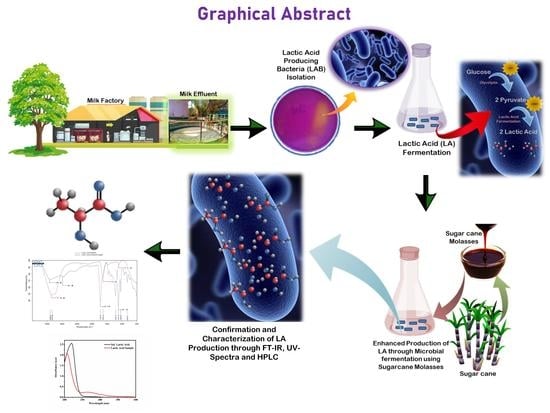
Studies on Optimization of Sustainable Lactic Acid Production by Bacillus amyloliquefaciens from Sugarcane Molasses through Microbial Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation, Screening of Lactic Acid Producing Bacteria
2.2. Screening for Best LAB Isolates
2.3. Pilot Scale Test of LA Production Using Sugarcane Molasses as Carbon Source
2.4. Effect of Sugarcane Molasses as Carbon Sources on LA Production
2.5. Molecular Identification and Phylogenetic Tree Construction of LAB
2.6. Downstream Processing of LA
2.7. Characterization of LA
2.7.1. UV-Vis Spectrophotometer Analysis of LA
2.7.2. Thin Layer Chromatographic for the Identification of LA
2.7.3. Analysis of LA Using HPLC
2.8. Statistical Analysis
3. Result and Discussion
3.1. Isolation, Screening of Lactic Acid Producing Bacteria
3.2. Screening for Best LAB Isolates
3.3. Pilot Scale Test of LA Production Using Sugarcane Molasses as Carbon Sources
3.4. Molecular Identification and Phylogenetic Tree Construction of LAB
3.4.1. UV-Vis Spectrophotometer Analysis of LA
3.4.2. Thin Layer Chromatographic for the Identification of LA
3.5. Effect of Sugarcane Molasses Concentration on LA Production
3.5.1. Fourier Transforms Infrared Spectroscopy (FTIR) Analysis of Lactic Acid
3.5.2. HPLC Analysis of Lactic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LA | Lactic Acid |
| SM | Sugarcane Molasses |
| BCP | Bromocresol Purple |
| MRS | de Man, Rogosa & Sharpe |
| mMRS | Modified MRS |
| FM | Fermentation Media |
| LAB | Lactic acid producing bacteria |
References
- Dumbrepatil, A.; Adsul, M.; Chaudhari, S.; Khire, J.; Gokhale, D. Utilization of molasses sugar for lactic acid production by Lactobacillus delbrueckii subsp. delbrueckii mutant Uc-3 in batch fermentation. Appl. Environ. Microbiol. 2008, 74, 333–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yoshida, M.; Vadlani, P.V. Biosynthesis of d-lactic acid from lignocellulosic biomass. Biotechnol. Lett. 2018, 40, 1167–1179. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, W.; Luo, J.; Wan, Y. Exploring the potential of lactic acid production from lignocellulosic hydrolysates with various ratios of hexose versus pentose by Bacillus coagulans IPE22. Bioresour. Technol. 2018, 261, 342–349. [Google Scholar] [CrossRef]
- Lin, H.T.V.; Huang, M.Y.; Kao, T.Y.; Lu, W.J.; Lin, H.J.; Pan, C.L. Production of lactic acid from seaweed hydrolysates via lactic acid bacteria fermentation. Fermentation 2020, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Din, N.A.S.; Lim, S.J.; Maskat, M.Y.; Mutalib, S.A.; Zaini, N.A.M. Lactic acid separation and recovery from fermentation broth by ion-exchange resin: A review. Bioresour. Bioprocess 2021, 8, 31. [Google Scholar] [CrossRef]
- Thygesen, A.; Tsapekos, P.; Alvarado-Morales, M.; Angelidaki, I. Valorization of municipal organic waste into purified lactic acid. Bioresour. Technol. 2021, 342, 125933. [Google Scholar] [CrossRef] [PubMed]
