vet. Intern. Med. 2007, 21, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Viviano, K.R. Update on Immununosuppressive Therapies for Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 1149–1170. [Google Scholar] [CrossRef]
- Westermarck, E.; Skrzypczak, T.; Harmoinen, J.; Steiner, J.M.; Ruaux, C.G.; Williams, D.A.; Eerola, E.; Sundbäck, P.; Rinkinen, M. Tylosin-Responsive Chronic Diarrhea in Dogs. J. Vet. Intern. Med. 2005, 19, 177–186. [Google Scholar] [CrossRef]
- Pietra, M.; Fracassi, F.; Diana, A.; Gazzotti, T.; Bettini, G.; Peli, A.; Morini, M.; Pagliuca, G.; Roncada, P. Plasma Concentrations and Therapeutic Effects of Budesonide in Dogs with Inflammatory Bowel Disease. Am. J. Vet. Res. 2013, 74, 78–83. [Google Scholar] [CrossRef]
- Grønvold, A.-M.R.; L’abée-Lund, T.M.; Sørum, H.; Skancke, E.; Yannarell, A.C.; Mackie, R.I. Changes in Fecal Microbiota of Healthy Dogs Administered Amoxicillin. FEMS Microbiol. Ecol. 2010, 71, 313–326. [Google Scholar] [CrossRef]
- Francino, M.P. Antibiotics and the Human Gut Microbiome: Dysbioses and Accumulation of Resistances. Front. Microbiol. 2015, 6, 1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manchester, A.C.; Webb, C.B.; Blake, A.B.; Sarwar, F.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Long-Term Impact of Tylosin on Fecal Microbiota and Fecal Bile Acids of Healthy Dogs. J. Vet. Intern. Med. 2019, 33, 2605–2617. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.M.; Pinchbeck, G.; McIntyre, K.M.; Nuttall, T.; McEwan, N.; Dawson, S.; Williams, N.J. Routine Antibiotic Therapy in Dogs Increases the Detection of Antimicrobial-Resistant Faecal Escherichia Coli. J. Antimicrob. Chemother. 2018, 73, 3305–3316. [Google Scholar] [CrossRef] [PubMed]
- Dickson, A.; Smith, M.; Smith, F.; Park, J.; King, C.; Currie, K.; Langdridge, D.; Davis, M.; Flowers, P. Understanding the Relationship between Pet Owners and Their Companion Animals as a Key Context for Antimicrobial Resistance-Related Behaviours: An Interpretative Phenomenological Analysis. Health Psychol. Behav. Med. 2019, 7, 45–61. [Google Scholar] [CrossRef] [Green Version]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory Bowel Disease and Cancer: The Role of Inflammation, Immunosuppression, and Cancer Treatment. World J. Gastroenterol. 2016, 22, 4794–4801. [Google Scholar] [CrossRef]
- Hall, E.J.; Day, M.J. Diseases of the Small Intestine. In Textbook of Veterinary Internal Medicine, 8th ed.; Ettinger, S.J., Feldman, E.C., Cote, E., Eds.; Elsevier: St. Louis, MO, USA, 2017; Volume 2, pp. 3643–3759. [Google Scholar]
- Larussa, T.; Imeneo, M.; Luzza, F. Potential Role of Nutraceutical Compounds in Inflammatory Bowel Disease. World J. Gastroenterol. 2017, 23, 2483–2492. [Google Scholar] [CrossRef]
- Gaschen, F.P.; Merchant, S.R. Adverse Food Reactions in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 361–379. [Google Scholar] [CrossRef]
- Hall, E.J. Antibiotic-Responsive Diarrhea in Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 273–286. [Google Scholar] [CrossRef]
- Simpson, K.W.; Jergens, A.E. Pitfalls and Progress in the Diagnosis and Management of Canine Inflammatory Bowel Disease. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 381–398. [Google Scholar] [CrossRef]
- Jergens, A.E.; Simpson, K.W. Inflammatory Bowel Disease in Veterinary Medicine. Front. Biosci. 2012, 4, 1404–1419. [Google Scholar] [CrossRef]
- German, A.J.; Hall, E.J.; Day, M.J. Chronic Intestinal Inflammation and Intestinal Disease in Dogs. J. Vet. Intern. Med. 2003, 17, 8–20. [Google Scholar] [CrossRef]
- Heilmann, R.M.; Allenspach, K. Pattern-Recognition Receptors: Signaling Pathways and Dysregulation in Canine Chronic Enteropathies-Brief Review. J. Vet. Diagn. Investig. 2017, 29, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N.; Kittana, H.; Gomes-Neto, J.C.; Hussein, H. Mucosal Immunity and Gut Microbiota in Dogs with Chronic Enteropathy. Res. Vet. Sci. 2019, 122, 156–164. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Burgener, I.A.; König, A.; Allenspach, K.; Sauter, S.N.; Boisclair, J.; Doherr, M.G.; Jungi, T.W. Upregulation of Toll-like Receptors in Chronic Enteropathies in Dogs. J. Vet. Intern. Med. 2008, 22, 553–560. [Google Scholar] [CrossRef] [PubMed]
- McMahon, L.A.; House, A.K.; Catchpole, B.; Elson-Riggins, J.; Riddle, A.; Smith, K.; Werling, D.; Burgener, I.A.; Allenspach, K. Expression of Toll-like Receptor 2 in Duodenal Biopsies from Dogs with Inflammatory Bowel Disease Is Associated with Severity of Disease. Vet. Immunol. Immunopathol. 2010, 135, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Kathrani, A.; House, A.; Catchpole, B.; Murphy, A.; German, A.; Werling, D.; Allenspach, K. Polymorphisms in the TLR4 and TLR5 Gene Are Significantly Associated with Inflammatory Bowel Disease in German Shepherd Dogs. PLoS ONE 2010, 5, e15740. [Google Scholar] [CrossRef] [PubMed]
- Okanishi, H.; Hayashi, K.; Sakamoto, Y.; Sano, T.; Maruyama, H.; Kagawa, Y.; Watari, T. NOD2 mRNA Expression and NFkappaB Activation in Dogs with Lymphocytic Plasmacytic Colitis. J. Vet. Intern. Med. 2013, 27, 439–444. [Google Scholar] [CrossRef]
- Kathrani, A.; Lee, H.; White, C.; Catchpole, B.; Murphy, A.; German, A.; Werling, D.; Allenspach, K. Association between Nucleotide Oligomerisation Domain Two (Nod2) Gene Polymorphisms and Canine Inflammatory Bowel Disease. Vet. Immunol. Immunopathol. 2014, 161, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, Z.H.; Bogdanovski, D.A.; Barratt-Stopper, P.; Paglinco, S.R.; Antonioli, L.; Rolandelli, R.H. Crohn’s Disease and Ulcerative Colitis Show Unique Cytokine Profiles. Cureus 2017, 9, e1177. [Google Scholar] [CrossRef] [Green Version]
- German, A.J.; Helps, C.R.; Hall, E.J.; Day, M.J. Cytokine mRNA Expression in Mucosal Biopsies from German Shepherd Dogs with Small Intestinal Enteropathies. Dig. Dis. Sci. 2000, 45, 7–17. [Google Scholar] [CrossRef]
- Peters, I.R.; Helps, C.R.; Calvert, E.L.; Hall, E.J.; Day, M.J. Cytokine mRNA Quantification in Duodenal Mucosa from Dogs with Chronic Enteropathies by Real-Time Reverse Transcriptase Polymerase Chain Reaction. J. Vet. Intern. Med. 2005, 19, 644–653. [Google Scholar] [CrossRef]
- Jergens, A.E.; Sonea, I.M.; O’Connor, A.M.; Kauffman, L.K.; Grozdanic, S.D.; Ackermann, M.R.; Evans, R.B. Intestinal Cytokine mRNA Expression in Canine Inflammatory Bowel Disease: A Meta-Analysis with Critical Appraisal. Comp. Med. 2009, 59, 153–162. [Google Scholar]
- Heilmann, R.M.; Suchodolski, J.S. Is Inflammatory Bowel Disease in Dogs and Cats Associated with a Th1 or Th2 Polarization? Vet. Immunol. Immunopathol. 2015, 168, 131–134. [Google Scholar] [CrossRef]
- Halpern, M.D.; Denning, P.W. The Role of Intestinal Epithelial Barrier Function in the Development of NEC. Tissue Barriers 2015, 3, e1000707. [Google Scholar] [CrossRef] [Green Version]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, S.H.; Proud, F.J.; Rutgers, H.C.; Markwell, P.; Adam, A.; Batt, R.M. A Blood Test for Intestinal Permeability and Function: A New Tool for the Diagnosis of Chronic Intestinal Disease in Dogs. Clin. Chim. Acta 1997, 264, 103–115. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ohno, K.; Uetsuka, K.; Nakashima, K.; Setoguchi, A.; Fujino, Y.; Tsujimoto, H. Measurement of Intestinal Mucosal Permeability in Dogs with Lymphocytic-Plasmacytic Enteritis. J. Vet. Med. Sci. 2007, 69, 745–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allenspach, K.; Steiner, J.M.; Shah, B.N.; Berghoff, N.; Ruaux, C.; Williams, D.A.; Blum, J.W.; Gaschen, F. Evaluation of Gastrointestinal Permeability and Mucosal Absorptive Capacity in Dogs with Chronic Enteropathy. Am. J. Vet. Res. 2006, 67, 479–483. [Google Scholar] [CrossRef]
