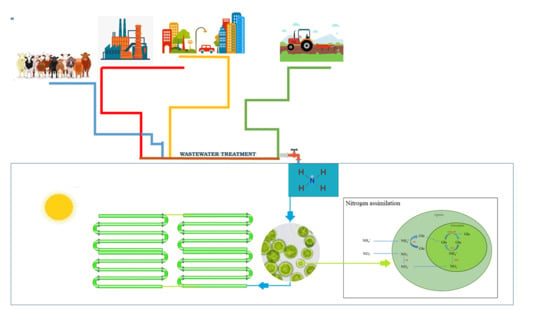
Ammonium Utilization in Microalgae: A Sustainable Method for Wastewater Treatment
Abstract
:1. Introduction
2. Nitrogen Sources for Microalgae and Ammonium Utilization
3. The Equilibrium Ammonium/Ammonia and Effect on Microalgae
4. Wastewater and Ammonium Content
5. Microalgae in Wastewater Treatment for Ammonium Removal
6. Extremophilic Microalgae in WWT
7. High-Value Molecules from Microalgae Exploited in WWT
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- De Morais, M.G.; de Fontoura Prates, D.; Moreira, J.B.; Duarte, J.H.; Costa, J.A.V. Phycocyanin from microalgae: Properties, extraction and purification, with some recent applications. Ind. Biotechnol. 2018, 14, 30–37. [Google Scholar] [CrossRef]
- Bottone, C.; Camerlingo, R.; Miceli, R.; Salbitani, G.; Sessa, G.; Pirozzi, G.; Carfagna, S. Antioxidant and anti-proliferative properties of extracts from heterotrophic cultures of Galdieria sulphuraria. Nat. Prod. Res. 2019, 15, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vidanarachchi, J.K.; Kurukulasuriya, M.S.; Malshani Samaraweera, A.; Silva, K.F.S.T. Applications of Marine Nutraceuticals in Dairy Products. Adv. Food Nutr. Res. 2012, 65, 457–478. [Google Scholar] [PubMed]
- De Jesus Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, F.; Macedo, A.; Malcata, F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef] [Green Version]
- Napolitano, G.; Fasciolo, G.; Salbitani, G.; Venditti, P. Chlorella sorokiniana Dietary Supplementation Increases Antioxidant Capacities and Reduces ROS Release in Mitochondria of Hyperthyroid Rat Liver. Antioxidants 2020, 9, 883. [Google Scholar] [CrossRef]
- Wu, H.Y.; Hu, H.Y.; Yu, Y.; Zhang, T.Y.; Zhu, S.F.; Zhuang, L.L.; Zhang, X.; Lu, Y. Microalgal species for suitable biomass/lipid production using wastewater as resources: A review. Renew. Sustain. Energy Rev. 2014, 33, 675–688. [Google Scholar] [CrossRef]
- Sun, X.M.; Ren, L.J.; Zhao, Q.Y.; Ji, X.J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef] [Green Version]
- Metting, F.B. Biodiversity and application of microalgae. J. Microbiol. Bioech. 1996, 17, 477–489. [Google Scholar] [CrossRef]
- Salbitani, G.; Barone, C.M.A.; Carfagna, S. Effect of bicarbonate on growth of the oleaginous microalga Botryococcus braunii. Int. J. Plant. Biol. 2019, 10, 8273. [Google Scholar] [CrossRef] [Green Version]
- Serive, B.; Kaas, R.; Bérard, J.B.; Pasquet, V.; Picot, L.; Cadoret, J.P. Selection and optimisation of a method for efficient metabolites extraction from microalgae. Bioresour. Technol. 2012, 124, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Nagao, N.; Kasan, N.A.; Khatoon, H.; Rahman, N.A.; Takahashi, K.; Furuya, K.; Yamada, Y.; Wahid, M.E.A.; Jusoh, M. Bioprospecting of indigenous marine microalgae with ammonium tolerance from aquaculture ponds for microalgae cultivation with ammonium-rich wastewaters. J. Biotechnol. 2020, 323, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, G.; Demurtas, O.; Ferrante, P. Le microalghe come bio-fabbriche per composti ad elevato valore aggiunto. ENEA Mag. EAI Speciale Biotecnologie per lo Sviluppo Sostenibile 2013, I, 61–66. [Google Scholar]
- Salbitani, G.; Bolinesi, F.; Affuso, M.; Carraturo, F.; Mangoni, O.; Carfagna, S. Rapid and positive effect of bicarbonate addition on growth and photosynthetic efficiency of the green microalgae Chlorella sorokiniana (Chlorophyta, Trebouxiophyceae). Appl. Sci. 2020, 10, 4515. [Google Scholar] [CrossRef]