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.V.N.; Devaraj, K.; Mani, Y.; Devaraj, T.; Subramanian, S. Production of optically pure lactic acid by microbial fermentation: A review. Environ. Chem. Lett. 2021, 19, 539–556. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Taher, H. A review on the lactic acid fermentation from low-cost renewable materials: Recent developments and challenges. Environ. Technol. Innov. 2020, 20, 101138. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J.K.; Yang, Z.X.; Han, L.; Tan, T. Utilization of white rice bran for production of l-lactic acid. Biomass Bioenergy 2012, 39, 53–58. [Google Scholar] [CrossRef]
- Monavari, S.; Galbe, M.; Zacchi, G. Influence of impregnation with lactic acid on sugar yields from steam pretreatment of sugarcane bagasse and spruce, for bioethanol production. Biomass Bioenergy 2011, 35, 3115–3122. [Google Scholar] [CrossRef]
- Srivastava, A.; Poonia, A.; Tripathi, A.D.; Singh, R.P.; Srivastava, S.K. Optimization of nutritional supplements for enhanced lactic acid production utilizing sugar refinery by-products. Ann. Microbiol. 2014, 64, 1211–1221. [Google Scholar] [CrossRef]
- Chen, H.; Su, Z.; Wang, Y.; Wang, B.; Si, Z.; Lu, J.; Su, C.; Ren, W.; Chen, H.; Cai, D.; et al. Lactic acid production from pretreated corn stover with recycled streams. Process Biochem. 2020, 91, 132–140. [Google Scholar] [CrossRef]
- Flores-Albino, B.; Arias, L.; Gómez, J.; Castillo, A.; Gimeno, M.; Shirai, K. Chitin and L(+)-lactic acid production from crab (Callinectes bellicosus) wastes by fermentation of Lactobacillus sp. B2 using sugar cane molasses as carbon source. Bioprocess Biosyst. Eng. 2012, 35, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.A.; Schneider, R.; Lunelli, B.H.; Rossell, C.E.V.; Filho, R.M.I.; Venus, J. A simple biorefinery concept to produce 2g-lactic acid from Sugar Beet Pulp (SBP): A high-value target approach to valorize awaste stream. Molecules 2020, 25, 2113. [Google Scholar] [CrossRef]
- de Oliveira, R.A.; Filho, R.M.; Rossell, C.E.V. High lactic acid production from molasses and hydrolysed sugarcane bagasse. Chem. Eng. Trans. 2016, 50, 307–312. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, B. L(+)-lactic acid production using sugarcane molasses and waste potato starch: An alternative approach. In Proceedings of the 31st Annual Australian Society of Sugar Cane Technologists Conference 2009, ASSCT 2009, Balina, Australia, 5–8 May 2009; pp. 588–596. [Google Scholar]
- Luo, R.; Zhang, Y.; Wang, F.; Liu, K.; Huang, G.; Zheng, N.; Wang, J. Effects of sugar cane molasses addition on the fermentation quality, microbial community, and tastes of alfalfa silage. Animals 2021, 11, 355. [Google Scholar] [CrossRef]
- John, R.P.; Gangadharan, D.; Nampoothiri, K.M. Genome shuffling of Lactobacillus delbrueckii mutant and Bacillus amyloliquefaciens through protoplasmic fusion for l-lactic acid production from starchy wastes. Bioresour. Technol. 2008, 99, 8008–8015. [Google Scholar] [CrossRef]
- Elezi, O.; Kourkoutas, Y.; Koutinas, A.A.; Kanellaki, M.; Bezirtzoglou, E.; Barnett, Y.A.; Nigam, P. Food additive lactic acid production by immobilized cells of Lactobacillus brevis on delignified cellulosic material. J. Agric. Food Chem. 2003, 51, 5285–5289. [Google Scholar] [CrossRef]
- Chen, P.T.; Hong, Z.S.; Cheng, C.L.; Ng, I.S.; Lo, Y.C.; Nagarajan, D.; Chang, J.S. Exploring fermentation strategies for enhanced lactic acid production with polyvinyl alcohol-immobilized Lactobacillus plantarum 23 using microalgae as feedstock. Bioresour. Technol. 2020, 308, 123266. [Google Scholar] [CrossRef]