- Viggiano, D.; Ianiro, G.; Vanella, G.; Bibbò, S.; Bruno, G.; Simeone, G.; Mele, G. Gut Barrier in Health and Disease: Focus on Childhood. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1077–1085. [Google Scholar] [PubMed]
- Kilian, E.; Suchodolski, J.S.; Hartmann, K.; Mueller, R.S.; Wess, G.; Unterer, S. Long-Term Effects of Canine Parvovirus Infection in Dogs. PLoS ONE 2018, 13, e0192198. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Dowd, S.E.; Suchodolski, J.S.; Middelbos, I.S.; Vester, B.M.; Barry, K.A.; Nelson, K.E.; Torralba, M.; Henrissat, B.; Coutinho, P.M.; et al. Phylogenetic and Gene-Centric Metagenomics of the Canine Intestinal Microbiome Reveals Similarities with Humans and Mice. ISME J. 2011, 5, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Benno, Y.; Nakao, H.; Uchida, K.; Mitsuoka, T. Impact of the Advances in Age on the Gastrointestinal Microflora of Beagle Dogs. J. Vet. Med. Sci. 1992, 54, 703–706. [Google Scholar] [CrossRef] [Green Version]
- Mentula, S.; Harmoinen, J.; Heikkilä, M.; Westermarck, E.; Rautio, M.; Huovinen, P.; Könönen, E. Comparison between Cultured Small-Intestinal and Fecal Microbiotas in Beagle Dogs. Appl. Environ. Microbiol. 2005, 71, 4169–4175. [Google Scholar] [CrossRef] [Green Version]
- Suchodolski, J.S.; Camacho, J.; Steiner, J.M. Analysis of Bacterial Diversity in the Canine Duodenum, Jejunum, Ileum, and Colon by Comparative 16S rRNA Gene Analysis. FEMS Microbiol. Ecol. 2008, 66, 567–578. [Google Scholar] [CrossRef]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut Biogeography of the Bacterial Microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Montalto, M.; D’Onofrio, F.; Gallo, A.; Cazzato, A.; Gasbarrini, G. Intestinal Microbiota and Its Functions. Dig. Liver Dis. Suppl. 2009, 3, 30–34. [Google Scholar] [CrossRef]
- Bauer, H.; Horowitz, R.E.; Levenson, S.M.; Popper, H. The Response of the Lymphatic Tissue to the Microbial Flora. Studies on Germfree Mice. Am. J. Pathol. 1963, 42, 471–483. [Google Scholar]
- Round, J.L.; Mazmanian, S.K. The Gut Microbiota Shapes Intestinal Immune Responses during Health and Disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Chung, H.; Pamp, S.J.; Hill, J.A.; Surana, N.K.; Edelman, S.M.; Troy, E.B.; Reading, N.C.; Villablanca, E.J.; Wang, S.; Mora, J.R.; et al. Gut Immune Maturation Depends on Colonization with a Host-Specific Microbiota. Cell 2012, 149, 1578–1593. [Google Scholar] [CrossRef] [Green Version]
- Tremaroli, V.; Bäckhed, F. Functional Interactions between the Gut Microbiota and Host Metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Ridlon, J.M.; Kang, D.-J.; Hylemon, P.B. Bile Salt Biotransformations by Human Intestinal Bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wielen, N.; Hee, B.; Wang, J.; Hendriks, W.; Gilbert, M. Impact of Fermentable Protein, by Feeding High Protein Diets, on Microbial Composition, Microbial Catabolic Activity, Gut Health and beyond in Pigs. Microorganisms 2020, 8, 1735. [Google Scholar] [CrossRef]
- Binder, H.J. Role of Colonic Short-Chain Fatty Acid Transport in Diarrhea. Annu. Rev. Physiol. 2010, 72, 297–313. [Google Scholar] [CrossRef]
- Scheppach, W. Effects of Short Chain Fatty Acids on Gut Morphology and Function. Gut 1994, 35, S35–S38. [Google Scholar] [CrossRef] [Green Version]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 2016, 165, 1708–1720. [Google Scholar] [CrossRef] [Green Version]
- Roediger, W.E. The Starved Colon—Diminished Mucosal Nutrition, Diminished Absorption, and Colitis. Dis. Colon Rectum 1990, 33, 858–862. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and Medium-Chain Fatty Acids in Energy Metabolism: The Cellular Perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herschel, D.A.; Argenzio, R.A.; Southworth, M.; Stevens, C.E. Absorption of Volatile Fatty Acid, Na, and H2O by the Colon of the Dog. Am. J. Vet. Res. 1981, 42, 1118–1124. [Google Scholar]
- Stevens, C.E.; Hume, I.D. Contributions of Microbes in Vertebrate Gastrointestinal Tract to Production and Conservation of Nutrients. Physiol. Rev. 1998, 78, 393–427. [Google Scholar] [CrossRef] [Green Version]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe, W.L. Short Chain Fatty Acids and Their Receptors: New Metabolic Targets. Transl. Res. 2013, 161, 131–140. [Google Scholar] [CrossRef]
- Kvietys, P.R.; Granger, D.N. Effect of Volatile Fatty Acids on Blood Flow and Oxygen Uptake by the Dog Colon. Gastroenterology 1981, 80, 962–969. [Google Scholar] [CrossRef]
- Mcmanus, C.M.; Michel, K.E.; Simon, D.M.; Washabau, R.J. Effect of Short-Chain Fatty Acids on Contraction of Smooth Muscle in the Canine Colon. Am. J. Vet. Res. 2002, 63, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, M.; Kotlo, K.U.; Dudeja, P.K.; Layden, B.T. Role of Short Chain Fatty Acid Receptors in Intestinal Physiology and Pathophysiology. Compr. Physiol. 2018, 8, 1091–1115. [Google Scholar] [CrossRef]
- Sun, Y.; O’Riordan, M.X.D. Regulation of Bacterial Pathogenesis by Intestinal Short-Chain Fatty Acids. Adv. Appl. Microbiol. 2013, 85, 93–118. [Google Scholar] [CrossRef] [Green Version]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, Probiotics, Synbiotics, Paraprobiotics and Postbiotic Compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Urrego, M.I.G.; Pedreira, R.S.; Santos, K.D.; Ernandes, M.C.; Santos, J.P.F.; Vendramini, T.H.A.; Eberlin, M.N.; Balieiro, J.C.; Pontieri, C.F.F.; Brunetto, M.A. Dietary Protein Sources and Their Effects on Faecal Odour and the Composition of Volatile Organic Compounds in Faeces of French Bulldogs. J. Anim. Physiol. Anim. Nutr. 2021, 105, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, B.S.; Roberts-Thomson, I.C.; Pannall, P.R.; Roediger, W.E. Impaired Sulphation of Phenol by the Colonic Mucosa in Quiescent and Active Ulcerative Colitis. Gut 1991, 32, 46–49. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.C.; Visek, W.J. Colon Mucosal Cell Damage by Ammonia in Rats. J. Nutr. 1991, 121, 887–893. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, J. Indole as an Intercellular Signal in Microbial Communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef]
- Bansal, T.; Alaniz, R.C.; Wood, T.K.; Jayaraman, A. The Bacterial Signal Indole Increases Epithelial-Cell Tight-Junction Resistance and Attenuates Indicators of Inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jayaraman, A.; Wood, T.K. Indole Is an Inter-Species Biofilm Signal Mediated by SdiA. BMC Microbiol. 2007, 7, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, T.; Englert, D.; Lee, J.; Hegde, M.; Wood, T.K.; Jayaraman, A. Differential Effects of Epinephrine, Norepinephrine, and Indole on Escherichia Coli O157:H7 Chemotaxis, Colonization, and Gene Expression. Infect. Immun. 2007, 75, 4597–4607. [Google Scholar] [CrossRef] [Green Version]
- Hirakawa, H.; Inazumi, Y.; Masaki, T.; Hirata, T.; Yamaguchi, A. Indole Induces the Expression of Multidrug Exporter Genes in Escherichia Coli. Mol. Microbiol. 2005, 55, 1113–1126. [Google Scholar] [CrossRef]
