1. Introduction
Dyes/pigments sourced from plants, animals, fruits, insects, minerals, etc. have a long history of application as colorants and for other uses. However, their dominance in the long run declined due to the advent of synthetic colorants during the course of modern industrial revolution. This trend of gradual shift toward artificial colorants was because of their low cost and stability as well as their easy production of diverse shades of colors and for other prospective uses [
1]. Consequently, synthetic colorants took the place of natural colorants and are still dominating the industry despite their toxic and harmful effects to humans and the environment [
2]. However, with more awareness of ecological and human health, natural colorants have gained attention of industries for a considerable period owing to their biofriendly prospective application in diversified fields over other synthetic variants. Being easy to extract from prospective natural sources, colors formed by innate dyes and pigments are brilliant. They are not only eco-friendly but also nontoxic and nonallergic with antimicrobial chattels as well, making them safer for humans too. Thus, they are much better for the environment and for human use.
There is universal awareness of the promotion of biocolorants from natural sources. By tradition, coloring mediators are derived from flora sources, such as berries, turmeric, indigo, and saffron [
3]. Vegetations can also generate colorants, and they were exploited in making innate colorants prior to the advent of artificial dyes, although in very small yields and with little eco-efficiency [
2]. Indeed, the use of vegetations to produce colorants is not environmentally friendly or sustainable due to the huge quantity of biomass expected to be accumulated in such a process. Similarly, animal sources for production of colorants are also not environmentally friendly or sustainable. Nevertheless, innate pigments sourced from microbes perform a vital part in the coloring industry owing to their ease of production, enhanced yield via strain development, absence of seasonal variation constraints, low production cost, etc. [
4].
More meticulous studies and precise investigations are required to access the real prospect and readiness of dye-yielding microbial sources and for the propagation of economically and commercially viable species. Biotechnological and other recent techniques play an important role in enhancing the trait and extent of innate dye production [
5]. Employing ideal biotechnological approaches, such as fermentation of microorganisms including fungi and bacteria, could be a precious source for developing natural colorants. Microorganisms yield a great diversity of durable pigments, such as carotenoids, flavonoids, quinones, and rubramines. In addition, the fermentation of microorganisms emits a higher yield of pigments and lesser dregs compared to the use of flora and fauna for pigment production [
6]. Therefore, the biosynthesis of dyes and pigments through fermentation progression has attracted additional interest in recent years [
7,
8]. Biosynthesized pigments could function as the main chromophores for more chemical conversion, which could lead to a wide range of colors [
9]. Moreover, a few innate colorants, particularly anthraquinone variety compounds, have revealed notable antibacterial pursuit in addition to providing brilliant colors [
10], which means they could serve as efficient dyes for producing colored antimicrobial textiles.
Among the microbial pigments, bacterial pigments have already emerged as possessing a vast potential for industrial development [
11]. Unlike food colorants, textile colorants are not restricted by safety criteria, so many bacterial pigments can be applied in textiles. Indeed, biotechnology plays a major role in the mass production of dyes [
12]. Darshan and Manonmani [
13] showed that prodigiosin from marine
Serratia sp. could be applied as innate dye for tinting color to a range of textile materials, and the color was durable even after washing. Similarly, Alihosseini et al. [
14] described the potential of the red pigment prodigiosin from
Vibrio sp. and tested it as a dye to color many fibers, including wool, nylon, and silk. Kanelli et al. [
15] used violet pigment from
Janthinobacterium lividum for tinting and produced excellent color tone on silk, cotton, wool, nylon, and vinylon. Ahmad et al. [
16] reported that prodigiosin from
Serratia marcescens and violacein from
Chromobacterium violaceum have the ability to dye various fabrics, such as cotton, silk, rayon, satin, and polyester. They also found that prodigiosin could be better used to dye acrylic, and violacein could be used to dye rayon and satin. Kumar et al. [
17] demonstrated that prodigiosin (
Vibrio sp. and
Serrtia sp.) and violacein (
Chromobacterium violaceum) can be used in the textile industry for tinting of all fibers, including cotton, wool, silk, nylon, and acrylic fibers. The eco-friendly, antimicrobial, and antioxidant properties of bacterial pigments add to their positive effects toward textile tinting [
4,
17].
