Incubation under Climate Warming Affects Behavioral Lateralisation in Port Jackson Sharks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Egg Collection and Incubation
2.3. Husbandry and Rearing
2.4. Procedure
2.5. Data Analysis
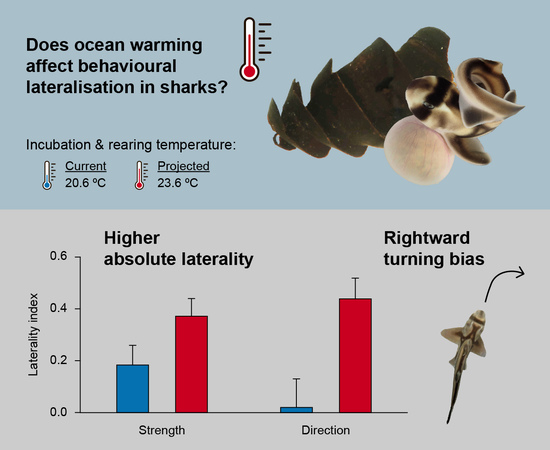
3. Results
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.-L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.; Johns, T.; Krinner, G. Long-term climate change: Projections, commitments and irreversibility. In Climate Change 2013: The Physical Science Basis; IPCC Working Group I Contribution to AR5; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 1029–1136. [Google Scholar]
- Pörtner, H.-O.; Karl, D.M.; Boyd, P.W.; Cheung, W.; Lluch-Cota, S.E.; Nojiri, Y.; Schmidt, D.N.; Zavialov, P.O.; Alheit, J.; Aristegui, J. Ocean systems. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects; Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 411–484. [Google Scholar]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Alexander, L.V.; Allen, S.K.; Bindoff, N.L.; Bréon, F.-M.; Church, J.A.; Cubasch, U.; Emori, S. Climate Change 2013: The Physical Science Basis; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 33–115. [Google Scholar]
- Cripps, I.L.; Munday, P.L.; McCormick, M.I. Ocean acidification affects prey detection by a predatory reef fish. PLoS ONE 2011, 6, e22736. [Google Scholar] [CrossRef] [PubMed]
- Munday, P.L.; Cheal, A.J.; Dixson, D.L.; Rummer, J.L.; Fabricius, K.E. Behavioural impairment in reef fishes caused by ocean acidification at CO2 seeps. Nat. Clim. Chang. 2014, 4, 487. [Google Scholar] [CrossRef]
- Simpson, S.D.; Munday, P.L.; Wittenrich, M.L.; Manassa, R.; Dixson, D.L.; Gagliano, M.; Yan, H.Y. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 2011, 7, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Dixson, D.L.; Munday, P.L.; Jones, G.P. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 2010, 13, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.; Baptista, M.; Lopes, V.M.; Pegado, M.R.; Ricardo Paula, J.; Trübenbach, K.; Leal, M.C.; Calado, R.; Repolho, T. Early-life exposure to climate change impairs tropical shark survival. Proc. R. Soc. B Biol. Sci. 2014, 281. [Google Scholar] [CrossRef] [PubMed]
- Pistevos, J.C.; Nagelkerken, I.; Rossi, T.; Olmos, M.; Connell, S.D. Ocean acidification and global warming impair shark hunting behaviour and growth. Sci. Rep. 2015, 5, 16293. [Google Scholar] [CrossRef] [PubMed]
- Donelson, J.M.; Munday, P.L.; McCormick, M.I.; Pankhurst, N.W.; Pankhurst, P.M. Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Mar. Ecol. Prog. Ser. 2010, 401, 233–243. [Google Scholar] [CrossRef]
- Munday, P.L.; Kingsford, M.J.; O’Callaghan, M.; Donelson, J.M. Elevated temperature restricts growth potential of the coral reef fish acanthochromis polyacanthus. Coral Reefs 2008, 27, 927–931. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Crawley, N.; Lunde, I.G.; Munday, P.L. Elevated temperature reduces the respiratory scope of coral reef fishes. Glob. Chang. Biol. 2009, 15, 1405–1412. [Google Scholar] [CrossRef]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate change and distribution shifts in marine fishes. Science 2005, 308, 1912–1915. [Google Scholar] [CrossRef] [PubMed]
- Soengas, J.L.; Aldegunde, M. Energy metabolism of fish brain. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 131, 271–296. [Google Scholar] [CrossRef]
- Brown, C. Experience and learning in changing environments. In Behavioural Responses to a Changing World: Mechanisms and Consequences; Candolin, U., Wong, B.B., Eds.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Sheridan, J.A.; Bickford, D. Shrinking body size as an ecological response to climate change. Nat. Clim. Chang. 2011, 1, 401. [Google Scholar] [CrossRef]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains: The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Vallortigara, G.; Versace, E. Laterality at the neural, cognitive, and behavioral levels. In Apa Handbook of Comparative Psychology: Basic Concepts, Methods, Neural Substrate, and Behavior, Vol. 1; American Psychological Association: Washington, DC, USA, 2017; pp. 557–577. [Google Scholar]