- Chant, E.L.; Summers, D.K. Indole Signalling Contributes to the Stable Maintenance of Escherichia Coli Multicopy Plasmids. Mol. Microbiol. 2007, 63, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Vega, N.M.; Allison, K.R.; Khalil, A.S.; Collins, J.J. Signaling-Mediated Bacterial Persister Formation. Nat. Chem. Biol. 2012, 8, 431–433. [Google Scholar] [CrossRef]
- Darkoh, C.; Plants-Paris, K.; Bishoff, D.; DuPont, H.L. Clostridium Difficile Modulates the Gut Microbiota by Inducing the Production of Indole, an Interkingdom Signaling and Antimicrobial Molecule. mSystems 2019, 4, e00346-18. [Google Scholar] [CrossRef] [Green Version]
- Banoglu, E.; Jha, G.G.; King, R.S. Hepatic Microsomal Metabolism of Indole to Indoxyl, a Precursor of Indoxyl Sulfate. Eur. J. Drug Metab. Pharm. 2001, 26, 235–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The Uremic Toxicity of Indoxyl Sulfate and P-Cresyl Sulfate: A Systematic Review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.-J.; Hylemon, P.B. Consequences of Bile Salt Biotransformations by Intestinal Bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef] [Green Version]
- Gadaleta, R.M.; Oldenburg, B.; Willemsen, E.C.L.; Spit, M.; Murzilli, S.; Salvatore, L.; Klomp, L.W.J.; Siersema, P.D.; van Erpecum, K.J.; van Mil, S.W.C. Activation of Bile Salt Nuclear Receptor FXR Is Repressed by Pro-Inflammatory Cytokines Activating NF-ΚB Signaling in the Intestine. Biochim. Biophys. Acta 2011, 1812, 851–858. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; van Erpecum, K.J.; Oldenburg, B.; Willemsen, E.C.L.; Renooij, W.; Murzilli, S.; Klomp, L.W.J.; Siersema, P.D.; Schipper, M.E.I.; Danese, S.; et al. Farnesoid X Receptor Activation Inhibits Inflammation and Preserves the Intestinal Barrier in Inflammatory Bowel Disease. Gut 2011, 60, 463–472. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Chen, W.-D.; Yu, D.; Forman, B.M.; Huang, W. The G-Protein-Coupled Bile Acid Receptor, Gpbar1 (TGR5), Negatively Regulates Hepatic Inflammatory Response through Antagonizing Nuclear Factor κ Light-Chain Enhancer of Activated B Cells (NF-ΚB) in Mice. Hepatology 2011, 54, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Ward, J.B.J.; Lajczak, N.K.; Kelly, O.B.; O’Dwyer, A.M.; Giddam, A.K.; Ní Gabhann, J.; Franco, P.; Tambuwala, M.M.; Jefferies, C.A.; Keely, S.; et al. Ursodeoxycholic Acid and Lithocholic Acid Exert Anti-Inflammatory Actions in the Colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G550–G558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajouz, H.; Mukherji, D.; Shamseddine, A. Secondary Bile Acids: An Underrecognized Cause of Colon Cancer. World J. Surg. Oncol. 2014, 12, 164. [Google Scholar] [CrossRef] [Green Version]
- Sartor, R.B. Microbial Influences in Inflammatory Bowel Diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Minamoto, Y. Gastrointestinal Microorganisms in Cats and Dogs: A Brief Review. Arch. Med. Vet. 2013, 45, 111–124. [Google Scholar] [CrossRef]
- Brüssow, H. Problems with the Concept of Gut Microbiota Dysbiosis. Microb. Biotechnol. 2020, 13, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of Inflammation-Driven Bacterial Dysbiosis in the Gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Lobionda, S.; Sittipo, P.; Kwon, H.Y.; Lee, Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorganisms 2019, 7, 271. [Google Scholar] [CrossRef] [Green Version]
- Xenoulis, P.G.; Palculict, B.; Allenspach, K.; Steiner, J.M.; Van House, A.M.; Suchodolski, J.S. Molecular-Phylogenetic Characterization of Microbial Communities Imbalances in the Small Intestine of Dogs with Inflammatory Bowel Disease. FEMS Microbiol. Ecol. 2008, 66, 579–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchodolski, J.S.; Xenoulis, P.G.; Paddock, C.G.; Steiner, J.M.; Jergens, A.E. Molecular Analysis of the Bacterial Microbiota in Duodenal Biopsies from Dogs with Idiopathic Inflammatory Bowel Disease. Vet. Microbiol. 2010, 142, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Dowd, S.E.; Wilke, V.; Steiner, J.M.; Jergens, A.E. 16S rRNA Gene Pyrosequencing Reveals Bacterial Dysbiosis in the Duodenum of Dogs with Idiopathic Inflammatory Bowel Disease. PLoS ONE 2012, 7, e39333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassmann, E.; White, R.; Atherly, T.; Wang, C.; Sun, Y.; Khoda, S.; Mosher, C.; Ackermann, M.; Jergens, A. Alterations of the Ileal and Colonic Mucosal Microbiota in Canine Chronic Enteropathies. PLoS ONE 2016, 11, e0147321. [Google Scholar] [CrossRef] [Green Version]
- Kotlowski, R.; Bernstein, C.N.; Sepehri, S.; Krause, D.O. High Prevalence of Escherichia Coli Belonging to the B2+D Phylogenetic Group in Inflammatory Bowel Disease. Gut 2007, 56, 669–675. [Google Scholar] [CrossRef] [Green Version]
- De la Fuente, M.; Franchi, L.; Araya, D.; Díaz-Jiménez, D.; Olivares, M.; Álvarez-Lobos, M.; Golenbock, D.; González, M.-J.; López-Kostner, F.; Quera, R.; et al. Escherichia Coli Isolates from Inflammatory Bowel Diseases Patients Survive in Macrophages and Activate NLRP3 Inflammasome. Int. J. Med. Microbiol. 2014, 304, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Baeza, Y.; Hyde, E.R.; Suchodolski, J.S.; Knight, R. Dog and Human Inflammatory Bowel Disease Rely on Overlapping yet Distinct Dysbiosis Networks. Nat. Microbiol. 2016, 1, 16177. [Google Scholar] [CrossRef]
- Barko, P.C.; McMichael, M.A.; Swanson, K.S.; Williams, D.A. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2018, 32, 9–25. [Google Scholar] [CrossRef]
- Omori, M.; Maeda, S.; Igarashi, H.; Ohno, K.; Sakai, K.; Yonezawa, T.; Horigome, A.; Odamaki, T.; Matsuki, N. Fecal Microbiome in Dogs with Inflammatory Bowel Disease and Intestinal Lymphoma. J. Vet. Med. Sci. 2017, 79, 1840–1847. [Google Scholar] [CrossRef] [Green Version]
- Minamoto, Y.; Otoni, C.C.; Steelman, S.M.; Büyükleblebici, O.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Alteration of the Fecal Microbiota and Serum Metabolite Profiles in Dogs with Idiopathic Inflammatory Bowel Disease. Gut Microbes 2015, 6, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Bresciani, F.; Minamoto, Y.; Suchodolski, J.S.; Galiazzo, G.; Vecchiato, C.G.; Pinna, C.; Biagi, G.; Pietra, M. Effect of an Extruded Animal Protein-Free Diet on Fecal Microbiota of Dogs with Food-Responsive Enteropathy. J. Vet. Intern. Med. 2018, 32, 1903–1910. [Google Scholar] [CrossRef] [Green Version]
- Kalenyak, K.; Isaiah, A.; Heilmann, R.M.; Suchodolski, J.S.; Burgener, I.A. Comparison of the Intestinal Mucosal Microbiota in Dogs Diagnosed with Idiopathic Inflammatory Bowel Disease and Dogs with Food-Responsive Diarrhea before and after Treatment. FEMS Microbiol. Ecol. 2018, 94, fix173. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Verbrugghe, A.; Lourenço, M.; Janssens, G.P.J.; Liu, D.J.X.; Van de Wiele, T.; Eeckhaut, V.; Van Immerseel, F.; Van de Maele, I.; Niu, Y.; et al. Does Canine Inflammatory Bowel Disease Influence Gut Microbial Profile and Host Metabolism? BMC Vet. Res. 2016, 12, 114. [Google Scholar] [CrossRef] [Green Version]
- Blake, A.B.; Guard, B.C.; Honneffer, J.B.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Altered Microbiota, Fecal Lactate, and Fecal Bile Acids in Dogs with Gastrointestinal Disease. PLoS ONE 2019, 14, e0224454. [Google Scholar] [CrossRef] [Green Version]
- Suchodolski, J.S. Diagnosis and Interpretation of Intestinal Dysbiosis in Dogs and Cats. Vet. J. 2016, 215, 30–37. [Google Scholar] [CrossRef]
