Antimicrobial and Antibiofilm Effects of Peptides from Venom of Social Wasp and Scorpion on Multidrug-Resistant Acinetobacter baumannii
Abstract
:1. Introduction
2. Results
2.1. Biofilm Formation by A. baumannii Clinical Isolates
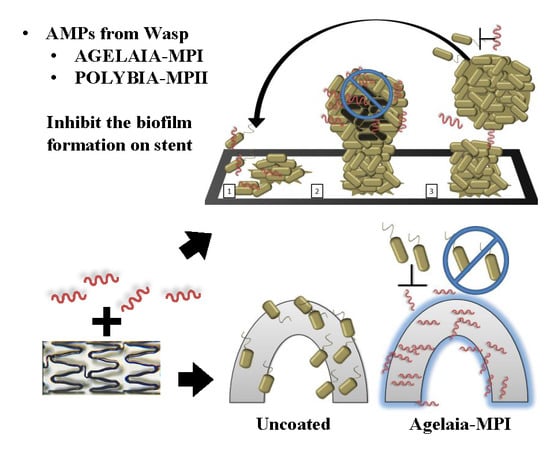
2.2. Adhesion and Early Formation of Biofilm on Cobalt–Chromium Vascular Stent
2.3. Determination of Minimum Inhibitory Concentration of Antimicrobial Peptides against Isolates of A. baumannii
2.4. Impact of Antimicrobial Peptides on Bacterial Biofilm Formation
2.5. Effect of the Agelaia-MPI and Polybia-MPII Peptides on Mature Biofilm and on the Dispersion of Adherent Cells
2.6. SEM Analysis of the Activity of the Agelaia-MPI and Polybia-MPII Wasp Peptides against AB 72 Isolate Biofilm adhered to the Vascular Stent
2.7. Inhibition of Bacterial adherence on the Cobalt–hromium Stent Coated with PEG Mixed with Agelaia-MPI
2.8. Effect of Antimicrobial Peptides on Staphylococcus Biofilm Formation
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacterial Strains and Growth Conditions
5.2. Analysis of Bacterial Biofilm Formation by A. baumannii Isolates by Colorimetric Dyes in 96-Well Polystyrene Culture Plates
5.3. Adhesion of A. baumannii to Abiotic Surfaces
5.4. Peptides and Computational Analysis
5.5. Determination of Minimum Inhibitory Concentration (MIC)
5.6. Determination of the Minimal Inhibitory Concentration for Biofilm Formation
5.7. Analysis of the Removal of Mature Bacterial Biofilm by Antimicrobial Peptides
5.8. Inhibition of the Dispersion of Bacteria from the Biofilm by the Antimicrobial Peptides
5.9. Stent Biofilm Formation Inhibition by Agelaia-MPI Complexed with Polyethylene Glycol (PEG)
5.10. Analysis of Bacterial Biofilm Formation of Staphylococcus Strains by Colorimetry
5.11. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Minardi, D.; Ghiselli, R.; Cirioni, O.; Giacometti, A.; Kamysz, W.; Orlando, F.; Silvestri, C.; Parri, G.; Kamysz, E.; Scalise, G.; et al. The antimicrobial peptide Tachyplesin III coated alone and in combination with intraperitoneal piperacillin-tazobactam prevents ureteral stent Pseudomonas infection in a rat subcutaneous pouch model. Peptides 2007, 28, 2293–2298. [Google Scholar] [CrossRef] [PubMed]
- De Breij, A.; Riool, M.; Kwakman, P.H.S.; de Boer, L.; Cordfunke, R.A.; Drijfhout, J.W.; Cohen, O.; Emanuel, N.; Zaat, S.A.J.; Nibbering, P.H.; et al. Prevention of Staphylococcus aureus biomaterial-associated infections using a polymer-lipid coating containing the antimicrobial peptide OP-145. J. Control. Release 2016, 222, 1–8. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Bosman, W.M.P.F.; Borger van der Burg, B.L.S.; Schuttevaer, H.M.; Thoma, S.; Hedeman Joosten, P.P. Infections of intravascular bare metal stents: A case report and review of literature. Eur. J. Vasc. Endovasc. Surg. 2014, 47, 87–99. [Google Scholar] [CrossRef]
- Dalal, J.J.; Digrajkar, A.; Hastak, M.; Mulay, A.; Lad, V.; Wani, S. Coronary stent infection—A grave, avoidable complication. IHJ Cardiovasc. Case Rep. 2017, 1, 77–79. [Google Scholar] [CrossRef]
- Reddy, K.V.C.; Sanzgiri, P.; Thanki, F.; Suratkal, V. Coronary stent infection: Interesting cases with varied presentation. J. Cardiol. Cases 2018, 19, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Elieson, M.; Mixon, T.; Carpenter, J. Coronary stent infections. Tex. Heart Inst. J. 2012, 39, 884–889. [Google Scholar]