- Vallortigara, G.; Rogers, L.J. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005, 28, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J.; Andrew, R. Comparative Vertebrate Lateralization; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Bisazza, A.; Brown, C. Lateralization of cognitive functions in fish. In Fish Cognition and Behavior; Wiley: Oxford, UK, 2011; pp. 298–324. [Google Scholar]
- Dadda, M.; Bisazza, A. Lateralized female topminnows can forage and attend to a harassing male simultaneously. Behav. Ecol. 2006, 17, 358–363. [Google Scholar] [CrossRef]
- Bisazza, A.; Cantalupo, C.; Capocchiano, M.; Vallortigara, G. Population lateralisation and social behaviour: A study with 16 species of fish. Later. Asymmet. Body Brain Cogn. 2000, 5, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Bisazza, A.; de Santi, A. Lateralization of aggression in fish. Behav. Brain Res. 2003, 141, 131–136. [Google Scholar] [CrossRef]
- Bibost, A.-L.; Brown, C. Laterality influences schooling position in rainbowfish, melanotaenia spp. PLoS ONE 2013, 8, e80907. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, E.E.; Vila Pouca, C.; Brown, C. Laterality strength is linked to stress reactivity in port jackson sharks (heterodontus portusjacksoni). Behav. Brain Res. 2016, 305, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Green, L.; Jutfelt, F. Elevated carbon dioxide alters the plasma composition and behaviour of a shark. Biol. Lett. 2014, 10, 20140538. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J.; Zucca, P.; Vallortigara, G. Advantages of having a lateralized brain. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, S420–S422. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.; Hoare, D.; Krause, S.; Hemelrijk, C.K.; Rubenstein, D.I. Leadership in fish shoals. Fish Fish. 2000, 1, 82–89. [Google Scholar] [CrossRef]
- Bisazza, A.; Dadda, M. Enhanced schooling performance in lateralized fishes. Proc. R. Soc. Lond. B Biol. Sci. 2005, 272, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Dadda, M.; Bisazza, A. Does brain asymmetry allow efficient performance of simultaneous tasks? Anim. Behav. 2006, 72, 523–529. [Google Scholar] [CrossRef]
- Sovrano, V.A.; Dadda, M.; Bisazza, A. Lateralized fish perform better than nonlateralized fish in spatial reorientation tasks. Behav. Brain Res. 2005, 163, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Bibost, A.L.; Brown, C. Laterality influences cognitive performance in rainbowfish melanotaenia duboulayi. Anim. Cogn. 2014, 17, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Domenici, P.; Allan, B.; McCormick, M.I.; Munday, P.L. Elevated carbon dioxide affects behavioural lateralization in a coral reef fish. Biol. Lett. 2011. [Google Scholar] [CrossRef] [PubMed]
- Domenici, P.; Allan, B.J.; Watson, S.-A.; McCormick, M.I.; Munday, P.L. Shifting from right to left: The combined effect of elevated CO2 and temperature on behavioural lateralization in a coral reef fish. PLoS ONE 2014, 9, e87969. [Google Scholar] [CrossRef] [PubMed]
- Jutfelt, F.; de Souza, K.B.; Vuylsteke, A.; Sturve, J. Behavioural disturbances in a temperate fish exposed to sustained high-CO2 levels. PLoS ONE 2013, 8, e65825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundin, J.; Jutfelt, F. Effects of elevated carbon dioxide on male and female behavioural lateralization in a temperate goby. R. Soc. Open Sci. 2018, 5, 171550. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Morais, P.; Pimentel, M.; Rosa, R.; Munday, P.; Gonçalves, E.; Faria, A. Behavioural lateralization and shoaling cohesion of fish larvae altered under ocean acidification. Mar. Biol. 2016, 163, 243. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Dixson, D.L.; Domenici, P.; McCormick, M.I.; Sørensen, C.; Watson, S.-A.; Munday, P.L. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat. Clim. Chang. 2012, 2, 201. [Google Scholar] [CrossRef]
- Rosa, R.; Pimentel, M.; Galan, J.G.; Baptista, M.; Lopes, V.M.; Couto, A.; Guerreiro, M.; Sampaio, E.; Castro, J.; Santos, C. Deficit in digestive capabilities of bamboo shark early stages under climate change. Mar. Biol. 2016, 163, 60. [Google Scholar] [CrossRef]
- Di Santo, V.; Bennett, W.A. Effect of rapid temperature change on resting routine metabolic rates of two benthic elasmobranchs. Fish Physiol. Biochem. 2011, 37, 929–934. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, R.H.; O’Gower, A.K. Life history and underwater studies of a heterodont shark. Ecol. Monogr. 1971, 41, 271–289. [Google Scholar] [CrossRef]