- Makielski, K.; Cullen, J.; O’Connor, A.; Jergens, A.E. Narrative Review of Therapies for Chronic Enteropathies in Dogs and Cats. J. Vet. Intern. Med. 2019, 33, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allenspach, K.; Culverwell, C.; Chan, D. Long-Term Outcome in Dogs with Chronic Enteropathies: 203 Cases. Vet. Rec. 2016, 178, 368. [Google Scholar] [CrossRef] [Green Version]
- Kawano, K.; Shimakura, H.; Nagata, N.; Masashi, Y.; Suto, A.; Suto, Y.; Uto, S.; Ueno, H.; Hasegawa, T.; Ushigusa, T.; et al. Prevalence of Food-Responsive Enteropathy among Dogs with Chronic Enteropathy in Japan. J. Vet. Med. Sci. 2016, 78, 1377–1380. [Google Scholar] [CrossRef] [Green Version]
- Volkmann, M.; Steiner, J.M.; Fosgate, G.T.; Zentek, J.; Hartmann, S.; Kohn, B. Chronic Diarrhea in Dogs—Retrospective Study in 136 Cases. J. Vet. Intern. Med. 2017, 31, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Guilford, W.G.; Matz, M.E. The Nutritional Management of Gastrointestinal Tract Disorders in Companion Animals. N. Z. Vet. J. 2003, 51, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, A.; Hesta, M.; Millet, S.; Janssens, G.P.J. Food Allergy in Dogs and Cats: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 259–273. [Google Scholar] [CrossRef]
- Outerbridge, C.A. Nutritional Management of Skin Diseases. In Applied Veterinary Clinical Nutrition; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 157–174. ISBN 978-1-118-78566-9. [Google Scholar]
- Cave, N.J. Hydrolyzed Protein Diets for Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 1251–1268. [Google Scholar] [CrossRef] [PubMed]
- Rudinsky, A.J.; Rowe, J.C.; Parker, V.J. Nutritional Management of Chronic Enteropathies in Dogs and Cats. J. Am. Vet. Med. Assoc. 2018, 253, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Puigdemont, A.; Brazís, P.; Serra, M.; Fondati, A. Immunologic Responses against Hydrolyzed Soy Protein in Dogs with Experimentally Induced Soy Hypersensitivity. Am. J. Vet. Res. 2006, 67, 484–488. [Google Scholar] [CrossRef]
- Serra, M.; Brazís, P.; Fondati, A.; Puigdemont, A. Assessment of IgE Binding to Native and Hydrolyzed Soy Protein in Serum Obtained from Dogs with Experimentally Induced Soy Protein Hypersensitivity. Am. J. Vet. Res. 2006, 67, 1895–1900. [Google Scholar] [CrossRef]
- Masuda, K.; Sato, A.; Tanaka, A.; Kumagai, A. Hydrolyzed Diets May Stimulate Food-Reactive Lymphocytes in Dogs. J. Vet. Med. Sci. 2020, 82, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Cave, N. Nutritional Management of Gastrointestinal Diseases. In Applied Veterinary Clinical Nutrition; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 175–219. ISBN 978-1-118-78566-9. [Google Scholar]
- Mandigers, P.J.J.; Biourge, V.; van den Ingh, T.S.G.; Ankringa, N.; German, A.J. A Randomized, Open-Label, Positively-Controlled Field Trial of a Hydrolyzed Protein Diet in Dogs with Chronic Small Bowel Enteropathy. J. Vet. Intern. Med. 2010, 24, 1350–1357. [Google Scholar] [CrossRef]
- Hall, E.J. Diseases of the Large Intestine. In Textbook of Veterinary Internal Medicine, 8th ed.; Ettinger, S.J., Feldman, E.C., Cote, E., Eds.; Elsevier: St. Louis, MO, USA, 2017; Volume 2, pp. 3842–3851. [Google Scholar]
- Kathrani, A. Dietary and Nutritional Approaches to the Management of Chronic Enteropathy in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 123–136. [Google Scholar] [CrossRef]
- Craven, M.D.; Washabau, R.J. Comparative Pathophysiology and Management of Protein-Losing Enteropathy. J. Vet. Intern. Med. 2019, 33, 383–402. [Google Scholar] [CrossRef]
- Wernimont, S.M.; Radosevich, J.; Jackson, M.I.; Ephraim, E.; Badri, D.V.; MacLeay, J.M.; Jewell, D.E.; Suchodolski, J.S. The Effects of Nutrition on the Gastrointestinal Microbiome of Cats and Dogs: Impact on Health and Disease. Front. Microbiol. 2020, 11, 1266. [Google Scholar] [CrossRef]
- Hooda, S.; Minamoto, Y.; Suchodolski, J.S.; Swanson, K.S. Current State of Knowledge: The Canine Gastrointestinal Microbiome. Anim. Health Res. Rev. 2012, 13, 78–88. [Google Scholar] [CrossRef]
- Huang, Z.; Pan, Z.; Yang, R.; Bi, Y.; Xiong, X. The Canine Gastrointestinal Microbiota: Early Studies and Research Frontiers. Gut Microbes 2020, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Middelbos, I.S.; Boler, B.M.V.; Qu, A.; White, B.A.; Swanson, K.S.; Fahey, G.C., Jr. Phylogenetic Characterization of Fecal Microbial Communities of Dogs Fed Diets with or without Supplemental Dietary Fiber Using 454 Pyrosequencing. PLoS ONE 2010, 5, e9768. [Google Scholar] [CrossRef] [Green Version]
- Panasevich, M.R.; Kerr, K.R.; Dilger, R.N.; Fahey, G.C.; Guérin-Deremaux, L.; Lynch, G.L.; Wils, D.; Suchodolski, J.S.; Steer, J.M.; Dowd, S.E.; et al. Modulation of the Faecal Microbiome of Healthy Adult Dogs by Inclusion of Potato Fibre in the Diet. Br. J. Nutr. 2015, 113, 125–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermingham, E.N.; Maclean, P.; Thomas, D.G.; Cave, N.J.; Young, W. Key Bacterial Families (Clostridiaceae, Erysipelotrichaceae and Bacteroidaceae) Are Related to the Digestion of Protein and Energy in Dogs. PeerJ 2017, 5, e3019. [Google Scholar] [CrossRef] [PubMed]
- Herstad, K.M.V.; Gajardo, K.; Bakke, A.M.; Moe, L.; Ludvigsen, J.; Rudi, K.; Rud, I.; Sekelja, M.; Skancke, E. A Diet Change from Dry Food to Beef Induces Reversible Changes on the Faecal Microbiota in Healthy, Adult Client-Owned Dogs. BMC Vet. Res. 2017, 13, 147. [Google Scholar] [CrossRef]
- Li, Q.; Lauber, C.L.; Czarnecki-Maulden, G.; Pan, Y.; Hannah, S.S. Effects of the Dietary Protein and Carbohydrate Ratio on Gut Microbiomes in Dogs of Different Body Conditions. mBio 2017, 8, e01703-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauf, S.; de la Fuente, G.; Newbold, C.J.; Salas-Mani, A.; Torre, C.; Abecia, L.; Castrillo, C. Effect of Dietary Fat to Starch Content on Fecal Microbiota Composition and Activity in Dogs. J. Anim. Sci. 2018, 96, 3684–3698. [Google Scholar] [CrossRef]
- Dolan, K.T.; Chang, E.B. Diet, Gut Microbes, and the Pathogenesis of Inflammatory Bowel Diseases. Mol. Nutr. Food Res. 2017, 61, 1600129. [Google Scholar] [CrossRef]
- Alessandri, G.; Milani, C.; Mancabelli, L.; Mangifesta, M.; Lugli, G.A.; Viappiani, A.; Duranti, S.; Turroni, F.; Ossiprandi, M.C.; van Sinderen, D.; et al. Metagenomic Dissection of the Canine Gut Microbiota: Insights into Taxonomic, Metabolic and Nutritional Features. Environ. Microbiol. 2019, 21, 1331–1343. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium Prausnitzii Is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilla, R.; Suchodolski, J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2020, 6, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Prebiotic Evaluation of Cocoa-Derived Flavanols in Healthy Humans by Using a Randomized, Controlled, Double-Blind, Crossover Intervention Study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Depauw, S.; Bosch, G.; Hesta, M.; Whitehouse-Tedd, K.; Hendriks, W.H.; Kaandorp, J.; Janssens, G.P.J. Fermentation of Animal Components in Strict Carnivores: A Comparative Study with Cheetah Fecal Inoculum. J. Anim. Sci. 2012, 90, 2540–2548. [Google Scholar] [CrossRef] [Green Version]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why Definitions Matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Biagi, G.; Cipollini, I.; Grandi, M.; Zaghini, G. Influence of Some Potential Prebiotics and Fibre-Rich Foodstuffs on Composition and Activity of Canine Intestinal Microbiota. Anim. Feed Sci. Technol. 2010, 159, 50–58. [Google Scholar] [CrossRef]