- Chookietwattana, K. Lactic Acid Production from Simultaneous Saccharification and Fermentation of Cassava Starch by Lactobacillus Plantarum MSUL 903. APCBEE Procedia 2014, 8, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Dahiya, D.K.; Puniya, A.K. Isolation, molecular characterization and screening of indigenous lactobacilli for their abilities to produce bioactive conjugated linoleic acid (CLA). J. Food Sci. Technol. 2017, 54, 792–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.M.; Lee, Y. A differential medium for lactic acid-producing bacteria in a mixed culture. Lett. Appl. Microbiol. 2008, 46, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Yadav, N.; Nain, L.; Khare, S.K. A simple downstream processing protocol for the recovery of lactic acid from the fermentation broth. Bioresour. Technol. 2020, 318, 124260. [Google Scholar] [CrossRef] [PubMed]
- Borshchevskaya, L.N.; Gordeeva, T.L.; Kalinina, A.N.; Sineokii, S.P. Spectrophotometric determination of lactic acid. J. Anal. Chem. 2016, 71, 755–758. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, J.; Li, W. L-(+) lactic acid production by Rhizopus oryzae using pretreated dairy manure as carbon and nitrogen source. Biomass Bioenergy 2012, 47, 442–450. [Google Scholar] [CrossRef]
- Karnaouri, A.; Asimakopoulou, G.; Kalogiannis, K.G.; Lappas, A.; Topakas, E. Efficient D-lactic acid production by Lactobacillus delbrueckii subsp. bulgaricus through conversion of organosolv pretreated lignocellulosic biomass. Biomass Bioenergy 2020, 140, 105672. [Google Scholar] [CrossRef]
- Lee, K.Y.; So, J.S.; Heo, T.R. Thin layer chromatographic determination of organic acids for rapid identification of bifidobacteria at genus level. J. Microbiol. Methods 2001, 45, 1–6. [Google Scholar] [CrossRef]
- Xu, K.; Xu, P. Efficient production of l-lactic acid using co-feeding strategy based on cane molasses/glucose carbon sources. Bioresour. Technol. 2014, 153, 23–29. [Google Scholar] [CrossRef]
- Alonso, S.; Herrero, M.; Rendueles, M.; Díaz, M. Residual yoghurt whey for lactic acid production. Biomass Bioenergy 2010, 34, 931–938. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, Z.; Zheng, Y.; Zhou, J.; Xiu, Z. Efficient production of lactic acid from sugarcane molasses by a newly microbial consortium CEE-DL15. Process Biochem. 2019, 81, 132–138. [Google Scholar] [CrossRef]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Direct lactic acid fermentation: Focus on simultaneous saccharification and lactic acid production. Biotechnol. Adv. 2009, 27, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ding, S.; Li, Z.; Tan, T. L-lactic acid production by Lactobacillus casei fermentation with corn steep liquor-supplemented acid-hydrolysate of soybean meal. Biotechnol. J. 2006, 1, 1453–1458. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, M.; Blanco-Catalá, J.; Schneider, R.; Turon, X.; Venus, J. High L(+)-lactic acid productivity in continuous fermentations using bakery waste and lucerne green juice as renewable substrates. Bioresour. Technol. 2020, 316, 123949. [Google Scholar] [CrossRef]