- Kim, G.; Mujtaba, G.; Lee, K. Effects of nitrogen sources on cell growth and biochemical composition of marine chlorophyte Tetraselmis sp. for lipid production. Algae 2016, 31, 257–266. [Google Scholar] [CrossRef]
- Carfagna, S.; Bottone, C.; Cataletto, P.R.; Petriccione, M.; Pinto, G.; Salbitani, G.; Vona, V.; Pollio, A.; Ciniglia, C. Impact of sulfur starvation in autotrophic and heterotrophic cultures of the Extremophilic Microalga Galdieria phlegrea (Cyanidiophyceae). Plant Cell Physiol. 2016, 57, 1890–1898. [Google Scholar] [CrossRef] [Green Version]
- Salbitani, G.; Del Prete, S.; Bolinesi, F.; Mangoni, O.; De Luca, V.; Carginale, V.; Donald, W.A.; Supuran, C.T.; Carfagna, S.; Capasso, C. Use of an immobilized thermostable α-CA (SspCA) for enhancing the metabolic efficiency of the freshwater green microalga Chlorella sorokiniana. J. Enzym. Inhib. Med. Chem. 2020, 35, 913–920. [Google Scholar] [CrossRef] [Green Version]
- Salbitani, G.; Carfagna, S. Different behavior between autotrophic and heterotrophic Galdieria sulphuraria (Rhodophyta) cells to nitrogen starvation and restoration. Impact on pigment and free amino acid contents. Int. J. Plant. Biol. 2020, 11, 8567. [Google Scholar] [CrossRef]
- Markou, G.; Vandamme, D.; Muylaert, K. Microalgal and cyanobacterial cultivation: The supply of nutrients. Water Res. 2014, 65, 186–202. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, M.; Agarwal, A.; Arya, M.C.; Ahmed, Z. Influence of nitrogen sources on biomass productivity of microalgae Scenedesmus bijugatus. Bioresour. Technol. 2013, 131, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Ruangsomboon, S. Effects of different media and nitrogen sources and levels on growth and lipid of green microalga Botryococcus braunii KMITL and its biodiesel properties based on fatty acid composition. Bioresour. Technol. 2015, 191, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The Use of Microalgae for Coupling Wastewater Treatment with CO2 Biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Yuan, Q. Removal of nitrogen from wastewater using microalgae and microalgae–bacteria consortia. Cogent. Environ. Sci. 2016, 2, 1275089. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae wastewater treatment: Biological and technological approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, M.R.; Othman, M.H.D.; Samah, R.A.; Puteh, M.H.; Ismail, A.F.; Mustafa Rahman, M.A.; Jaafar, J. Current trends and future prospects of ammonia removal in wastewater: A comprehensive review on adsorptive membrane development. Sep. Purif. Technol. 2019, 213, 114–132. [Google Scholar] [CrossRef]
- Mandal, S.; Shurin, J.B.; Efroymson, R.A.; Mathews, T.J. Functional divergence in nitrogen uptake rates explains diversity–productivity relationship in microalgal communities. Ecosphere 2018, 9, e02228. [Google Scholar] [CrossRef]
- Sanz-Luque, E.; Chamizo-Ampudia, A.; Llamas, A.; Galvan, A.; Fernandez, E. Understanding nitrate assimilation and its regulation in microalgae. Front. Plant. Sci. 2015, 26, 899. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Y.; Norton, J.M.; Stark, J.M.; Reeve, J.R.; Habteselassie, M.Y. Ammonia-oxidizing bacteria are more responsive than archaea to nitrogen source in an agricultural soil. Soil Biol. Biochem. 2016, 96, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar] [CrossRef]
