Digitally Printed AgNPs Doped TiO2 on Commercial Porcelain-Grès Tiles: Synergistic Effects and Continuous Photocatalytic Antibacterial Activity
Abstract
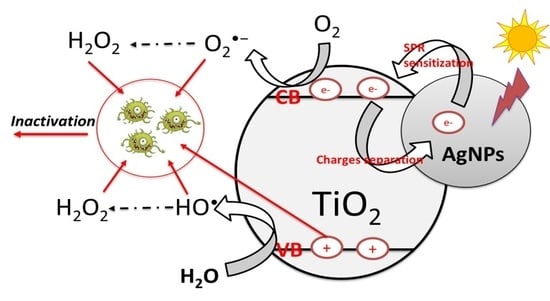
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Anti-Bacterial Ag@TiO2 Photoactive Porcelain-Grès Tiles
2.3. Characteristics of Ag@TiO2 Photoactive Porcelain-Grès Tiles
2.4. Antibacterial Experiments
2.4.1. Photocatalytic Properties
2.4.2. Bacterial Inactivation and Used Light Sources
2.4.3. Stereomicroscopy and Live/Dead Bacteria
2.4.4. Micro-Oxidation (Local pH) and Interfacial Potential at the Surface of the Coated Tiles
2.4.5. ROS Quenchers
3. Results and Discussion
3.1. Photocatalyst Characterization
3.2. E. coli Inactivation under Different Solar Light Intensities
3.3. Different Initial Concentration of Bacteria
3.4. Bacterial Inactivation under Indoor Light
3.5. Stereomicroscopy and Live/Dead Bacteria at the Interface Of Tiles
3.6. Interfacial Potential and Micro-pH at the Tiles’ Surface under Low Intensity Solar Simulated Light (50 mW/cm2)
3.7. ROS Quenchers
4. Comparison of the Current Research and a Standard Test According to ISO22196:2011
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Poon, C.S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Sacchi, B.; Capelli, S.; Pirola, C.; Cerrato, G.; Morandi, S.; Capucci, V. Micro-sized TiO2 as photoactive catalyst coated on industrial porcelain grès tiles to photodegrade drugs in water. Environ. Sci. Pollut. Res. 2018, 25, 20348–20353. [Google Scholar] [CrossRef]
- Bianchi, C.; Pirola, C.; Gatto, S.; Nucci, S.; Minguzzi, A.; Cerrato, G.; Biella, S.; Capucci, V. New surface properties in porcelain gres tiles with a look to human and environmental safety. Adv. Mater. Sci. Eng. 2012, 2012, 970182. [Google Scholar] [CrossRef] [Green Version]
- Gatto, S. Photocatalytic Activity Assessment of Micro-Sized TiO2 Used As Powders and As Starting Material for Porcelain Gres Tiles Production. Ph.D. Thesis, University of Milan, Milan, Italy, December 2014. [Google Scholar]
- Bianchi, C.L.; Pirola, C.; Stucchi, M.; Cerrato, G.; Galli, F.; di Michele, A.; Biella, S.; Chen, W.-F.; Koshy, P.; Sorrell, C. Photocatalytic TiO2: From Airless Jet Spray Technology to Digital Inkjet Printing; Yang, D., Ed.; IntechOpen: London, UK, 2018; pp. 261–279. [Google Scholar]
- Klasen, H. A historical review of the use of silver in the treatment of burns. II. Renewed interest for silver. Burns 2000, 26, 131–138. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.Y. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Russell, A.; Hugo, W. 7 Antimicrobial Activity and Action of Silver. In Progress in Medicinal Chemistry; Elsevier Science B.V.: Amsterdam, The Netherlands, 1994; pp. 351–370. [Google Scholar]
- Wang, J.; Li, J.; Guo, G.; Wang, Q.; Tang, J.; Zhao, Y.; Qin, H.; Wahafu, T.; Shen, H.; Liu, X. Silver-nanoparticles-modified biomaterial surface resistant to staphylococcus: New insight into the antimicrobial action of silver. Sci. Rep. 2016, 6, 32699. [Google Scholar] [CrossRef] [Green Version]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Wong, M.S.; Chen, C.W.; Hsieh, C.C.; Hung, S.C.; Sun, D.S.; Chang, H.H. Antibacterial property of Ag nanoparticle-impregnated N-doped titania films under visible light. Sci. Rep. 2015, 5, 11978. [Google Scholar] [CrossRef] [Green Version]