- Lunelli, B.H.; Andrade, R.R.; Atala, D.I.P.; MacIel, M.R.W.; Filho, F.M.; Filho, R.M.I. Production of lactic acid from sucrose: Strain selection, fermentation, and kinetic modeling. Appl. Biochem. Biotechnol. 2010, 161, 227–237. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Tripathi, A.D.; Jha, A.; Poonia, A.; Sharma, N. Production, optimization and characterization of lactic acid by Lactobacillus delbrueckii NCIM 2025 from utilizing agro-industrial byproduct (cane molasses). J. Food Sci. Technol. 2015, 52, 3571–3578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, L.F.; de Lima, C.J.B.; Bernardo, M.P.; Contiero, J. D(-)-lactic acid production by leuconostoc mesenteroides B512 using different carbon and nitrogen sources. Appl. Biochem. Biotechnol. 2011, 164, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Chaisu, K.; Charles, A.L.; Guu, Y.-K.; Yen, T.-B.; Chiu, C.-H. Optimization Lactic Acid Production from Molasses Renewable Raw Material through Response Surface Methodology with Lactobacillus Casei M-15. APCBEE Procedia 2014, 8, 194–198. [Google Scholar] [CrossRef] [Green Version]
- Calabia, B.P.; Tokiwa, Y. Production of D-lactic acid from sugarcane molasses, sugarcane juice and sugar beet juice by Lactobacillus delbrueckii. Biotechnol. Lett. 2007, 29, 1329–1332. [Google Scholar] [CrossRef]
- Chen, H.; Chen, B.; Su, Z.; Wang, K.; Wang, B.; Wang, Y.; Si, Z.; Wu, Y.; Cai, D.; Qin, P. Efficient lactic acid production from cassava bagasse by mixed culture of Bacillus coagulans and lactobacillus rhamnosus using stepwise pH controlled simultaneous saccharification and co-fermentation. Ind. Crops Prod. 2020, 146, 112175. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Taher, H. Comparative study of lactic acid production from date pulp waste by batch and cyclic–mode dark fermentation. Waste Manag. 2021, 120, 585–593. [Google Scholar] [CrossRef]
- Ding, S.; Tan, T. l-lactic acid production by Lactobacillus casei fermentation using different fed-batch feeding strategies. Process Biochem. 2006, 41, 1451–1454. [Google Scholar] [CrossRef]
- Liang, S.; Jiang, W.; Song, Y.; Zhou, S.F. Improvement and Metabolomics-Based Analysis of d -Lactic Acid Production from Agro-Industrial Wastes by Lactobacillus delbrueckii Submitted to Adaptive Laboratory Evolution. J. Agric. Food Chem. 2020, 68, 7660–7669. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Gao, M.; Yin, Z.; Zhu, W.; Liu, S.; Wang, Q. Lactic acid and animal feeds production from Sophora flavescens residues by Rhizopus oryzae fermentation. Process Biochem. 2020, 92, 401–408. [Google Scholar] [CrossRef]
- Trakarnpaiboon, S.; Srisuk, N.; Piyachomkwan, K.; Yang, S.T.; Kitpreechavanich, V. L-Lactic acid production from liquefied cassava starch by thermotolerant Rhizopus microsporus: Characterization and optimization. Process Biochem. 2017, 63, 26–34. [Google Scholar] [CrossRef]
- Lu, Z.; Wei, M.; Yu, L. Enhancement of pilot scale production of l(+)-lactic acid by fermentation coupled with separation using membrane bioreactor. Process Biochem. 2012, 47, 410–415. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Wang, D.; Xing, J. Improving the lactic acid production of Actinobacillus succinogenes by using a novel fermentation and separation integration system. Process Biochem. 2014, 49, 1245–1250. [Google Scholar] [CrossRef]