- Pritchard, D.W.; Hurd, C.L.; Beardall, J.; Hepburn, C.D. Restricted use of nitrate and a strong preference for ammonium reflects the nitrogen ecophysiology of a light-limited red alga. J. Phycol. 2015, 51, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Lachman, S.; Mettler-Altmann, T.; Wacker, A. Nitrate or ammonium: Influences of nitrogen source on the physiology of a green alga. Ecol. Evol. 2018, 9, 1070–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellebust, J.A.; Ahmad, I. Regulation of Nitrogen Assimilation in Green Microalgae. Biol. Oceanogr. 1989, 6, 241–255. [Google Scholar]
- Chen, M.; Tang, H.; Ma, H.; Holland, T.C.; Ng, K.Y.; Salley, S.O. Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour. Technol. 2011, 102, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Müller, C.; Matt, P.; Gibon, Y.; Carillo, P.; Morcuende, R.; Scheible, W.R.; Krapp, A. Steps towards an integrated view of nitrogen metabolism. J. Exp. Bot. 2002, 53, 959–970. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.; Wu, K.; Fu, X. Nitrogen signaling and use efficiency in plants: What’s new? Curr. Opin. Plant. Biol. 2015, 27, 192–198. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Glass, A.D.; Yaeesh Siddiqi, M. Inhibition of nitrate uptake by ammonium in barley. Analysis Of component fluxes. Plant. Physiol. 1999, 120, 283–292. [Google Scholar] [CrossRef] [Green Version]
- L’Helguen, S.; Maguer, J.F.; Caradec, J. Inhibition kinetics of nitrate uptake by ammonium in size-fractionated oceanic phytoplankton communities: Implications for new production and f-ratio estimates. J. Plankton Res. 2008, 30, 1179–1188. [Google Scholar] [CrossRef]
- Wheeler, P.A.; Kokkinakis, S.A. Ammonium recycling limits nitrate use in the oceanic subarctic. Pacific. Limnol. Oceanogr. 1990, 35, 1267–1278. [Google Scholar] [CrossRef]
- Holzer, H. Regulation of enzymes by enzyme-catalized chemical modification. Advanc. Enzymol. 1969, 32, 297–326. [Google Scholar]
- Watzer, B.; Forchhammer, K. Cyanophycin synthesis optimizes nitrogen utilization in the unicellular cyanobacterium Synechocystis sp. PCC6803. Appl. Environ. Microbiol. 2018, 84, e01298-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, W.; Zhai, J.; Wei, H.; Wang, Q. Effect of ammonium nitrogen on microalgal growth, biochemical composition and photosynthetic performance in mixotrophic cultivation. Bioresour Technol. 2019, 273, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kankanamge, N.R.; Chow, C.; Welsh, D.T.; Li, T.; Teasdale, P.R. Removing ammonium from water and wastewater using cost-effective adsorbents: A review. J. Environ. Sci. 2018, 63, 174–197. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, W.; Chen, H.; Zhan, J.; He, C.; Wang, Q. Ammonium Nitrogen Tolerant Chlorella Strain Screening and Its Damaging Effects on Photosynthesis. Front. Microbiol. 2019, 9, 3250. [Google Scholar] [CrossRef]
- Collos, Y.; Harrison, P.J. Acclimatation and toxicity of high ammonium concentrations to unicellular algae. Mar. Pollut. Bull. 2014, 80, 8–23. [Google Scholar] [CrossRef]
- Erikson, R.J. An evaluation of mathematical models for the effects of pH and temperature on ammonia toxicity to aquatic organisms. Water Res. 1985, 19, 1047–1058. [Google Scholar] [CrossRef]
- Bower, C.E.; Bidwell, J.P. Ionization of Ammonia in Seawater: Effects of Temperature, pH, and Salinity. JFRBC 1978, 35, 1012–1016. [Google Scholar] [CrossRef]