- Stabryla, L.M.; Johnston, K.A.; Millstone, J.E.; Gilbertson, L.M. Emerging investigator series: it’s not all about the ion: Support for particle-specific contributions to silver nanoparticle antimicrobial activity. Environ. Sci. 2018, 5, 2047–2068. [Google Scholar] [CrossRef]
- Kędziora, A.; Speruda, M.; Krzyżewska, E.; Rybka, J.; Łukowiak, A.; Bugla-Płoskońska, G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018, 19, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco-Torgal, F.; Jalali, S. Nanotechnology: Advantages and drawbacks in the field of construction and building materials. Constr. Build. Mater. 2011, 25, 582–590. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-González, V.; Hernández-Gordillo, A. Silver-Based Photocatalysts: A Special Class, Nanophotocatalysis and Environmental Applications; Springer Nature Switzerland AG: Basel, Switzerland, 2019; pp. 221–239. [Google Scholar]
- Rtimi, S.; Dionysiou, D.D.; Pillai, S.C.; Kiwi, J. Advances in catalytic/photocatalytic bacterial inactivation by nano Ag and Cu coated surfaces and medical devices. Appl. Catal. B 2019, 240, 291–318. [Google Scholar] [CrossRef]
- Rtimi, S.; Konstantinidis, S.; Britun, N.; Bensimon, M.; Khmel, I.; Nadtochenko, V. Extracellular bacterial inactivation proceeding without Cu-ion release: Drastic effects of the applied plasma energy on the performance of the Cu-polyester (PES) samples. Appl. Catal. B 2018, 239, 245–253. [Google Scholar] [CrossRef]
- Cerrato, G.; Galli, F.; Boffito, D.C.; Operti, L.; Bianchi, C.L. Correlation preparation parameters/activity for microTiO2 decorated with SilverNPs for NOx photodegradation under LED light. Appl. Catal. B 2019, 253, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, C.L.; Cerrato, G.; Pirola, C.; Galli, F.; Capucci, V. Photocatalytic porcelain grés large slabs digitally coated with AgNPs-TiO2. Environ. Sci. Pollut. Res. 2019, 26, 1–7. [Google Scholar] [CrossRef]
- Stucchi, M.; Bianchi, C.L.; Argirusis, C.; Pifferi, V.; Neppolian, B.; Cerrato, G.; Boffito, D. Ultrasound assisted synthesis of Ag-decorated TiO2 active in visible light. Ultrason. Sonochem. 2018, 40, 282–288. [Google Scholar] [CrossRef]
- Djellabi, R.; Yang, B.; Xiao, K.; Gong, Y.; Cao, D.; Sharif, H.M.A.; Zhao, X.; Zhu, C.; Zhang, J. Unravelling the Mechanistic Role of Ti-O-C Bonding Bridge at Titania/Lignocellulosic Biomass Interface for Cr (VI) Photoreduction Under Visible Light. J. Colloid Interface Sci. 2019, 553, 409–417. [Google Scholar] [CrossRef]
- Yoo, S.M.; Rawal, S.B.; Lee, J.E.; Kim, J.; Ryu, H.Y.; Park, D.W.; Lee, W.I. Size-dependence of plasmonic Au nanoparticles in photocatalytic behavior of Au/TiO2 and Au@ SiO2/TiO2. Appl. Catal. A 2015, 499, 47–54. [Google Scholar] [CrossRef]
- Wang, P.; Huang, B.; Dai, Y.; Whangbo, M.H. Plasmonic photocatalysts: Harvesting visible light with noble metal nanoparticles. Phys. Chem. Chem. Phys. 2012, 14, 9813–9825. [Google Scholar] [CrossRef]
- Djellabi, R.; Ghorab, M.F.; Smara, A.; Bianchi, C.L.; Cerrato, G.; Zhao, X.; Yang, B. Titania–Montmorillonite for the Photocatalytic Removal of Contaminants from Water: Adsorb & Shuttle Process, Green Materials for Wastewater Treatment; Springer Nature Switzerland AG: Basel, Switzerland, 2020; pp. 291–319. [Google Scholar]
- Chen, Y.-S.; Chao, B.-K.; Nagao, T.; Hsueh, C.-H. Effects of Ag particle geometry on photocatalytic performance of Ag/TiO2/reduced graphene oxide ternary systems. Mater. Chem. Phys. 2020, 240, 122216. [Google Scholar] [CrossRef]