- Zhao, W.; Huang, J.; Lv, C.; Hu, S.; Yao, S.; Mei, L.; Lei, Y. pH stabilization of lactic acid fermentation via the glutamate decarboxylation reaction: Simultaneous production of lactic acid and γ-aminobutyric acid. Process Biochem. 2015, 50, 1523–1527. [Google Scholar] [CrossRef]













| Sl. No | Fermentation Media/Substratum | Microorganisms | Lactic Acid Production |
|---|---|---|---|
| 1. | Corn-Steep Liquor with acid-hydrolysate Soybean meal | Lactobacillus casei LA-04-1 | 162.5 g/L [32,33] |
| 2. | Bakery waste hydrolysates and lucerne green juice | Bacillus coagulans | 62.2 g/L [34] |
| 3. | Diluted sugarcane molasses | Bacterial isolate 25 | 8.5 g/L [35] |
| 4. | Cane molasses | Lactobacillus delbrueckii NCIM 2025 | 84.50 g/L [36] |
| 5. | Sugar and yeast autolysate | L. mesenteroides B512 | 116.9 and 44.25 g/L [37] |
| 6. | Sugarcane molasses | Lactobacillus casei M-15 | 38.33 g/L [38] |
| 7. | Sugarcane molasses, sugarcane juice and sugar beet juice | Lactobacillus delbrueckii | 107 g/L, 120 g/L and 84 g/L, respectively [39] |
| 8. | Cassava bagasse | B. coagulans | 110 g/L [40] |
| 9. | Cassava bagasse | L. rhamnosus and B.coagulans | 112.5 g/L [40] |
| 10. | Date pulp | Indigenous Microbiota | 21.66 g/L [41] |
| 11. | Corn steep liquor | Lactobacillus casei | 180 g/L [42] |
| 12. | Agro-industrial wastes | Lactobacillus delbrueckii | 112.3 g/L [43] |
| 13. | Sophora flavescens residues | Rhizopus oryzae | 46.78 g/L [44] |
| 14. | Liquefied cassava starch | Rhizopus microsporus | 84.3 g/L (Batch) and 105–119 g/L (Fed Batch) [45] |
| 15. | Yam tuber reducing sugar, wheat bran hydrolysate and persimmon juice | Lactobacillus rhamnosus HG09F5-27 | 157.22 g/L [46] |
| 16. | Fermentation Broth | Actinobacillus succinogenes 130Z | 183.4 g/L [47] |
| 17. | Fermentation Broth | Rhizopus oryzae As3.819 | 80.2 g/L [48] |
| 18. | Fermentation Broth | Bacillus amyloliquefaciens J2V2AA | 49.17 g/L (This Study) |
| 19. | Sugarcane Molasses | Bacillus amyloliquefaciens J2V2AA | 178 g/L (This Study) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vignesh Kumar, B.; Muthumari, B.; Kavitha, M.; John Praveen Kumar, J.K.; Thavamurugan, S.; Arun, A.; Jothi Basu, M. Studies on Optimization of Sustainable Lactic Acid Production by Bacillus amyloliquefaciens from Sugarcane Molasses through Microbial Fermentation. Sustainability 2022, 14, 7400. https://doi.org/10.3390/su14127400
Vignesh Kumar B, Muthumari B, Kavitha M, John Praveen Kumar JK, Thavamurugan S, Arun A, Jothi Basu M. Studies on Optimization of Sustainable Lactic Acid Production by Bacillus amyloliquefaciens from Sugarcane Molasses through Microbial Fermentation. Sustainability. 2022; 14(12):7400. https://doi.org/10.3390/su14127400
Chicago/Turabian StyleVignesh Kumar, Balasubramanian, Balakrishnan Muthumari, Murugan Kavitha, John Kennedy John Praveen Kumar, Subbu Thavamurugan, Alagarsamy Arun, and Muthuramalingam Jothi Basu. 2022. "Studies on Optimization of Sustainable Lactic Acid Production by Bacillus amyloliquefaciens from Sugarcane Molasses through Microbial Fermentation" Sustainability 14, no. 12: 7400. https://doi.org/10.3390/su14127400