The textile industry generates around 1 trillion dollar worth of clothing and other fabric materials, contributing to about 7% of the total world exports, and is one of the biggest global polluters, consuming a high amount of chemicals [
18]. Therefore, there is a big scope for the use of innate colorants in products as substitute for artificial colorants, which are toxic and have adverse effects on all forms of life. In this backdrop, the textile industry has recently made a significant move toward innate colorants by gradually switching to them from synthetic colorants. The present environment therefore provides good scope for significant application of innate colorants in textile materials.
The central aim of the present research was to devise a plain process for biosynthesizing a bacterial pigment and applying it in textile dyeing. The intention is to bring bacterial colorants from the research field to the commercial world for mass production of biocolorants in innate dye markets. Therefore, we undertook this investigation as a probing study to analysis the dyeing properties and the inherent antimicrobial activities of pigment extracted from Serratia marcescens SB08, an insect-associated bacterium, with a view to developing protective clothing.
2. Materials and Methods
2.1. Origin and Identification of the Bacterium
The surroundings of Bharathiar University, Coimbatore, abutting the foothills of Western Ghats in Tamil Nadu state, India, are exceptional. The strategic geographically located landmass of the university in the west of Coimbatore is endowed with an invaluable natural treasure of many mammals, birds, insects, and reptiles. It is also part of the Nilgiri Biosphere Reserve (NBR) buffer zone [
19] and a flourishing ground for a rich variety of insects and other organisms. This distinct environment with a diversity of fauna and flora provided very good scope for selection of the required insect species for this study.
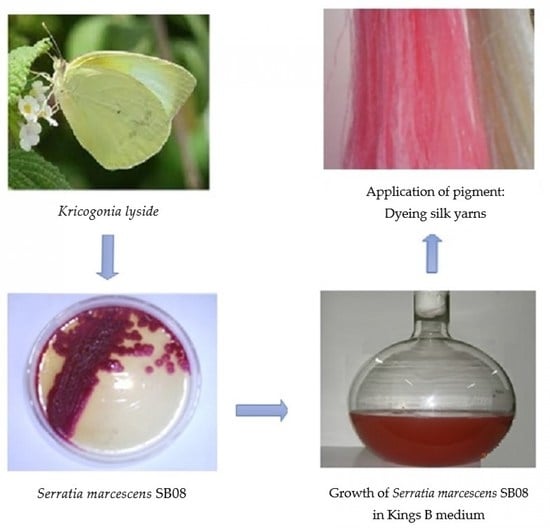
A total of 22 insect samples (black ant (Paratrechina longicornis), cricket (Gryllus assimilis), red ant (Monomorium pharaonis), cockroach (Periplaneta americana), dragonfly (Libellula luctosa), drone fly (Eristalis tenax), grasshopper (Tettigonia viridissima and Patanga japonica), hide beetle (Dermestes maculatus), honeybee (Apis mellifera), large yellow ant (Acanthomyops interjectus), silver fish (Lepisma saccharina), soil cockroach (Eublaberus distanti), spider (Achaearanea tepidariorum), stick insect (Carausius morosus), sulfur butterfly (Kricogonia lyside), wasp (Aleiodes indiscretus), waspmoth (Cosmosoma myrodora), weevil (Otiorhynchus sulcatus), wheel bug (Arilus cristatus), wolf spider (Trite planiceps), and wood cockroach (Parcoblatta virginica)) were collected and transferred to sterile glass containers and processed within 24 h. Insects were dissected under sterile conditions by disrupting the walls. The contents of the stomach were transferred to phosphate-buffered saline solutions in Eppendorf tubes, serially diluted and spread onto nutrient agar plates, and incubated for 48 h at 30 °C to record the total colony forming units (CFU/mL) [
20]. Among the isolates, the pigment-producing bacterium isolated from the enteric gut of sulfur butterfly (Kricogonia lyside) was selected for this study and identified by 16S rRNA gene sequencing. PCR amplification of the 16S rRNA gene was performed using two oligonucleotide primers, 5′-AGAGTTTGATCCTGGCTCAG-3′ and 5′-AAGGAGGTGATCCAGCCGCA-3′ (
http://rrna.uia.ac.be/primers/database.html, accessed on 11 March 2019), and identified as Serratia marcescens SB08 (GenBank Accession No. GQ465847).