- Swanson, K.S.; Fahey, G.C. Prebiotic Impacts on Companion Animals. In Prebiotics: Development & Application; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 213–236. ISBN 978-0-470-02315-0. [Google Scholar]
- Valcheva, R.; Dieleman, L.A. Prebiotics: Definition and Protective Mechanisms. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 27–37. [Google Scholar] [CrossRef]
- Shoaf, K.; Mulvey, G.L.; Armstrong, G.D.; Hutkins, R.W. Prebiotic Galactooligosaccharides Reduce Adherence of Enteropathogenic Escherichia Coli to Tissue Culture Cells. Infect. Immun. 2006, 74, 6920–6928. [Google Scholar] [CrossRef] [Green Version]
- Froebel, L.K.; Froebel, L.E.; Duong, T. Refined Functional Carbohydrates Reduce Adhesion of Salmonella and Campylobacter to Poultry Epithelial Cells in Vitro. Poult. Sci. 2020, 99, 7027–7034. [Google Scholar] [CrossRef]
- Bavington, C.; Page, C. Stopping Bacterial Adhesion: A Novel Approach to Treating Infections. Respiration 2005, 72, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Garssen, J. Evidence-Based Benefits of Specific Mixtures of Non-Digestible Oligosaccharides on the Immune System. Carbohydr. Polym. 2013, 93, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.M.; Meyer, D.; Pullens, G.; Faas, M.M.; Venema, K.; Ramasamy, U.; Schols, H.A.; de Vos, P. Toll-like Receptor 2 Activation by Β2→1-Fructans Protects Barrier Function of T84 Human Intestinal Epithelial Cells in a Chain Length-Dependent Manner. J. Nutr. 2014, 144, 1002–1008. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Folkerts, J.; Folkerts, G.; Maurer, M.; Braber, S. Microbiota-Dependent and -Independent Effects of Dietary Fibre on Human Health. Br. J. Pharmacol. 2020, 177, 1363–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molis, C.; Flourié, B.; Ouarne, F.; Gailing, M.F.; Lartigue, S.; Guibert, A.; Bornet, F.; Galmiche, J.P. Digestion, Excretion, and Energy Value of Fructooligosaccharides in Healthy Humans. Am. J. Clin. Nutr. 1996, 64, 324–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obermeier, S.; Rudloff, S.; Pohlentz, G.; Lentze, M.J.; Kunz, C. Secretion of 13C-Labelled Oligosaccharides into Human Milk and Infant’s Urine after an Oral [13C]Galactose Load. Isot. Environ. Health Stud. 1999, 35, 119–125. [Google Scholar] [CrossRef]
- Patra, A.K. Responses of Feeding Prebiotics on Nutrient Digestibility, Faecal Microbiota Composition and Short-Chain Fatty Acid Concentrations in Dogs: A Meta-Analysis. Animal 2011, 5, 1743–1750. [Google Scholar] [CrossRef] [Green Version]
- Karr-Lilienthal, L.K.; Grieshop, C.M.; Spears, J.K.; Patil, A.R.; Czarnecki-Maulden, G.L.; Merchen, N.R.; Fahey, G.C. Estimation of the Proportion of Bacterial Nitrogen in Canine Feces Using Diaminopimelic Acid as an Internal Bacterial Marker. J. Anim. Sci. 2004, 82, 1707–1712. [Google Scholar] [CrossRef]
- Pinna, C.; Biagi, G. The Utilisation of Prebiotics and Synbiotics in Dogs. Ital. J. Anim. Sci. 2014, 13, 3107. [Google Scholar] [CrossRef]
- Guarner, F. Prebiotics in Inflammatory Bowel Diseases. Br. J. Nutr. 2007, 98, S85–S89. [Google Scholar] [CrossRef] [Green Version]
- Looijer–van Langen, M.A.C.; Dieleman, L.A. Prebiotics in Chronic Intestinal Inflammation. Inflamm. Bowel Dis. 2009, 15, 454–462. [Google Scholar] [CrossRef] [Green Version]
- Casellas, F.; Borruel, N.; Torrejón, A.; Varela, E.; Antolin, M.; Guarner, F.; Malagelada, J.-R. Oral Oligofructose-Enriched Inulin Supplementation in Acute Ulcerative Colitis Is Well Tolerated and Associated with Lowered Faecal Calprotectin. Aliment. Pharmacol. Ther. 2007, 25, 1061–1067. [Google Scholar] [CrossRef]
- Lindsay, J.O.; Whelan, K.; Stagg, A.J.; Gobin, P.; Al-Hassi, H.O.; Rayment, N.; Kamm, M.A.; Knight, S.C.; Forbes, A. Clinical, Microbiological, and Immunological Effects of Fructo-Oligosaccharide in Patients with Crohn’s Disease. Gut 2006, 55, 348–355. [Google Scholar] [CrossRef]
- Jia, J.; Frantz, N.; Khoo, C.; Gibson, G.R.; Rastall, R.A.; McCartney, A.L. Investigation of the Faecal Microbiota Associated with Canine Chronic Diarrhoea. FEMS Microbiol. Ecol. 2010, 71, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Segarra, S.; Martínez-Subiela, S.; Cerdà-Cuéllar, M.; Martínez-Puig, D.; Muñoz-Prieto, A.; Rodríguez-Franco, F.; Rodríguez-Bertos, A.; Allenspach, K.; Velasco, A.; Cerón, J. Oral Chondroitin Sulfate and Prebiotics for the Treatment of Canine Inflammatory Bowel Disease: A Randomized, Controlled Clinical Trial. BMC Vet. Res. 2016, 12, 49. [Google Scholar] [CrossRef] [Green Version]
- Isidori, M.; Rueca, F.; Massacci, F.R.; Diaferia, M.; Giontella, A.; Caldin, M.; Furlanello, T.; Corbee, R.J.; Mannucci, G.; Pezzotti, G.; et al. The Use of Ascophyllum Nodosum and Bacillus Subtilis C-3102 in the Management of Canine Chronic Inflammatory Enteropathy: A Pilot Study. Animals 2021, 11, 3417. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed Nutraceuticals and Their Therapeutic Role in Disease Prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Xiao, J.; Bai, W. Bioactive Phytochemicals. Crit. Rev. Food Sci. Nutr. 2019, 59, 827–829. [Google Scholar] [CrossRef] [Green Version]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [Green Version]
- Mazieiro, R.; Frizon, R.R.; Barbalho, S.M.; Goulart, R.d.A. Is Curcumin a Possibility to Treat Inflammatory Bowel Diseases? J. Med. Food 2018, 21, 1077–1085. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, B.B. Activation of Transcription Factor NF-Kappa B Is Suppressed by Curcumin (Diferuloylmethane). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef] [Green Version]
- Abe, Y.; Hashimoto, S.; Horie, T. Curcumin Inhibition of Inflammatory Cytokine Production by Human Peripheral Blood Monocytes and Alveolar Macrophages. Pharmacol. Res. 1999, 39, 41–47. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. Biomed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Saxena, A.; Kaur, K.; Hegde, S.; Kalekhan, F.M.; Baliga, M.S.; Fayad, R. Dietary Agents and Phytochemicals in the Prevention and Treatment of Experimental Ulcerative Colitis. J. Tradit. Complement. Med. 2014, 4, 203–217. [Google Scholar] [CrossRef] [Green Version]
- Suskind, D.L.; Wahbeh, G.; Burpee, T.; Cohen, M.; Christie, D.; Weber, W. Tolerability of Curcumin in Pediatric Inflammatory Bowel Disease: A Forced-Dose Titration Study. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 277–279. [Google Scholar] [CrossRef] [Green Version]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Biological Activities and Potential Industrial Applications of Fucose Rich Sulfated Polysaccharides and Fucoidans Isolated from Brown Seaweeds: A Review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [Green Version]
- Fitton, J.H. Therapies from Fucoidan; Multifunctional Marine Polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef]
- Zhang, X.W.; Liu, Q.; Thorlacius, H. Inhibition of Selectin Function and Leukocyte Rolling Protects against Dextran Sodium Sulfate-Induced Murine Colitis. Scand. J. Gastroenterol. 2001, 36, 270–275. [Google Scholar] [CrossRef]
- Iraha, A.; Chinen, H.; Hokama, A.; Yonashiro, T.; Kinjo, T.; Kishimoto, K.; Nakamoto, M.; Hirata, T.; Kinjo, N.; Higa, F.; et al. Fucoidan Enhances Intestinal Barrier Function by Upregulating the Expression of Claudin-1. World J. Gastroenterol. 2013, 19, 5500–5507. [Google Scholar] [CrossRef] [PubMed]