- Whitcher, G.H.; Bertges, D.J.; Shukla, M. Peripheral vascular stent infection: Case report and review of literature. Ann. Vasc. Surg. 2018, 51, 326.e9–326.e15. [Google Scholar] [CrossRef]
- Desai, J.A.; Husain, S.F.; Islam, O.; Jin, A.Y. Carotid artery stent infection with Streptococcus agalactiae. Neurology 2010, 74, 344. [Google Scholar] [CrossRef]
- Sekhar, S.; Vupputuri, A.; Nair, R.C.; Palaniswamy, S.S.; Natarajan, K.U. Coronary stent infection successfully diagnosed using 18F-flurodeoxyglucose positron emission tomography computed tomography. Can. J. Cardiol. 2016, 32, 1575.e1–1575.e3. [Google Scholar] [CrossRef]
- Sudhakar, B.G.K. Pseudomonas aeruginosa septicemia resulting in coronary stent infection and coronary artery aneurysm and acute infective endocarditis of mitral valve causing severe mitral regurgitation—A case report. IHJ Cardiovasc. Case Rep. 2018, 2, 191–195. [Google Scholar] [CrossRef]
- Raychaudhuri, R.; Yu, W.; Hatanpaa, K.; Cavuoti, D.; Pride, G.L.; White, J. Basilar artery dissection treated by Neuroform stenting: Fungal stent infection. Surg. Neurol. 2009, 71, 477–480. [Google Scholar] [CrossRef]
- Soman, R.; Gupta, N.; Suthar, M.; Sunavala, A.; Shetty, A.; Rodrigues, C. Intravascular stent-related endocarditis due to rapidly growing mycobacteria: A new problem in the developing world. J. Assoc. Phys. India 2015, 63, 18–21. [Google Scholar]
- Wu, X.; Yin, T.; Tian, J.; Tang, C.; Huang, J.; Zhao, Y.; Zhang, X.; Deng, X.; Fan, Y.; Yu, D.; et al. Distinctive effects of CD34- and CD133-specific antibody-coated stents on re-endothelialization and in-stent restenosis at the early phase of vascular injury. Regen. Biomater. 2015, 2, 87–96. [Google Scholar] [CrossRef] [Green Version]
- DeCunha, J.; Janicki, C.; Enger, S.A. A retrospective analysis of catheter-based sources in intravascular brachytherapy. Brachytherapy 2017, 16, 586–596. [Google Scholar] [CrossRef]
- Abhyankar, A.D.; Thakkar, A.S. In vivo assessment of stent recoil of biodegradable polymer-coated cobalt–chromium sirolimus-eluting coronary stent system. Indian Heart J. 2012, 64, 541–546. [Google Scholar] [CrossRef]
- Buccheri, D.; Orrego, P.S.; Cortese, B. Drug-eluting stent treatment of left main coronary artery disease: The case for a sirolimus-eluting, autoexpandable alternative. An optical coherence tomography analysis. Int. J. Cardiol. 2015, 199, 119–120. [Google Scholar] [CrossRef]
- Ito, S.; Saeki, T. Coronary angioscopic imaging of in-stent restenosis after biolimus-eluting coronary stent implantation. J. Cardiol. Cases 2015, 12, 145–149. [Google Scholar] [CrossRef] [Green Version]
- Li, C.H.; Gao, B.L.; Wang, J.W.; Liu, J.F.; Li, H.; Yang, S.T.; Ren, C.F. Endovascular stent deployment in the management of lesions related to internal carotid artery redundancy. World Neurosurg. 2018, 116, e903–e912. [Google Scholar] [CrossRef]
- Omar, A.; Pendyala, L.K.; Ormiston, J.A.; Waksman, R. Review: Stent fracture in the drug-eluting stent era. Cardiovasc. Revasc. Med. 2016, 17, 404–411. [Google Scholar] [CrossRef]
- Zumstein, V.; Betschart, P.; Albrich, W.C.; Buhmann, M.T.; Ren, Q.; Schmid, H.P.; Abt, D. Biofilm formation on ureteral stents—Incidence, clinical impact and prevention. Swiss Med. Wkly. 2017, 147. [Google Scholar] [CrossRef]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Elbadawi, A.; Saad, M.; Elgendy, I.Y.; Zafar, A.; Chow, M.-Y. Multiple myocardial abscesses secondary to late stent infection. Cardiovasc. Pathol. 2017, 28, 1–2. [Google Scholar] [CrossRef]