- Körner, S.; Das, S.K.; Veenstra, S.; Vermaat, J.E. The effect of pH variation at the ammonium/ammonia equilibrium in wastewater and its toxicity to Lemna gibba. Aquatic Bot. 2001, 71, 71–78. [Google Scholar] [CrossRef]
- Gutierrez, J.; Kwan, T.A.; Zimmerman, J.B.; Peccia, J. Ammonia inhibition in oleaginous microalgae. Algal Res. 2016, 19, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Markou, G. Effect of light intensity on the degree of ammonia toxicity on PSII activity of Arthrospira platensis. Bioresour. Technol. 2016, 216, 453–461. [Google Scholar] [CrossRef]
- Nimptsch, J.; Pflugmacher, S. Ammonia triggers the promotion of oxidative stress in the aquatic macrophyte Myriophyllum mattogrossense. Chemosphere 2007, 66, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Zengh, H.; Wu, X.; Zou, G.; Zhou, T.; Liu, Y.; Ruan, R. Cultivation of Chlorella vulgaris in manure-free piggery wastewater with high-strength ammonium for nutrients removal and biomass production: Effect of ammonium concentration, carbon/nitrogen ratio and pH. Bioresour Technol. 2019, 273, 203–211. [Google Scholar]
- Chuka-ogwude, D.; Ogbonna, J.; Moheimani, N.R. A review on microalgal culture to treat anaerobic digestate food waste effluent. Algal Res. 2020, 47, 101841. [Google Scholar] [CrossRef]
- Arbib, Z.; Ruiz, J.; Alvarez, P.; Garrido, C.; Barragan, J.; Perales, J.A. Chlorella stigmatophora for urban wastewater nutrient removal and CO2 abatement. Int. J. Phytoremediat. 2012, 14, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karri, R.R.; Sahu, J.N.; Chimmiri, V. Critical review of abatement of ammonia from wastewater. J. Mol. Liq. 2018, 261, 21–31. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Surampalli, R.Y. Performance of anaerobic hybrid reactors for the treatment of complex phenolic wastewa-ters with biogas recirculation. Bioresour. Technol. 2013, 129, 26–32. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.; Gong, Z.; Yang, F. Removal of COD, phenols and ammonium from Lurgi coal gasification wastewater using A2O-MBR system. J. Hazard. Mater. 2012, 235, 78–84. [Google Scholar] [CrossRef]
- Jiang, Y.; McAdam, E.; Zhang, Y.; Heaven, S.; Banks, C.; Longhurst, P. Ammonia inhibition and toxicity in anaerobic digestion: A critical review. J. Water Process. Eng. 2019, 32, 100899. [Google Scholar] [CrossRef]
- Kotsyurbenko, O.R.; Chin, K.J.; Glagolev, M.V.; Stubner, S.; Simankova, M.V.; Nozhevnikova, A.N.; Conrad, R. Acetoclastic and hydrogenotrophic methane production and methanogenic populations in an acidic West-Siberian peat bog. Environ. Microbiol. 2004, 6, 1159–1173. [Google Scholar] [CrossRef]
- Ruìz-Marìn, A.; Mendoza-Espinosa, L.G.; Stephenson, T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour Technol. 2010, 101, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Lee, S.A.; Ko, S.R.; Oh, H.M.; Ahn, C.Y. Effects of photoperiod on nutrient removal, biomass production, and algal-bacterial population dynamics in lab-scale photobioreactors treating municipal wastewater. Water Res. 2015, 68, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, S.; Cao, S.; Miao, Y.; Jia, F.; Du, R.; Peng, Y. Biological nitrogen removal from sewage via anammox: Recent advances. Bioresour. Technol. 2016, 200, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Jafarinejad, S.; Park, H.; Mayton, H.; Walker, S.L.; Jiang, S.C. Concentrating ammonium in wastewater by forward osmosis using a surface modified nanofiltration membrane. Environ. Sci. Water Res. Technol. 2019, 5, 246–255. [Google Scholar] [CrossRef]