- Isidori, M.; Trabalza-Marinucci, M.; Rueca, F.; Cappelli, K.; Lepri, E.; Mecocci, S.; Scattini, G.; Pascucci, L. Ascophyllum nodosum-derived fucoidan modulates the intestinal expression of immune-inflammatory genes in a biopsy model of canine chronic enteropathy. In ASPA 24th Congress Book of Abstract. Ital. J. Anim. Sci. 2021, 20, 181. [Google Scholar] [CrossRef]
- Peritore, A.F.; Siracusa, R.; Crupi, R.; Cuzzocrea, S. Therapeutic Efficacy of Palmitoylethanolamide and Its New Formulations in Synergy with Different Antioxidant Molecules Present in Diets. Nutrients 2019, 11, 2175. [Google Scholar] [CrossRef] [Green Version]
- Chapman, K.D.; Venables, B.; Markovic, R.; Blair, R.W.; Bettinger, C. N-Acylethanolamines in Seeds. Quantification of Molecular Species and Their Degradation upon Imbibition. Plant Physiol. 1999, 120, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Re, G.; Barbero, R.; Miolo, A.; Di Marzo, V. Palmitoylethanolamide, Endocannabinoids and Related Cannabimimetic Compounds in Protection against Tissue Inflammation and Pain: Potential Use in Companion Animals. Vet. J. 2007, 173, 21–30. [Google Scholar] [CrossRef]
- Borrelli, F.; Romano, B.; Petrosino, S.; Pagano, E.; Capasso, R.; Coppola, D.; Battista, G.; Orlando, P.; Di Marzo, V.; Izzo, A.A. Palmitoylethanolamide, a Naturally Occurring Lipid, Is an Orally Effective Intestinal Anti-Inflammatory Agent. Br. J. Pharmacol. 2015, 172, 142–158. [Google Scholar] [CrossRef] [Green Version]
- Capasso, R.; Orlando, P.; Pagano, E.; Aveta, T.; Buono, L.; Borrelli, F.; Di Marzo, V.; Izzo, A.A. Palmitoylethanolamide Normalizes Intestinal Motility in a Model of Post-Inflammatory Accelerated Transit: Involvement of CB1 Receptors and TRPV1 Channels. Br. J. Pharmacol. 2014, 171, 4026–4037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, S.; Suchodolski, J. Understanding the Canine Intestinal Microbiota and Its Modification by Pro-, Pre- and Synbiotics—What Is the Evidence? Vet. Med. Sci. 2016, 2, 71–94. [Google Scholar] [CrossRef]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Kolida, S.; Gibson, G.R. Synbiotics in Health and Disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef] [Green Version]
- Marteau, P.; Seksik, P.; Lepage, P.; Doré, J. Cellular and Physiological Effects of Probiotics and Prebiotics. Mini-Rev. Med. Chem. 2004, 4, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Servin, A.L.; Coconnier, M.-H. Adhesion of Probiotic Strains to the Intestinal Mucosa and Interaction with Pathogens. Best Pract. Res. Clin. Gastroenterol. 2003, 17, 741–754. [Google Scholar] [CrossRef]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Edrington, T.S.; Anderson, R.C.; Harvey, R.B.; Genovese, K.J.; Kennedy, C.N.; Venn, D.W.; Nisbet, D.J. Probiotics, Prebiotics and Competitive Exclusion for Prophylaxis against Bacterial Disease. Anim. Health Res. Rev. 2008, 9, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Christensen, H.R.; Frøkiaer, H.; Pestka, J.J. Lactobacilli Differentially Modulate Expression of Cytokines and Maturation Surface Markers in Murine Dendritic Cells. J. Immunol. 2002, 168, 171–178. [Google Scholar] [CrossRef]
- Dogi, C.A.; Perdigón, G. Importance of the Host Specificity in the Selection of Probiotic Bacteria. J. Dairy Res. 2006, 73, 357–366. [Google Scholar] [CrossRef]
- Thomas, C.M.; Versalovic, J. Probiotics-Host Communication: Modulation of Signaling Pathways in the Intestine. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef]
- Biourge, V.; Vallet, C.; Levesque, A.; Sergheraert, R.; Chevalier, S.; Roberton, J.L. The Use of Probiotics in the Diet of Dogs. J. Nutr. 1998, 128, 2730S–2732S. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S.; Anderson, M.E.C. Preliminary Evaluation of Lactobacillus Rhamnosus Strain GG, a Potential Probiotic in Dogs. Can. Vet. J. 2002, 43, 771–774. [Google Scholar]
- Biagi, G.; Cipollini, I.; Pompei, A.; Zaghini, G.; Matteuzzi, D. Effect of a Lactobacillus Animalis Strain on Composition and Metabolism of the Intestinal Microflora in Adult Dogs. Vet. Microbiol. 2007, 124, 160–165. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex—32002R0178—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32002R0178 (accessed on 29 November 2021).
- EUR-Lex—02008R0429-20210327—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2008/429 (accessed on 29 November 2021).
- Anadón, A.; Martínez-Larrañaga, M.R.; Aranzazu Martínez, M. Probiotics for Animal Nutrition in the European Union. Regulation and Safety Assessment. Regul. Toxicol. Pharmacol. 2006, 45, 91–95. [Google Scholar] [CrossRef]
- Grześkowiak, Ł.; Endo, A.; Beasley, S.; Salminen, S. Microbiota and Probiotics in Canine and Feline Welfare. Anaerobe 2015, 34, 14–23. [Google Scholar] [CrossRef]
- Grześkowiak, Ł.; Endo, A.; Collado, M.C.; Pelliniemi, L.J.; Beasley, S.; Salminen, S. The Effect of Growth Media and Physical Treatments on the Adhesion Properties of Canine Probiotics. J. Appl. Microbiol. 2013, 115, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Grześkowiak, Ł.; Collado, M.C.; Beasley, S.; Salminen, S. Pathogen Exclusion Properties of Canine Probiotics Are Influenced by the Growth Media and Physical Treatments Simulating Industrial Processes. J. Appl. Microbiol. 2014, 116, 1308–1314. [Google Scholar] [CrossRef]
- Lahtinen, S.J. Probiotic Viability—Does It Matter? Microb. Ecol. Health Dis. 2012, 23, 18567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weese, J.S.; Martin, H. Assessment of Commercial Probiotic Bacterial Contents and Label Accuracy. Can. Vet. J. 2011, 52, 43–46. [Google Scholar]
- Jugan, M.C.; Rudinsky, A.J.; Parker, V.J.; Gilor, C. Use of Probiotics in Small Animal Veterinary Medicine. J. Am. Vet. Med. Assoc. 2017, 250, 519–528. [Google Scholar] [CrossRef]
- Sauter, S.N.; Allenspach, K.; Gaschen, F.; Gröne, A.; Ontsouka, E.; Blum, J.W. Cytokine Expression in an Ex Vivo Culture System of Duodenal Samples from Dogs with Chronic Enteropathies: Modulation by Probiotic Bacteria. Domest. Anim. Endocrinol. 2005, 29, 605–622. [Google Scholar] [CrossRef]
- Schmitz, S.; Henrich, M.; Neiger, R.; Werling, D.; Allenspach, K. Stimulation of Duodenal Biopsies and Whole Blood from Dogs with Food-Responsive Chronic Enteropathy and Healthy Dogs with Toll-like Receptor Ligands and Probiotic Enterococcus Faecium. Scand. J. Immunol. 2014, 80, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, S.; Werling, D.; Allenspach, K. Effects of Ex-Vivo and In-Vivo Treatment with Probiotics on the Inflammasome in Dogs with Chronic Enteropathy. PLoS ONE 2015, 10, e0120779. [Google Scholar] [CrossRef]
- Sauter, S.N.; Benyacoub, J.; Allenspach, K.; Gaschen, F.; Ontsouka, E.; Reuteler, G.; Cavadini, C.; Knorr, R.; Blum, J.W. Effects of Probiotic Bacteria in Dogs with Food Responsive Diarrhoea Treated with an Elimination Diet. J. Anim. Physiol. Anim. Nutr. 2006, 90, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, S.; Glanemann, B.; Garden, O.A.; Brooks, H.; Chang, Y.M.; Werling, D.; Allenspach, K. A Prospective, Randomized, Blinded, Placebo-Controlled Pilot Study on the Effect of Enterococcus Faecium on Clinical Activity and Intestinal Gene Expression in Canine Food-Responsive Chronic Enteropathy. J. Vet. Intern. Med. 2015, 29, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Guard, B.C.; Steiner, J.M.; Gaschen, F.P.; Olson, E.; Werling, D.; Allenspach, K.; Salavati Schmitz, S.; Suchodolski, J.S. Administration of a Synbiotic Containing Enterococcus Faecium Does Not Significantly Alter Fecal Microbiota Richness or Diversity in Dogs with and without Food-Responsive Chronic Enteropathy. Front. Vet. Sci. 2019, 6, 277. [Google Scholar] [CrossRef] [Green Version]