- Rodda, K.; Seymour, R. Functional morphology of embryonic development in the port jackson shark heterodontus portusjacksoni (meyer). J. Fish Biol. 2008, 72, 961–984. [Google Scholar] [CrossRef]
- Bisazza, A.; Pignatti, R.; Vallortigara, G. Laterality in detour behaviour: Interspecific variation in poeciliid fish. Anim. Behav. 1997, 54, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, 3.4.3; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Quaranta, A.; Siniscalchi, M.; Frate, A.; Iacoviello, R.; Buonavoglia, C.; Vallortigara, G. Lateralised behaviour and immune response in dogs: Relations between paw preference and interferon-γ, interleukin-10 and igg antibodies production. Behav. Brain Res. 2006, 166, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; Sasso, R.; Pepe, A.M.; Dimatteo, S.; Vallortigara, G.; Quaranta, A. Catecholamine plasma levels following immune stimulation with rabies vaccine in dogs selected for their paw preferences. Neurosci. Lett. 2010, 476, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Neveu, P.J. Cerebral lateralization and the immune system. In International Review of Neurobiology; Academic Press: Cambridge, MA, USA, 2002; Volume 52, pp. 303–323. [Google Scholar]
- Jozet-Alves, C.; Viblanc, V.A.; Romagny, S.; Dacher, M.; Healy, S.D.; Dickel, L. Visual lateralization is task and age dependent in cuttlefish, sepia officinalis. Anim. Behav. 2012, 83, 1313–1318. [Google Scholar] [CrossRef]
- Dharmaretnam, M.; Andrew, R.J. Age- and stimulus-specific use of right and left eyes by the domestic chick. Anim. Behav. 1994, 48, 1395–1406. [Google Scholar] [CrossRef]
- Brown, C.; Gardner, C.; Braithwaite, V.A. Population variation in lateralized eye use in the poeciliid brachyraphis episcopi. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2004, 271, S455–S457. [Google Scholar] [CrossRef] [PubMed]
- Bisazza, A.; Facchin, L.; Pignatti, R.; Vallortigara, G. Lateralization of detour behaviour in poeciliid fish: The effect of species, gender and sexual motivation. Behav. Brain Res. 1998, 91, 157–164. [Google Scholar] [CrossRef]
- Bibost, A.-L.; Kydd, E.; Brown, C. The effect of sex and early environment on the lateralization of the rainbowfish melanotaenia duboulayi. In Behavioral Lateralization in Vertebrates: Two Sides of the Same Coin; Csermely, D., Regolin, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 9–24. [Google Scholar]
- Rogers, L.J. Relevance of brain and behavioural lateralization to animal welfare. Appl. Anim. Behav. Sci. 2010, 127, 1–11. [Google Scholar] [CrossRef]


| Shark ID | Sex | Weight (g) | Treatment | # Right Turns | # Left Turns | LI | LS |
|---|---|---|---|---|---|---|---|
| C489 | M | 86 | C | 1 | 9 | −0.8 | 0.8 |
| C451 | M | 87 | C | 4 | 6 | −0.2 | 0.2 |
| C430 | M | 81 | C | 5 | 5 | 0 | 0 |
| C437 | M | 70 | C | 5 | 5 | 0 | 0 |
| C456 | F | 53 | C | 5 | 5 | 0 | 0 |
| C469 | M | 101 | C | 5 | 5 | 0 | 0 |
| C492 | F | 79 | C | 5 | 5 | 0 | 0 |
| C500 | F | 89 | C | 5 | 5 | 0 | 0 |
| C407 | M | 83 | C | 6 | 4 | 0.2 | 0.2 |
| C452 | F | 76 | C | 6 | 4 | 0.2 | 0.2 |
| C459 | M | 94 | C | 6 | 4 | 0.2 | 0.2 |
| C460 | F | 95 | C | 8 | 2 | 0.6 | 0.6 |
| ET455 | M | 64 | ET | 6 | 4 | 0.2 | 0.2 |
| ET369 | M | 50.5 | ET | 6 | 4 | 0.2 | 0.2 |
| ET373 | F | 59 | ET | 6 | 4 | 0.2 | 0.2 |
| ET480 | M | 64.5 | ET | 7 | 3 | 0.4 | 0.4 |
| ET400 | F | 78.5 | ET | 7 | 3 | 0.4 | 0.4 |
| ET433 | F | 79 | ET | 8 | 2 | 0.6 | 0.6 |
| ET468 | F | 62 | ET | 8 | 2 | 0.6 | 0.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vila Pouca, C.; Gervais, C.; Reed, J.; Brown, C. Incubation under Climate Warming Affects Behavioral Lateralisation in Port Jackson Sharks. Symmetry 2018, 10, 184. https://doi.org/10.3390/sym10060184
Vila Pouca C, Gervais C, Reed J, Brown C. Incubation under Climate Warming Affects Behavioral Lateralisation in Port Jackson Sharks. Symmetry. 2018; 10(6):184. https://doi.org/10.3390/sym10060184
Chicago/Turabian StyleVila Pouca, Catarina, Connor Gervais, Joshua Reed, and Culum Brown. 2018. "Incubation under Climate Warming Affects Behavioral Lateralisation in Port Jackson Sharks" Symmetry 10, no. 6: 184. https://doi.org/10.3390/sym10060184