- Babapour, E.; Haddadi, A.; Mirnejad, R.; Angaji, S.-A.; Amirmozafari, N. Biofilm formation in clinical isolates of nosocomial Acinetobacter baumannii and its relationship with multidrug resistance. Asian Pac. J. Trop. Biomed. 2016, 6, 528–533. [Google Scholar] [CrossRef] [Green Version]
- Longo, F.; Vuotto, C.; Donelli, G. Biofilm formation in Acinetobacter baumannii. New Microbiol. 2014, 37, 119–127. [Google Scholar]
- Liu, C.P.; Shih, S.C.; Wang, N.Y.; Wu, A.Y.; Sun, F.J.; Chow, S.F.; Chen, T.L.; Yan, T.R. Risk factors of mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteremia. J. Microbiol. Immunol. Infect. 2016, 49, 934–940. [Google Scholar] [CrossRef] [Green Version]
- Almasaudi, S.B. Acinetobacter spp. as nosocomial pathogens: Epidemiology and resistance features. Saudi J. Biol. Sci. 2018, 25, 586–596. [Google Scholar] [CrossRef]
- Deiham, B.; Douraghi, M.; Adibhesami, H.; Yaseri, M.; Rahbar, M. Screening of mutator phenotype in clinical strains of Acinetobacter baumannii. Microb. Pathog. 2017, 104, 175–179. [Google Scholar] [CrossRef]
- Djeribi, R.; Bouchloukh, W.; Jouenne, T.; Menaa, B. Characterization of bacterial biofilms formed on urinary catheters. Am. J. Infect. Control 2012, 40, 854–859. [Google Scholar] [CrossRef]
- Cobrado, L.; Silva-Dias, A.; Azevedo, M.M.; Pina-Vaz, C.; Rodrigues, A.G. In vivo antibiofilm effect of cerium, chitosan and hamamelitannin against usual agents of catheter-related bloodstream infections. J. Antimicrob. Chemother. 2013, 68, 126–130. [Google Scholar] [CrossRef]
- Silva, J.C.; Neto, L.M.; Neves, R.C.; Gonçalves, J.C.; Trentini, M.M.; Mucury-Filho, R.; Smidt, K.S.; Fensterseifer, I.C.; Silva, O.N.; Lima, L.D.; et al. Evaluation of the antimicrobial activity of the mastoparan Polybia-MPII isolated from venom of the social wasp Pseudopolybia vespiceps testacea (Vespidae, Hymenoptera). Int. J. Antimicrob. Agents 2017, 49, 167–175. [Google Scholar] [CrossRef]
- Pluzhnikov, K.A.; Kozlov, S.A.; Vassilevski, A.A.; Vorontsova, O.V.; Feofanov, A.V.; Grishin, E.V. Linear antimicrobial peptides from Ectatomma quadridens ant venom. Biochimie 2014, 107, 211–215. [Google Scholar] [CrossRef]
- Perumal Samy, R.; Stiles, B.G.; Franco, O.L.; Sethi, G.; Lim, L.H.K. Animal venoms as antimicrobial agents. Biochem. Pharmacol. 2017, 134, 127–138. [Google Scholar] [CrossRef]
- Garcia, F.; Villegas, E.; Espino-Solis, G.P.; Rodriguez, A.; Paniagua-Solis, J.F.; Sandoval-Lopez, G.; Possani, L.D.; Corzo, G. Antimicrobial peptides from arachnid venoms and their microbicidal activity in the presence of commercial antibiotics. J. Antibiot. 2013, 66, 3–10. [Google Scholar] [CrossRef]
- Harrison, P.L.; Abdel-Rahman, M.A.; Miller, K.; Strong, P.N. Antimicrobial peptides from scorpion venoms. Toxicon 2014, 88, 115–137. [Google Scholar] [CrossRef]
- Souza, B.M.D.; Cabrera, M.P.D.S.; Gomes, P.C.; Dias, N.B.; Stabeli, R.G.; Leite, N.B.; Neto, J.R.; Palma, M.S. Structure-activity relationship of mastoparan analogs: Effects of the number and positioning of Lys residues on secondary structure, interaction with membrane-mimetic systems and biological activity. Peptides 2015, 72, 164–174. [Google Scholar] [CrossRef]