- Rossi, G.; Pengo, G.; Caldin, M.; Palumbo Piccionello, A.; Steiner, J.M.; Cohen, N.D.; Jergens, A.E.; Suchodolski, J.S. Comparison of Microbiological, Histological, and Immunomodulatory Parameters in Response to Treatment with Either Combination Therapy with Prednisone and Metronidazole or Probiotic VSL#3 Strains in Dogs with Idiopathic Inflammatory Bowel Disease. PLoS ONE 2014, 9, e94699. [Google Scholar] [CrossRef]
- White, R.; Atherly, T.; Guard, B.; Rossi, G.; Wang, C.; Mosher, C.; Webb, C.; Hill, S.; Ackermann, M.; Sciabarra, P.; et al. Randomized, Controlled Trial Evaluating the Effect of Multi-Strain Probiotic on the Mucosal Microbiota in Canine Idiopathic Inflammatory Bowel Disease. Gut Microbes 2017, 8, 451–466. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, S.; Fracassi, F.; Bresciani, F.; Galuppi, R.; Diana, A.; Linta, N.; Bettini, G.; Morini, M.; Pietra, M. Effect of Saccharomyces Boulardii in Dog with Chronic Enteropathies: Double-Blinded, Placebo-Controlled Study. Vet. Rec. 2018, 182, 258. [Google Scholar] [CrossRef]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal Microbiota Transplantation: In Perspective. Ther. Adv. Gastroenterol. 2016, 9, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Borody, T.J.; Warren, E.F.; Leis, S.M.; Surace, R.; Ashman, O.; Siarakas, S. Bacteriotherapy Using Fecal Flora: Toying with Human Motions. J. Clin. Gastroenterol. 2004, 38, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Kassam, Z.; Lee, C.H.; Yuan, Y.; Hunt, R.H. Fecal Microbiota Transplantation for Clostridium Difficile Infection: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2013, 108, 500–508. [Google Scholar] [CrossRef]
- Quraishi, M.N.; Widlak, M.; Bhala, N.; Moore, D.; Price, M.; Sharma, N.; Iqbal, T.H. Systematic Review with Meta-Analysis: The Efficacy of Faecal Microbiota Transplantation for the Treatment of Recurrent and Refractory Clostridium Difficile Infection. Aliment. Pharmacol. Ther. 2017, 46, 479–493. [Google Scholar] [CrossRef] [Green Version]
- Honneffer, J.B.; Minamoto, Y.; Suchodolski, J.S. Microbiota Alterations in Acute and Chronic Gastrointestinal Inflammation of Cats and Dogs. World J. Gastroenterol. 2014, 20, 16489–16497. [Google Scholar] [CrossRef]
- Craig, J.M. Atopic Dermatitis and the Intestinal Microbiota in Humans and Dogs. Vet. Med. Sci. 2016, 2, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, H.; Huang, H.; Li, Y.; Chen, H.; He, J.; Du, Y.; Chen, Y.; Zhou, Y.; Nie, Y. Are There Potential Applications of Fecal Microbiota Transplantation beyond Intestinal Disorders? BioMed Res. Int. 2019, 2019, 3469754. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zou, Z.; Bian, X.; Huang, Y.; Wang, Y.; Yang, C.; Zhao, J.; Xie, L. Fecal Microbiota Transplantation Research Output from 2004 to 2017: A Bibliometric Analysis. PeerJ 2019, 7, e6411. [Google Scholar] [CrossRef] [PubMed]
- Khoruts, A.; Sadowsky, M.J. Understanding the Mechanisms of Faecal Microbiota Transplantation. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 508–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaitman, J.; Gaschen, F. Fecal Microbiota Transplantation in Dogs. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 219–233. [Google Scholar] [CrossRef] [PubMed]
- DuPont, H.L.; Jiang, Z.-D.; DuPont, A.W.; Utay, N.S. Abnormal Intestinal Microbiome in Medical Disorders and Potential Reversibility by Fecal Microbiota Transplantation. Dig. Dis. Sci. 2020, 65, 741–756. [Google Scholar] [CrossRef] [Green Version]
- Burz, S.D.; Abraham, A.-L.; Fonseca, F.; David, O.; Chapron, A.; Béguet-Crespel, F.; Cénard, S.; Le Roux, K.; Patrascu, O.; Levenez, F.; et al. A Guide for Ex Vivo Handling and Storage of Stool Samples Intended for Fecal Microbiota Transplantation. Sci. Rep. 2019, 9, 8897. [Google Scholar] [CrossRef]
- Papanicolas, L.E.; Choo, J.M.; Wang, Y.; Leong, L.E.X.; Costello, S.P.; Gordon, D.L.; Wesselingh, S.L.; Rogers, G.B. Bacterial Viability in Faecal Transplants: Which Bacteria Survive? EBioMedicine 2019, 41, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Bojanova, D.P.; Bordenstein, S.R. Fecal Transplants: What Is Being Transferred? PLoS Biol. 2016, 14, e1002503. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, J.; Liao, M.; Li, W.; Zou, J.; Han, X.; Kuang, M.; Shen, W.; Li, H. Beneficial Effects of Fecal Microbiota Transplantation on Ulcerative Colitis in Mice. Dig. Dis. Sci. 2016, 61, 2262–2271. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, Z.; Ji, P.; Ma, M.; Guo, J.; Jiang, S. Effect of Fecal Microbiota Transplantation on Experimental Colitis in Mice. Exp. Ther. Med. 2019, 17, 2581–2586. [Google Scholar] [CrossRef]
- Elinav, E.; Strowig, T.; Kau, A.L.; Henao-Mejia, J.; Thaiss, C.A.; Booth, C.J.; Peaper, D.R.; Bertin, J.; Eisenbarth, S.C.; Gordon, J.I.; et al. NLRP6 Inflammasome Is a Regulator of Colonic Microbial Ecology and Risk for Colitis. Cell 2011, 145, 745–757. [Google Scholar] [CrossRef] [Green Version]
- Niederwerder, M.C. Fecal Microbiota Transplantation as a Tool to Treat and Reduce Susceptibility to Disease in Animals. Vet. Immunol. Immunopathol. 2018, 206, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chaitman, J.; Ziese, A.-L.; Pilla, R.; Minamoto, Y.; Blake, A.B.; Guard, B.C.; Isaiah, A.; Lidbury, J.A.; Steiner, J.M.; Unterer, S.; et al. Fecal Microbial and Metabolic Profiles in Dogs With Acute Diarrhea Receiving Either Fecal Microbiota Transplantation or Oral Metronidazole. Front. Vet. Sci. 2020, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.; Barko, P.C.; Biggs, P.J.; Gedye, K.R.; Midwinter, A.C.; Williams, D.A.; Burchell, R.K.; Pazzi, P. One Dog’s Waste Is Another Dog’s Wealth: A Pilot Study of Fecal Microbiota Transplantation in Dogs with Acute Hemorrhagic Diarrhea Syndrome. PLoS ONE 2021, 16, e0250344. [Google Scholar] [CrossRef] [PubMed]
- Bottero, E.; Benvenuti, E.; Ruggiero, P. Fecal microbiota transplantation (FMT) in 16 dogs with idiopatic IBD. Veterinaria 2017, 31, 31–45. [Google Scholar]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Improvement in Clinical Symptoms and Fecal Microbiome After Fecal Microbiota Transplantation in a Dog with Inflammatory Bowel Disease. Vet. Med. 2019, 10, 197–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berlanda, M.; Innocente, G.; Simionati, B.; Di Camillo, B.; Facchin, S.; Giron, M.C.; Savarino, E.; Sebastiani, F.; Fiorio, F.; Patuzzi, I. Faecal Microbiome Transplantation as a Solution to Chronic Enteropathies in Dogs: A Case Study of Beneficial Microbial Evolution. Animals 2021, 11, 1433. [Google Scholar] [CrossRef]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Fecal Microbiota Transplantation as a New Treatment for Canine Inflammatory Bowel Disease. Biosci. Microbiota Food Health 2021, 40, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Golchin, A.; Farahany, T.Z. Biological Products: Cellular Therapy and FDA Approved Products. Stem Cell Rev. Rep. 2019, 15, 166–175. [Google Scholar] [CrossRef]
- Kang, M.-H.; Park, H.-M. Challenges of Stem Cell Therapies in Companion Animal Practice. J. Vet. Sci. 2020, 21, e42. [Google Scholar] [CrossRef] [PubMed]
- Gugjoo, M.B.; Amarpal, A.; Sharma, G.T. Mesenchymal Stem Cell Basic Research and Applications in Dog Medicine. J. Cell. Physiol. 2019, 234, 16779–16811. [Google Scholar] [CrossRef]