- Prieto, P.; Nistor, V.; Nouneh, K.; Oyama, M.; Abd-Lefdil, M.; Díaz, R. XPS study of silver, nickel and bimetallic silver–nickel nanoparticles prepared by seed-mediated growth. Appl. Surf. Sci. 2012, 258, 8807–8813. [Google Scholar] [CrossRef]
- Rtimi, S.; Sanjines, R.; Pulgarin, C.; Houas, A.; Lavanchy, J.-C.; Kiwi, J. Coupling of narrow and wide band-gap semiconductors on uniform films active in bacterial disinfection under low intensity visible light: Implications of the interfacial charge transfer (IFCT). J. Hazard. Mater. 2013, 260, 860–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rtimi, S.; Pascu, M.; Sanjines, R.; Pulgarin, C.; Ben-Simon, M.; Houas, A.; Lavanchy, J.C.; Kiwi, J. ZrNO–Ag co-sputtered surfaces leading to E. coli inactivation under actinic light: Evidence for the oligodynamic effect. Appl. Catal. B 2013, 138, 113–121. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhang, L.; An, T.; Li, G.; Yip, H.Y.; Wong, P.K. Comparative study of visible-light-driven photocatalytic mechanisms of dye decolorization and bacterial disinfection by B–Ni-codoped TiO2 microspheres: The role of different reactive species. Appl. Catal. B 2011, 108, 108–116. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, A.; Li, Y.; Zhang, L.; Yip, H.Y.; Zhao, H.; An, T.; Wong, P.K. Naturally occurring sphalerite as a novel cost-effective photocatalyst for bacterial disinfection under visible light. Environ. Sci. Technol. 2011, 45, 5689–5695. [Google Scholar] [CrossRef] [PubMed]
- ISO 22196. Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces; International Organization for Standardization Geneva: Geneva, Switzerland, 2011. [Google Scholar]











| BET Surface Area (m2 g−1) | Particle Size Range (nm) | XPS Ti 2p3/2 (eV) | XPS OH/Otot | Band Gap (eV) |
|---|---|---|---|---|
| 12 ± 2 m2 g−1 | 110–130 | 458.4 | 0.32 | 3.15 |
| Test | Time (h) | UM—Unit Measurements | Results |
|---|---|---|---|
| N. bacteria inoculated on NON photocatalytic tiles (U0) | 0 | Log (cell/cm2) | 4.50 |
| N. bacteria inoculated on photocatalytic tiles (A0) | 0 | Log (cell/cm2) | 4.45 |
| N. bacteria inoculated on NON photocatalytic tiles (Ut) | 24 | Log (cell/cm2) | 5.72 |
| N. bacteria inoculated on photocatalytic tiles (At) | 24 | Log (cell/cm2) | 1.00 |
| Antibacterial activity R * | Log10 | 4.72 | |
| Antibacterial activity R * | % | 99.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, C.L.; Cerrato, G.; Bresolin, B.M.; Djellabi, R.; Rtimi, S. Digitally Printed AgNPs Doped TiO2 on Commercial Porcelain-Grès Tiles: Synergistic Effects and Continuous Photocatalytic Antibacterial Activity. Surfaces 2020, 3, 11-25. https://doi.org/10.3390/surfaces3010002
Bianchi CL, Cerrato G, Bresolin BM, Djellabi R, Rtimi S. Digitally Printed AgNPs Doped TiO2 on Commercial Porcelain-Grès Tiles: Synergistic Effects and Continuous Photocatalytic Antibacterial Activity. Surfaces. 2020; 3(1):11-25. https://doi.org/10.3390/surfaces3010002
Chicago/Turabian StyleBianchi, Claudia Letizia, Giuseppina Cerrato, Bianca Maria Bresolin, Ridha Djellabi, and Sami Rtimi. 2020. "Digitally Printed AgNPs Doped TiO2 on Commercial Porcelain-Grès Tiles: Synergistic Effects and Continuous Photocatalytic Antibacterial Activity" Surfaces 3, no. 1: 11-25. https://doi.org/10.3390/surfaces3010002