- Wang, K.; Yan, J.; Dang, W.; Xie, J.; Yan, B.; Yan, W.; Sun, M.; Zhang, B.; Ma, M.; Zhao, Y.; et al. Dual antifungal properties of cationic antimicrobial peptides polybia-MPI: Membrane integrity disruption and inhibition of biofilm formation. Peptides 2014, 56, 22–29. [Google Scholar] [CrossRef]
- Das Neves, R.C.; Trentini, M.M.; de Castro e Silva, J.; Simon, K.S.; Bocca, A.L.; Silva, L.P.; Mortari, M.R.; Kipnis, A.; Junqueira-Kipnis, A.P. Antimycobacterial activity of a new peptide polydim-I isolated from neotropical social Wasp Polybia dimorpha. PLoS ONE 2016, 11, e0149729. [Google Scholar] [CrossRef]
- Moerman, L.; Bosteels, S.; Noppe, W.; Willems, J.; Clynen, E.; Schoofs, L.; Thevissen, K.; Tytgat, J.; Van Eldere, J.; Van Der Walt, J.; et al. Antibacterial and antifungal properties of ??-helical, cationic peptides in the venom of scorpions from southern Africa. Eur. J. Biochem. 2002, 269, 4799–4810. [Google Scholar] [CrossRef]
- O’Brien-Simpson, N.M.; Hoffmann, R.; Chia, C.S.B.S.B.; Wade, J.D.D.; Brien-Simpson, N.M.O.; Hoffmann, R.; Chia, C.S.B.S.B.; Wade, J.D.D. (Eds.) Antimicrobial and Anticancer Peptides; Frontiers Research Topics; Frontiers Media: Lausanne, Switzerland, 2018; Volume 6, ISBN 9782889454709. [Google Scholar]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta Biomembr. 2016, 1858, 1044–1060. [Google Scholar] [CrossRef]
- Castilho, S.R.A.; Godoy, C.S.D.M.; Guilarde, A.O.; Cardoso, J.L.; André, M.C.P.; Junqueira-Kipnis, A.P.; Kipnis, A. Acinetobacter baumannii strains isolated from patients in intensive care units in Goiânia, Brazil: Molecular and drug susceptibility profiles. PLoS ONE 2017, 12, e0176790. [Google Scholar] [CrossRef]
- Mendes, M.A.; De Souza, B.M.; Marques, M.R.; Palma, M.S. Structural and biological characterization of two novel peptides from the venom of the neotropical social wasp Agelaia pallipes pallipes. Toxicon 2004, 44, 67–74. [Google Scholar] [CrossRef]
- De Souza, B.M.; Marques, M.R.; Tomazela, D.M.; Eberlin, M.N.; Mendes, M.A.; Palma, M.S. Mass spectrometric characterization of two novel inflammatory peptides from the venom of the social waspPolybia paulista. Rapid Commun. Mass Spectrom. 2004, 18, 1095–1102. [Google Scholar] [CrossRef]
- Silva, É.C.N.; Camargos, T.S.; Maranhão, A.Q.; Silva-Pereira, I.; Silva, L.P.; Possani, L.D.; Schwartz, E.F. Cloning and characterization of cDNA sequences encoding for new venom peptides of the Brazilian scorpion Opisthacanthus cayaporum. Toxicon 2009. [Google Scholar] [CrossRef]
- Kaufmann, B.A.; Kaiser, C.; Pfisterer, M.E.; Bonetti, P.O. Coronary stent infection: A rare but severe complication of percutaneous coronary intervention. Swiss Med. Wkly. 2005, 135, 483–487. [Google Scholar]
- Sanchez, C.J.; Mende, K.; Beckius, M.L.; Akers, K.S.; Romano, D.R.; Wenke, J.C.; Murray, C.K. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect. Dis. 2013, 13, 1. [Google Scholar] [CrossRef]
- Espinal, P.; Martí, S.; Vila, J. Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J. Hosp. Infect. 2012, 80, 56–60. [Google Scholar] [CrossRef]
- Bravo, Z.; Chapartegui-González, I.; Lázaro-Díez, M.; Ramos-Vivas, J. Acinetobacter pittii biofilm formation on inanimate surfaces after long-term desiccation. J. Hosp. Infect. 2018, 98, 74–82. [Google Scholar] [CrossRef]