- Hackett, C.H. Assessing the Function of Mesenchymal Stromal Cells: All That Glitters Is Not Gold. Vet. J. 2013, 195, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.; Patel, T. The Mesenchymal Stem Cell Secretome as an Acellular Regenerative Therapy for Liver Disease. J. Gastroenterol. 2019, 54, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, A.M.; Dow, S.W. Concise Review: Stem Cell Trials Using Companion Animal Disease Models. Stem Cells 2016, 34, 1709–1729. [Google Scholar] [CrossRef]
- Dias, I.E.; Pinto, P.O.; Barros, L.C.; Viegas, C.A.; Dias, I.R.; Carvalho, P.P. Mesenchymal Stem Cells Therapy in Companion Animals: Useful for Immune-Mediated Diseases? BMC Vet. Res. 2019, 15, 358. [Google Scholar] [CrossRef] [Green Version]
- Song, W.-J.; Li, Q.; Ryu, M.-O.; Ahn, J.-O.; Bhang, D.H.; Jung, Y.C.; Youn, H.-Y. TSG-6 Released from Intraperitoneally Injected Canine Adipose Tissue-Derived Mesenchymal Stem Cells Ameliorate Inflammatory Bowel Disease by Inducing M2 Macrophage Switch in Mice. Stem Cell Res. Ther. 2018, 9, 91. [Google Scholar] [CrossRef]
- Song, W.-J.; Li, Q.; Ryu, M.-O.; Nam, A.; An, J.-H.; Jung, Y.C.; Ahn, J.-O.; Youn, H.-Y. Canine Adipose Tissue-Derived Mesenchymal Stem Cells Pre-Treated with TNF-Alpha Enhance Immunomodulatory Effects in Inflammatory Bowel Disease in Mice. Res. Vet. Sci. 2019, 125, 176–184. [Google Scholar] [CrossRef]
- An, J.-H.; Li, Q.; Bhang, D.-H.; Song, W.-J.; Youn, H.-Y. TNF-α and INF-γ Primed Canine Stem Cell-Derived Extracellular Vesicles Alleviate Experimental Murine Colitis. Sci. Rep. 2020, 10, 2115. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Merino, E.M.; Usón-Casaús, J.M.; Duque-Carrasco, J.; Zaragoza-Bayle, C.; Mariñas-Pardo, L.; Hermida-Prieto, M.; Vilafranca-Compte, M.; Barrera-Chacón, R.; Gualtieri, M. Safety and Efficacy of Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells for Treatment of Dogs with Inflammatory Bowel Disease: Endoscopic and Histological Outcomes. Vet. J. 2015, 206, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Merino, E.M.; Usón-Casaús, J.M.; Zaragoza-Bayle, C.; Duque-Carrasco, J.; Mariñas-Pardo, L.; Hermida-Prieto, M.; Barrera-Chacón, R.; Gualtieri, M. Safety and Efficacy of Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells for Treatment of Dogs with Inflammatory Bowel Disease: Clinical and Laboratory Outcomes. Vet. J. 2015, 206, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, J.I.; Duque, F.J.; Usón-Casaús, J.M.; Ruiz, P.; Nieto, E.L.; Pérez-Merino, E.M. Effects of Allogeneic Mesenchymal Stem Cell Transplantation in Dogs with Inflammatory Bowel Disease Treated with and without Corticosteroids. Animals 2021, 11, 2061. [Google Scholar] [CrossRef]

| Reference | Inclusion Diagnosis | Experimental Setting * | Probiotic Strain(s)/Treatment | Probiotic Dosage | Time § | Main Outcomes |
|---|---|---|---|---|---|---|
| Sauter et al. (2005) [206] | CIE | Ex vivo study | L. acidophilus NCC 2628, L. acidophilus NCC 2766, L. johnsonii NCC 2767 | 1 × 107 CFU/mL of medium | 36 h | Increased IL-10 mRNA and protein expression; decreased ratio of TNF-α/IL-10, IFN-γ/IL-10 and IL-12p40/IL-10 mRNA levels. |
| Schmitz et al. (2014) [207] | FRE | Ex vivo study | E. faecium NCIMB 10415 | 1 × 107 CFU/mL of medium | 5 h | Increased TNF-α protein expression from whole blood in both groups. TNF-α protein responses opposite in blood and biopsies. |
| Schmitz et al. (2015b) [208] | CIE | Ex vivo study | E. faecium NCIMB 10415 | 1 × 107 CFU/mL of medium | 5 h | No effect on NLRP3, casp-1, IL-1β and IL-18 gene and protein expression. |
| FRE | In vivo placebo-controlled randomised trial | E. faecium NCIMB 10415 + FOSs + gum Arabic + hydrolysed protein diet | 1 × 109 CFU/dog/day | 42 days | ||
| Sauter et al. (2006) [209] | FRE | In vivo placebo-controlled randomised trial | L. acidophilus NCC 2628, L. acidophilus NCC 2766, L. johnsonii NCC 2767 + novel protein diet | 1 × 1010 CFU/dog/day (of each strain) | 28 days | Decreased duodenal IL-10 and increased colonic IFN-γ mRNA expression; † increased numbers of Lactobacillus spp.; † detection of L. johnsonii NCC 2767 in 5 of 8 dogs after probiotic supplementation; no significant differences in clinical response between groups. |
| Schmitz et al. (2015a) [210] | FRE | In vivo placebo-controlled randomised trial | E. faecium NCIMB 10415 + FOSs + gum Arabic + hydrolysed protein diet | 1 × 109 CFU/dog/day | 42 days | No significant differences in clinical efficacy and histology score between groups. No effect on TLR-2, -4, -5, -9; IL-17A; IL-22; IL-23p19; RORC; IL-2; IL-12p35; TNF-α; IL-4; IFN-γ; IL-10; TGF β; IL-1β; IL-18; NLRP3; casp-1; TFF1; TFF3 and PPAR-γ mRNA expression. |
| Pilla et al. (2019) [211] | FRE | In vivo placebo-controlled randomised trial | E. faecium NCIMB 10415 + FOSs + gum Arabic + hydrolysed protein diet | 1 × 109 CFU/dog/day | 42 days | Small increase in faecal species diversity; no significant differences in microbial community composition between groups. |
| Westermarck et al. (2005) [8] | ARE | In vivo uncontrolled study | L. rhamnosus ATCC 53103 | 1 × 1010 CFU/dog/day | ≤30 days | Failure to avoid recurrence of diarrhoea in 9 of 9 dogs. |
| Isidori et al. (2021) [162] | ARE + IRE | In vivo uncontrolled study | B. subtilis DSM 15544 | 125 × 109 CFU/10 kg BW/day | 30 days | No significant differences in clinical outcome between pre- and post-treatment. Increased faecal concentrations of butyric acid. † |
| Rossi et al. (2014) [212] | IRE | In vivo comparative randomised trial | L. plantarum DSM 24730, S. thermophiles DSM 24731, B. breve DSM 24732, L. paracasei DSM 24733, L. delbrueckii subsp. bulgaricus DSM 24734, L. acidophilus DSM 24735, B. longum DSM 24736, B. infantis DSM 24737 | 112–225 × 109 CFU/10 kg BW/day | 60 days | Decreased clinical and histological scores and reduced proinflammatory CD3+ T-cell infiltration in both study groups; increased FoxP3+ immunosuppressive cells and relative abundance of genus Faecalibacterium. |
| White et al. (2017) [213] | IRE | In vivo placebo-controlled randomised trial | L. plantarum DSM 24730, S. thermophiles DSM 24731, B. breve DSM 24732, L. paracasei DSM 24733, L. delbrueckii subsp. bulgaricus DSM 24734, L. acidophilus DSM 24735, B. longum DSM 24736, B. infantis DSM 24737 + prednisone + elimination diet | 112–225 × 109 CFU/10 kg BW/day | 56 days | Increased E-cadherin, occludin and zonulin protein expression. |
| D’Angelo et al. (2018) [214] | IRE | In vivo placebo-controlled nonrandomised trial | S. boulardii + dietary therapy + antibiotics + steroids ± immunosuppressors | 1 × 109 CFU/kg BW/twice a day | 60 days | Lower clinical activity index, stool frequency, stool consistency; higher body condition score. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isidori, M.; Corbee, R.J.; Trabalza-Marinucci, M. Nonpharmacological Treatment Strategies for the Management of Canine Chronic Inflammatory Enteropathy—A Narrative Review. Vet. Sci. 2022, 9, 37. https://doi.org/10.3390/vetsci9020037
Isidori M, Corbee RJ, Trabalza-Marinucci M. Nonpharmacological Treatment Strategies for the Management of Canine Chronic Inflammatory Enteropathy—A Narrative Review. Veterinary Sciences. 2022; 9(2):37. https://doi.org/10.3390/vetsci9020037
Chicago/Turabian StyleIsidori, Marco, Ronald Jan Corbee, and Massimo Trabalza-Marinucci. 2022. "Nonpharmacological Treatment Strategies for the Management of Canine Chronic Inflammatory Enteropathy—A Narrative Review" Veterinary Sciences 9, no. 2: 37. https://doi.org/10.3390/vetsci9020037