2.2. Cultivation, Extraction, and Purification of the Pigment
The culture
Serratia marcescens SB08 cultivated in Kings B medium contained the following (per liter of deionized water): peptone: 20.0 g; K
2HPO
4: 1.5 g; and MgSO
4: 1.5 g in Hafkins flask. The pH of the medium was adjusted to 7 using 1 N HCl or 1 N NaOH. The flask was inoculated with 10
6 cells/mL of
S. marcescens SB08 grown at 30 °C under static conditions. After an incubation period of 3 days, the pigments were filtered using sterilized gauze cloth. Finally, 95% (
v/
v) methanol (2 volumes) were added to the culture broth, and the resulting mixture was kept at 30 °C for 30 min on the rotary shaker at 200×
g. The methanolic mixture was centrifuged at 5000×
g for 15 min, and the pellets were dispersed in the remaining volume of methanol and centrifuged again. The supernatant was recovered, and the filtrate was dispersed in the leftover volume of methanol and centrifuged at 5000×
g for 5 min. Finally, the supernatant was collected and filtered through a Whatman No.1 filter paper and diluted with 95% (
v/
v) methanol to a dilution factor of 20. The absorption spectrum was observed at 300–600 nm using Hitachi 3210 spectrometer (Hitachi Ltd., Tokyo, Japan) [
21], and the purified pigment was concentrated in a rotary evaporator and lyophilized. The optical density (OD) measured at 535 nm and multiplied by the above dilution factor gives the quantum of the red pigment production [
22].
2.3. Structural Identification of the Pigment
The pigment extracted with methanol was filtered and concentrated under reduced pressure. It was fractionated by thin-layer chromatography, and the red pigment components were separated and subjected to different types of instrumental analysis, namely High Performance Liquid Chromatography (HPLC), Gas Chromatography–Mass Spectroscopy (GC–MS), Fourier Transform Infrared Spectroscopy (FTIR) and Nuclear Magnetic Resonance (NMR), to determine their chemical structure. The pigment was identified as prodigiosin.
2.4. Textile Dyeing
Raw cotton, pure silk, and China silk yarns were subdivided into standard specimens (2 cm length) in agreement with the Italian Association of Textile Colorists. Dyeing was carried out by textile tad with an optimized concentration of the pigment in 100 mL deionized water for 120 min in an orbital shaker operated at 70 °C, 100 rpm/min, with 1.0 g sodium sulfate [
23] at pH 5. The colored samples were washed with normal water to eliminate the unfixed pigment in a bath of liquid ratio (L:R = 40:1) using nonionic detergent (3 g/L) for 30 min at 50 °C and then air dried. The dye bath pH was monitored with a pH meter and adjusted with dilute solutions of 1 M sodium carbonate.
2.5. Optimization of Dyeing Conditions
The different yarn samples were tinted at various ranges of affecting parameters, namely pigment concentration (2–14% owl), pH (4–9), retention time (20–120 min), temperature (20–90 °C), and fixed salt concentration (1.0 g/L). The best conditions for dyeing were derived as follows: pigment concentration of 5% owl, pH 6, temperature of 70 °C for pure silk and 60 °C for China silk and cotton, and retention time of 100 min. All experiments were performed in triplicate.
2.6. Pigment Exhaustion
The process concentration and the residual pigment in the exhausted process liquid (before and after dyeing) were analyzed (Hitachi 3210 spectrometer). The percentage of pigment exhaustion was calculated using the following equation:
where Cg is the concentration of pigment used, and Ct is the concentration of pigment in the spent liquid.
2.7. Color Measurement Analysis
Quantification of pigment-tinted yarn color was carried out as per the Commission Internationale de l’Eclairage (CIE) system of color measurement with 100 standard observer data. L*, a*, b*, c*, and h values for both the grain shade of the dyed yarns were obtained using Datacolor SF 600 spectrophotometer (Datacolor, Dietikon, Switzerland). The values L*, a*, b*, c*, and h are the variables in the CIELAB color space and explained in
Table 1.
2.8. Assessment of Visual Color
Dyed yarn samples were exposed to visual valuation for consistency of color, penetration of shade, color shift from control, and overall appearance by the standard tactile evaluation technique. The different yarn samples were rated on a scale of 0–10 points for each functional property, with 0 as the lowest and 10 as the highest. The average rating was calculated for each parameter and used for comparison studies.
2.9. Determination of Fastness Properties
Dyed yarn samples were tested for washing, rubbing, and light fastness after conditioning according to IS 6191–1971 (LF: 4) (Indian Standards IS 6191, 1971).
2.10. Antimicrobial Activity of Pigments
2.10.1. Test Organisms
The common pathogenic bacteria Bacillus subtilis MTCC2388, Escherichia coli MTCC443, Klebsiella pneumonia MTCC109, Proteus vulgaris MTCC1771, and Pseudomonas aeruginosa MTCC1688 were selected and used as test organisms in the study. The organisms were inoculated in 50 mL nutrient broth and incubated at 37 °C for 24 h. This broth was used for seeding the agar plates.