- Munier, A.-L.; Biard, L.; Rousseau, C.; Legrand, M.; Lafaurie, M.; Lomont, A.; Donay, J.-L.; de Beaugrenier, E.; Flicoteaux, R.; Mebazaa, A.; et al. Incidence, risk factors, and outcome of multidrug-resistant Acinetobacter baumannii acquisition during an outbreak in a burns unit. J. Hosp. Infect. 2017, 97, 226–233. [Google Scholar] [CrossRef]
- Furtado, A.D.; Bhat, S.P.S.; Peer, S.M.; Chikkatur, R. Infected pseudoaneurysm involving a drug-eluting stent. Interact. Cardiovasc. Thorac. Surg. 2011. [Google Scholar] [CrossRef]
- Venkatesan, A.M.; Kundu, S.; Sacks, D.; Wallace, M.J.; Wojak, J.C.; Rose, S.C.; Clark, T.W.I.; D’Othee, B.J.; Itkin, M.; Jones, R.S.; et al. Practice guideline for adult antibiotic prophylaxis during vascular and interventional radiology procedures. J. Vasc. Interv. Radiol. 2010, 21, 1611–1630. [Google Scholar] [CrossRef]
- Schoenkerman, A.B.; Lundstrom, R.J. Coronary stent infections: A case series. Catheter. Cardiovasc. Interv. 2009, 73, 74–76. [Google Scholar] [CrossRef]
- Vila-Farrés, X.; López-Rojas, R.; Pachón-Ibáñez, M.E.; Teixidó, M.; Pachón, J.; Vila, J.; Giralt, E. Sequence-activity relationship, and mechanism of action of mastoparan analogues against extended-drug resistant Acinetobacter baumannii. Eur. J. Med. Chem. 2015, 101, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Wiradharma, N.; Xu, K.; Ji, Z.; Bi, S.; Li, L.; Yang, Y.Y.; Fan, W. Cationic amphiphilic alpha-helical peptides for the treatment of carbapenem-resistant Acinetobacter baumannii infection. Biomaterials 2012, 33, 8841–8847. [Google Scholar] [CrossRef]
- Lin, C.H.; Lee, M.C.; Tzen, J.T.C.; Lee, H.M.; Chang, S.M.; Tu, W.C.; Lin, C.F. Efficacy of mastoparan-AF alone and in combination with clinically used antibiotics on nosocomial multidrug-resistant Acinetobacter baumannii. Saudi J. Biol. Sci. 2017, 24, 1023–1029. [Google Scholar] [CrossRef]
- Lin, M.-F. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J. Clin. Cases 2014, 2, 787. [Google Scholar] [CrossRef]
- Wang, Y.C.; Kuo, S.C.; Yang, Y.S.; Lee, Y.T.; Chiu, C.H.; Chuang, M.F.; Lin, J.C.; Chang, F.Y.; Chen, T.L. Individual or combined effects of meropenem, imipenem, sulbactam, colistin, and tigecycline on biofilm-embedded Acinetobacter baumannii and biofilm architecture. Antimicrob. Agents Chemother. 2016, 60, 4670–4676. [Google Scholar] [CrossRef]
- Andreassen, S.; Zalounina, A.; Paul, M.; Sanden, L.; Leibovici, L. Interpretative reading of the antibiogram—Semi-naïve Bayesian approach. Artif. Intell. Med. 2015, 65, 209–217. [Google Scholar] [CrossRef]
- Ammann, C.G.; Neuhauser, D.; Eberl, C.; Nogler, M.; Coraça-Huber, D. Tolerance towards gentamicin is a function of nutrient concentration in biofilms of patient-isolated Staphylococcus epidermidis. Folia Microbiol. 2018, 63, 299–305. [Google Scholar] [CrossRef]
- Saint Jean, K.D.; Henderson, K.D.; Chrom, C.L.; Abiuso, L.E.; Renn, L.M.; Caputo, G.A. Effects of hydrophobic amino acid substitutions on antimicrobial peptide behavior. Probiotics Antimicrob. Proteins 2018, 10, 408–419. [Google Scholar] [CrossRef]
- Andreev, K.; Martynowycz, M.W.; Huang, M.L.; Kuzmenko, I.; Bu, W.; Kirshenbaum, K.; Gidalevitz, D. Hydrophobic interactions modulate antimicrobial peptoid selectivity towards anionic lipid membranes. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1414–1423. [Google Scholar] [CrossRef]