- Cruz, H.; Luckman, P.; Seviour, T.; Verstraete, W.; Laycock, B.; Pikaar, I. Rapid removal of ammonium from domestic wastewater using polymer hydrogels. Sci. Rep. 2018, 8, 2912. [Google Scholar] [CrossRef] [Green Version]
- Posadas, E.; Alcántara, C.; García-Encina, P.A.; Gouveia, L.; Guieysse, B.; Norvill, Z.; Acién, F.G.; Markou, G.; Congestri, R.; Koreiviene, J.; et al. 3—microalgae cultivation in wastewater. In Microalgae-Based Biofuels and Bioproducts; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing: Lincolnshire, IL, USA, 2017; pp. 67–91. [Google Scholar]
- García, D.; de Godos, I.; Domínguez, C.; Turiel, S.; Bolado, S.; Muñoz, R. A systematic comparison of the potential of microalgae-bacteria and purple phototrophic bacteria consortia for the treatment of piggery wastewater. Bioresour Technol. 2019, 276, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; An, J.; Lee, Y.S.; Choi, K.; Kim, J.Y. Uncertainty-based concentration estimation of chlortetracycline antibiotics in swine farms and risk probability assessment for agricultural application of manure. J. Hazard. Mater. 2021, 402, 123763. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Burton, F.L.; Stensel, H.D.; Metcalf Eddy, Inc.; Burton, F. Wastewater Engineering: Treatment and Reuse; McGraw-Hill Education: New York, NY, USA, 2003. [Google Scholar]
- Cai, T.; Park, S.Y.; Li, Y. Nutrient recovery from wastewater streams by microalgae: Status and prospects. Renew. Sustain. Energy Rev. 2013, 19, 360–369. [Google Scholar] [CrossRef]
- Yalei, Z.; Chunmin, Z.; Xuefei, L.; Jinpeng, L. Cultivation of Chlorella pyrenoidosa in soybean processing wastewater. Bioresour Technol. 2011, 102, 9884–9890. [Google Scholar]
- Muylaert, K.; Beuckels, A.; Depraetere, O.; Foubert, I.; Markou, G.; Vandamme, D. Wastewater as a Source of Nutrients for Microalgae Biomass Production. In Biomass and Biofuels from Microalgae; Moheimani, N., McHenry, M., de Boer, K., Bahri, P., Eds.; Biofuel and Biorefinery Technologies; Springer: Cham, Switzerland, 2015; p. 2. [Google Scholar]
- Raper, E.; Fisher, R.; Anderson, D.R.; Stephenson, T.; Soares, A. Nitrogen removal from coke making wastewater through a pre-denitrification activated sludge process. Sci. Total Environ. 2019, 666, 31–38. [Google Scholar] [CrossRef]
- Reges, K.D.; Costa, A.G.; Cunha, V.T.; Portela, J.C.; Batista, R.; Mendonça, V.; Pereira, J.O.; Gurgel, G.C.; Oliveira, J.F.; Freire, F.G.; et al. Growing, Production and Quality of Thornless Cactus Irrigated With Dairies Effluent. J. Agric. Sci. 2019, 11, 175. [Google Scholar] [CrossRef] [Green Version]
- Gil-Pulido, B.; Tarpey, E.; Almeida, E.L.; Finnegan, W.; Zhan, X.; Dobson, A.; O’Leary, N. Evaluation of dairy processing wastewater biotreatment in an IASBR system: Aeration rate impacts on performance and microbial ecology. Biotechnol. Rep. 2018, 19, e00263. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Chen, P.H.; Yu, R.F.; Ho, W.N. Treating ammonium-rich wastewater with sludge from water treatment plant to produce ammonium alum. Sustain. Environ. Res. 2016, 26, 63–69. [Google Scholar] [CrossRef] [Green Version]
- Brown, T.; Simpson, J. Managing phosphorous inputs to urban lakes: I. Determining the trophic state of your lake. Watershed Prot. Tech. 2001, 3, 771–781. [Google Scholar]