2.10.2. Antimicrobial Screening Test
Mueller–Hinton agar medium was prepared and autoclaved at 121 °C for 15 min. Sterilized agar medium was dispensed uniformly onto sterilized petriplates. The plates were seeded with appropriate cultures. An amount of 10 mg of pigment was impregnated onto a small disc of filter paper (diameter 5.0 mm) and placed on top of the seeded medium. The plates were incubated at 37 °C for 24 h, and the zone of inhibition (diameter) was recorded in each case.
In the second set of experiments, concentration of pigment impregnated (5, 10, 20, and 40 mg) onto a disc of filter paper was varied to study its effect on the growth of microbes and the minimum inhibitory concentration (MIC) of the pigment.
In the third set of experiments, the antimicrobial activity of dyed specimens was tested. A 1 inch yarn (dyed and undyed) was introduced in the 100 mL nutrient broth inoculated with the desired microbe and incubated at 37 °C for 24 h. The reduction of bacterial growth by pigment was expressed as follows:
where
R is the percentage reduction in bacterial population,
B is the absorbance (660 nm) of the media inoculated with microbe and undyed yarn, and
A is the absorbance (660 nm) of the media inoculated with microbe and dyed yarn.
3. Results and Discussion
The present study was embarked upon to unravel the potential of an insect gut microorganism and exploit its bioactive compound for textile dyeing. A better understanding of insect–microbe interactions and their mutual contributions may lead to new strategies to further study their relationship and also to explore the bioactive potential of novel gut microbes awaiting discovery. In the present investigation, among the 22 insect species collected from the local environment and subjected to isolation and enumeration of bacteria from the insect enteric guts,
Serratia marcescens SB08 from the enteric gut of sulfur butterfly exhibited its ability to produce dark red color pigment (
Figure 1).
3.1. Identification of the Pigment
The pigment extracted from
Serratia marcescens SB08 gave an Rf value of 0.95 in Thin Layer Chromatography (TLC). The Rf value of the extracted pigment from
Serratia marcescens KH1R was 0.64–0.96 [
24]. Mohammed et al. [
25] reported that the Rf value of prodigiosin was 0.73. Srimathi et al. [
26] reported that the Rf value of prodigiosin from
Serratia marcescens isolated from rhizosphere soil samples in Salem and Namakkal districts, Tamil Nadu, India, was 0.78. This indicates that although the microbes are of the same species of
S. marcescens, the Rf value of pigments produced by different strains within the same species shows variation.
HPLC analysis of the pigment in a Bondapack C18 column (2.5 × 10 cm) isocratically eluted with a mixture of methanol/water (7:3,
v/
v) showed the pigment in a single peak corresponding to prodigiosin with approximately 95% purity at the retention time of 19.51 min (
Figure 2a). Gas chromatogram of the pigment extract showed that it had a strong peak at 266 mass units and metastable ion peak at 323 (
Figure 2b). The molecular mass of the pigment on GC–MS was 323.0 Da as
m/z 323.0 (M + H)
+, as shown in
Figure 2c, which corresponds to that of prodigiosin (C
20H
25N
3O) [
27]. Similarly, Silva et al. [
28] reported that the red pigment from
S. marcescens has a molecular weight of 323
m/z and characterized it as prodigiosin. Yang et al. [
29] in their study reported that prodigiosin from
Microcystis aeruginosa had a molecular weight of 323
m/z. There have been no earlier reports on GC for prodigiosin. In the study by Lin et al. [
30], prodigiosin from
Serratia marcescens FZSF02 showed a main peak at the molecular weight of 323.9. Therefore, the findings of this study could be a base for future investigation of prodigiosin.
FTIR absorption spectra in KBr for the pigment was dominated by very strong bands at 2925.46 (aromatic CH) and 1402.75 (aromatic C=C) cm
−1. The main absorption peak included 3430.08, 2925.46, 1715.91, 1402.75, 1262.61, 1091.83, 802.78, and 638.90 (
Figure 2d). The spectra of the red pigment showed similarities to the spectra of prodigiosin [
31]. The peaks at 2925 (aromatic CH) are due to the asymmetrical stretching of methylene groups. Earlier findings by Song et al. [
32] showed that FTIR absorption in KBr for the red pigment isolated from
Serratia spp. KH95 was dominated by very strong bands at 2928 (aromatic CH) and 1602 (aromatic C=C) cm
−1, except that the relative intensities were reversed and the first band was possibly a pyrrolenine (C=N). This indicates that the pattern of the pigment from
Serratia marcescens SB08 is similar to that of prodigiosin.