- Mishra, B.; Lushnikova, T.; Golla, R.M.; Wang, X.; Wang, G. Design and surface immobilization of short anti-biofilm peptides. Acta Biomater. 2017, 49, 316–328. [Google Scholar] [CrossRef]
- Gabriel, M.; Nazmi, K.; Veerman, E.C.; Amerongen, A.V.N.; Zentner, A. Preparation of LL-37-grafted titanium surfaces with bactericidal activity. Bioconjug. Chem. 2006. [Google Scholar] [CrossRef]
- Mishra, B.; Basu, A.; Chua, R.R.Y.; Saravanan, R.; Tambyah, P.A.; Ho, B.; Chang, M.W.; Leong, S.S.J. Site specific immobilization of a potent antimicrobial peptide onto silicone catheters: Evaluation against urinary tract infection pathogens. J. Mater. Chem. B 2014. [Google Scholar] [CrossRef]
- Bagheri, M.; Beyermann, M.; Dathe, M. Immobilization reduces the activity of surface-bound cationic antimicrobial peptides with no influence upon the activity spectrum. Antimicrob. Agents Chemother. 2009, 53, 1132–1141. [Google Scholar] [CrossRef]
- Costa, F.; Carvalho, I.F.; Montelaro, R.C.; Gomes, P.; Martins, M.C.L. Covalent immobilization of antimicrobial peptides (AMPs) onto biomaterial surfaces. Acta Biomater. 2011, 7, 1431–1440. [Google Scholar] [CrossRef] [Green Version]
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Feng, X.; Sambanthamoorthy, K.; Palys, T.; Paranavitana, C. The human antimicrobial peptide LL-37 and its fragments possess both antimicrobial and antibiofilm activities against multidrug-resistant Acinetobacter baumannii. Peptides 2013, 49, 131–137. [Google Scholar] [CrossRef]
- Kwasny, S.M.; Opperman, T.J. Static biofilm cultures of gram positive pathogens grown in a microtiter format used for anti-biofilm drug discovery. Curr. Protoc. Pharmacol. 2010, 50, 13A.8.1–13A.8.23. [Google Scholar] [CrossRef]








| Peptide | Sequence | Venom source | Hydrophobicity | First Description |
|---|---|---|---|---|
| Agelaia-MPI | INWLKLGKAIIDAL | Agelaia pallipes pallipes | 0.781 | [44] |
| Polybia-MPII | INWLKLGKMVIDAL | Pseudopolybia vespiceps testacea | 0.740 | [45] |
| Polydim-I | AVAGEKLWLLPHLLKMLLTPTP | Polybia dimorpha | 0.791 | [38] |
| Con10 | FWSFLVKAASKILPSLIGGGDDNKSSS | Opisthacanthus cayaporum | 0.435 | [46] |
| NDBP-5.8 | GILGKIWEGVKSLI | Opisthacanthus cayaporum | 0.686 | [46] |
| Peptide | Isolate AB 02 1 | Isolate AB 53 | Isolate AB 72 |
|---|---|---|---|
| Agelaia-MPI | 25 | 6.25 | 12.5 |
| Polybia-MPII | 25 | 12.5 | 25 |
| Polydim-I | >25 | >25 | >25 |
| Con 10 | 25 | 12.5 | 12.5 |
| NDBP 5.8 | >25 | >25 | >25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
das Neves, R.C.; Mortari, M.R.; Schwartz, E.F.; Kipnis, A.; Junqueira-Kipnis, A.P. Antimicrobial and Antibiofilm Effects of Peptides from Venom of Social Wasp and Scorpion on Multidrug-Resistant Acinetobacter baumannii. Toxins 2019, 11, 216. https://doi.org/10.3390/toxins11040216
das Neves RC, Mortari MR, Schwartz EF, Kipnis A, Junqueira-Kipnis AP. Antimicrobial and Antibiofilm Effects of Peptides from Venom of Social Wasp and Scorpion on Multidrug-Resistant Acinetobacter baumannii. Toxins. 2019; 11(4):216. https://doi.org/10.3390/toxins11040216
Chicago/Turabian Styledas Neves, Rogério Coutinho, Márcia Renata Mortari, Elisabeth Ferroni Schwartz, André Kipnis, and Ana Paula Junqueira-Kipnis. 2019. "Antimicrobial and Antibiofilm Effects of Peptides from Venom of Social Wasp and Scorpion on Multidrug-Resistant Acinetobacter baumannii" Toxins 11, no. 4: 216. https://doi.org/10.3390/toxins11040216