- Kastner, F.; Buchwald, R.; Biedermann, R. Occurrence of Aeshna viridis in marsh ditches in relation to habitat conditions (Odonata: Aeshnidae). Int. J. Odonatol. 2018, 21, 205–219. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Liang, H.; Wang, J. A critical review on ammonium recovery from wastewater for sustainable wastewater management. Bioresour. Technol. 2018, 268, 749–758. [Google Scholar] [CrossRef]
- Khanzada, Z.T.; Övez, S. Growing Fresh Water Microalgae in High Ammonium Landfill Leachate. Am. AJMA 2018, 6, 40–51. [Google Scholar] [CrossRef]
- Mayo, A.W.; Hanai, E.E. Dynamics of nitrogen transformation and removal in a pilot high rate pond. J. Water Resour. Prot. 2014, 6, 433–445. [Google Scholar] [CrossRef] [Green Version]
- Fito, J.; Alemu, K. Microalgae–bacteria consortium treatment technology for municipal wastewater management. Nanotechnol. Environ. Eng. 2019, 4, 4. [Google Scholar] [CrossRef]
- Freed, T. Wastewater industry moving toward enhanced nutrient removal standards. Water World 2007, 23, 1–3. [Google Scholar]
- Yang, J.; Xu, M.; Zhang, X.; Hu, Q.; Sommerfeld, M.; Chen, Y. Life-cycle analysis on biodiesel production from microalgae: Water footprint and nutrients balance. Bioresour. Technol. 2011, 102, 6633. [Google Scholar] [CrossRef]
- Judd, S.; van den Broeke, L.J.P.; Shurair, M.; Kuti, Y.; Znad, H. Algal remediation of CO2 and nutrient discharges: A review. Water Res. 2015, 87, 356–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markou, G.; Depraetere, O.; Muylaert, K. Effect of ammonia on the photosynthetic activity of Arthrospira and Chlorella: A study on chlorophyll fluorescence and electron transport. Algal Res. 2016, 16, 449–457. [Google Scholar] [CrossRef]
- Vargas, G.; Donoso-Bravo, A.; Vergara, C.; Ruiz-Filippi, G. Assessment of microalgae and nitrifiers activity in a consortium in a continuous operation and the effect of oxygen depletion. Electron. J. Biotechnol. 2016, 23, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Praveen, P.; Loh, K. Nutrient removal in an algal membrane photobioreactor: Effects of wastewater composition and light/dark cycle. Appl. Microbiol. Biotechnol. 2019, 103, 3571–3580. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Trejo, A.; Huss, V.A.R.; Hernandez, J.-P.; Bashan, Y. Chlorella sorokiniana UTEX 2805, a heat and intense, sunlight-tolerant microalga with potential for removing ammonium from wastewater. Bioresour Technol. 2008, 99, 4980–4989. [Google Scholar]
- Kobayashi, N.; Noel, E.A.; Barnes, A.; Watson, A.; Rosenberg, J.N.; Erickson, G.; Oyler, G.A. Characterization of three Chlorella sorokiniana strains in anaerobic digested effluent from cattle manure. Bioresour Technol. 2013, 150, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Hernández, D.; Riaño, B.; Coca, M.; García-González, M.C. Treatment of agro-industrial wastewater using microalgae–bacteria consortium combined with anaerobic digestion of the produced biomass. Bioresour Technol. 2013, 135, 598–603. [Google Scholar] [CrossRef]
- Moondra, N.; Jariwala, N.D.; Christian, R.A. Sustainable treatment of domestic wastewater through microalgae. Int. J. Phytoremediat. 2020, 22, 1480–1486. [Google Scholar] [CrossRef]
- Sydney, E.B.; da Silva, T.E.; Tokarski, A.; Novak, A.C.; de Carvalho, J.C.; Woiciecohwski, A.L.; Larroche, C.; Soccol, C.R. Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl. Energy 2011, 88, 3291–3294. [Google Scholar] [CrossRef]