In
1H-NMR spectrum, a chemical shift of the methoxy group in prodigiosin exhibited 4.04 ppm as singlet (
Figure 2e). The chemical shifts in CDCl
3 of carbon were 120.7, 117.00, 92.81, 126.95, 122.27, 165.79, 92.81, 147.72, 58.69, 116.03, 147.04, 125.16, 128.41, 126.95, 12.48, 25.32, 29.69, 31.41, 22.49, and 14.01 (
Figure 2f). The results of the above study indicated that the pigment was prodigiosin. Similar results were observed by Song et al. [
32] and the pigment identified from
Serratia spp. KH95 corresponded to this investigation. The NMR spectrum indicate the consistency of chemical shifts of methyl groups of prodigiosin-like pigments [
33]. It was therefore concluded that the pigment isolated from the
S. marcescens SB08 was prodigiosin.
3.2. Optimization of pH, Retention Time, and Temperature for Textile Dyeing
Conditions optimized for the usage of pigment to attain maximum exhaustion into the textile yarns confirmed that the following conditions were optimum: pigment concentration of 5% owl, salt concentration of 1.0 g/L in L:R ratio of 40:1 tested with relevant effect of pH, retention time, and temperature.
As shown in
Figure 3a, the exhaustion of pigment decreased in pure silk, China silk, and cotton at both high and low pH values. The pigment exhaustion was found to be maximum in pH 6 for pure silk, China silk, and cotton, and an additional increase in pH resulted in reduction in exhaustion of pigment. The effect of dye bath pH can be attributed to the correlation between dye structure and the dyed yarn. However, further increase in pH made the dye and yarn more anionic, which repelled each other and resulted in lesser dyeability with higher pH [
34].
The fixation of pigment to the yarn samples at different retention times is given in
Figure 3b. The results revealed that pigment exhaustion increased steadily according to the retention time. The yarn sample required a maximum of 100 min to bring about significant exhaustion in the dye bath. Hence, 100 min retention time was taken as optimum duration for pure silk, China silk, and cotton. The longer the dyeing time, the higher was the color strength until dye exhaustion attained equilibrium. There was a decrease in the color strength after further increase in time over 100 min. The decline in color strength could be attributed to the shift in equilibrium of the coloring component from yarn into the dye bath during longer dyeing times [
34].
The results attained for the exhaustion of pigment at various temperatures is shown in
Figure 3c. The pigment exhaustion increased with the increase in dyeing temperature and reached a maximum at 70 °C for pure silk and 60 °C for China silk and cotton, which declined at further temperatures. However, temperatures higher than 70 °C resulted in a decrease in color strength, which might be attributed to a decrease in dye molecule stability at higher temperatures [
34].
The optimized conditions, namely pigment concentration of 5% owl, salt concentration of 1.0 g/L, pH 6, retention time of 100 min, temperature of 70 °C for pure silk and 60 °C for China silk and cotton, resulted in maximum pigment exhaustion of 96.02% for pure silk, 90% for China silk, and 81.6% for cotton.
3.3. Color Analysis by Reflectance Measurement
Quantification of color value of the yarns dyed by conventional dyeing process with the pigment extracted from
Serratia marcescens SB08 was analyzed by reflectance measurement. The color values of the samples L*, a*, b*, c*, and h are shown in
Table 2. Noticeable development in the color strength was observed in pigment dyed with 5% owl. Sutlovic et al. [
35] have reported that the relationship between lightness and chroma is crucial for visual experience of total color appearance.
3.4. Visual Assessment of Yarn Samples
Visual appraisal for change in color from control, uniformity of color, depth of shade, and general appearance (
Figure 4) was carried out by the standard tactile assessment technique, and the values are given in
Table 2. The penetration of shade was moderate consistency for cotton yarns and had better uniformity in pure silk and China silk yarns at 5% owl. The strength of the pigment-dyed yarn sample was reasonably lower than the control. These results were in agreement with the reflectance measurement values. The uniformity of color, pigment diffusion, and shade were reasonable for the pigment-dyed yarn samples. A moderate improvement in the appearance of the yarn dyed with pigment (optimized conditions) was observed.
3.5. Fastness Properties of Pigment-Dyed Yarn Samples
The wide range of colors available with good fastness properties at moderate costs was the main reason for the replacement of natural dyes by their counterparts [
36]. Many colorants from synthetic sources can be harmful and cause allergies in humans [
37]; therefore, interest in natural dyes has increased considerably during the last few years.
The washing, rubbing, and light fastness properties of the optimized pigment-dyed yarn samples is given in
Table 3. The results showed that the fastness to washing and rubbing of the pigment-dyed yarns was inferior than the control yarns. In general, pigment-dyed yarn samples showed modest light fastness (rating of 3.5 on gray scale), equal to the standards. The effect of ageing (3 months) on the fastness of yarn samples was also studied. However, the value for light resistance was poor, which was not unpredicted given mordant was not employed during the natural yarn dyeing process. The usage of conventional metal mordents in dyeing with natural materials is a general practice [
38]. As the present study involved health sensitivity, metal mordents were not applied. It is significant to point out that a light fastness value of 3 is considered as acceptable by textile industries. The good light fastness properties of the pigment could be attributed to the strong intramolecular H-bonding.
Prodigiosins are natural tripyrrole red pigments and possess various biological activities. Lee et al. [
39] tested the effect of bacterial prodigiosin on human skin keratinocytes (HaCaT) and reported that prodigiosin did not cause cytotoxicity and increased the proliferation of HaCaT cells, thereby protecting against UV irradiation. Surawanshi et al. [
40] reported that prodigiosin protected the skin against UV radiation and suggested that prodigiosin could be used to develop natural materials that protects the skin. In this aspect, prodigiosin from
Serratia marcescens SB08 can be used in textiles for dyeing as there is no cytotoxicity on human skin keratinocytes, as reported earlier.
3.6. Antimicrobial Activity of Pigment
Textile materials and clothing are known to be susceptible to microbial attack as they provide large surface area and absorb moisture, which is required for microbial growth [
41]. Natural fibers have protein (keratin), cellulose, etc., which provide basic requirements such as moisture, oxygen, nutrients, and temperature for bacterial growth and multiplication. This often leads to objectionable odor, dermal infection, product deterioration, allergic response, and other related diseases [
42]. This necessitates the development of clothing that could provide a desired antimicrobial effect.
Preliminary screening showed that pigment from Serratia marcescens SB08 was effective against all the tested microbes except K. pneumoniae MTCC109 and P. vulgaris MTCC1771. A clear zone of inhibition was observed by the pigment for B. subtilis MTCC2388, E. coli MTCC443, and P. aeruginosa MTCC1688.
The effect of concentration of pigment on antimicrobial activity was further studied, and the results are summarized in
Table 4. The zone of inhibition (diameter) was recorded in each case. It is evident that with increasing concentration of dye, the zone of inhibition increased almost linearly. From the clear zone of inhibition obtained, it is apparent that pigment dyes are bactericidal in nature and not bacteriostatic.
Antimicrobial Activity of Pigment on Substrate
Antimicrobial activity of pigment on dyed substrates (pure silk, China silk, and cotton) was studied (
Figure 5). A reduction of 19.71% in bacterial growth (
P. aeruginosa MTCC1688) was seen in pure silk, followed by 16.92% and 16.42% in China silk and cotton samples, respectively. Further reduction of 18.44%, 14.13%, and 9.52% in
E.
coli MTCC443 was observed in pure silk, China silk, and cotton, respectively. Moreover, there was a reduction in bacterial growth (
B. subtilis MTCC2388) of 12.08%, 11.13%, and 10.17% in pure silk, China silk, and cotton, respectively. This is an interesting finding and requires more in depth investigation into the effect of pigment structure on antimicrobial property. It is obvious that antimicrobial properties are closely related to the dye structure, especially the presence of functional groups [
43].
4. Conclusions
Natural dyes and pigments have emerged as an important alternative to potentially harmful synthetic dyes. The application of these natural dyes and pigments in dyeing of cotton, silk, and wool samples has been reported in several studies. From an ecological viewpoint, the substitution of chemical dyes with natural products in textile dyeing may be feasible and may represent not only a strategy to reduce risk and pollutants but also an opportunity for new markets and new businesses, which can develop from inclusion of ecology in trade policy. This research focused on the application of pigment from S. marcescens SB08 for dyeing of pure silk, China silk, and cotton and proved its potential for dyeing efficiency.