- Kang, J.; Wen, Z. Use of microalgae for mitigating ammonia and CO2 emissions from animal production operations-Evaluation of gas removal efficiency and algal biomass composition. Algal Res. 2015, 11, 204–210. [Google Scholar] [CrossRef]
- Uggetti, E.; Sialve, E.; Latrille, E.; Steyer, J.P. Anaerobic digestate as substrate for microalgae culture: The role of ammonium concentration on the microalgae productivity. Bioresour Technol. 2014, 152, 437–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.L.; Bui, M.H. Removal of Nutrients from Fertilizer Plant Wastewater Using Scenedesmus sp.: Formation of Bioflocculation and Enhancement of Removal Efficiency. J. Chem. 2020, 2020, 9. [Google Scholar] [CrossRef] [Green Version]
- Henkanatte-Gedera, S.M.; Selvaratnam, T.; Karbakhshravari, M.; Myint, M.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: Laboratory to field scale demonstration. Algal Res. 2017, 24, 450–456. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.K.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters. Bioresour Technol. 2014, 156, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Sloth, J.K.; Jensen, H.C.; Pleissner, D.; Eriksen, N.T. Growth and phycocyanin synthesis in the heterotrophic microalga Galdieria sulphuraria on substrates made of food waste from restaurants and bakeries. Bioresour Technol. 2017, 238, 296–305. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Boto-Ordóñez, M.; Van Hulle, S.W.H.; Ferrer, I.; Garfí, M.; Rousseau, D.P.L. Natural pigments from microalgae grown in industrial wastewater. Bioresour Technol. 2020, 303, 122894. [Google Scholar] [CrossRef] [Green Version]
- Hernández, D.; Riaño, B.; Coca, M.; Solana, M.; Bertucco, A.; García-González, M.C. Microalgae cultivation in high rate algal ponds using slaughterhouse wastewater for biofuel applications. Chem. Eng. J. 2016, 285, 449–458. [Google Scholar] [CrossRef]
- Winayu, B.N.R.; Lai, K.T.; Hsueh, H.T.; Chu, H. Production of phycobiliprotein and carotenoid by efficient extraction from Thermosynechococcus sp. CL-1 cultivation in swine wastewater. Bioresour Technol. 2021, 319, 124125. [Google Scholar] [CrossRef]
- Rinna, F.; Buono, S.; Cabanelas, I.T.D.; Nascimento, I.A.; Sansone, G.; Barone, C.M.A. Wastewater treatment by microalgae can generate high quality biodiesel feedstock. J. Water Process. Eng. 2017, 18, 144–149. [Google Scholar] [CrossRef]
- Kolmert, A.; Johnson, D.B. Remediation of acidic waste waters using immobilised, acidophilic sulfate-reducing bacteria. J. Chem. Technol. Biotechnol. 2001, 76, 836–843. [Google Scholar] [CrossRef]
- Goyal, A.; Srivastava, V.C. Treatment of highly acidic wastewater containing high energetic compounds using dimensionally stable anode. Chem. Engin. J. 2017, 325, 289–299. [Google Scholar] [CrossRef]
- Hirooka, S.; Miyagishima, S. Cultivation of Acidophilic Algae Galdieria sulphuraria and Pseudochlorella sp. YKT1 in Media Derived from Acidic Hot Springs. Front. Microbiol. 2016, 7, 2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nancucheo, I.; Johnson, D.B. Acidophilic algae isolated from mine-impacted environments and their roles in sustaining heterotrophic acidophiles. Front. Microbial. 2012, 3, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carfagna, S.; Landi, V.; Coraggio, F.; Salbitani, G.; Vona, V.; Pinto, G.; Pollio, A.; Ciniglia, C. Different characteristics of C-phycocyanin (C-PC) in two strains of the extremophilic Galdieria phlegrea. Algal Res. 2018, 31, 406–412. [Google Scholar] [CrossRef]
- Salbitani, G.; Cipolletta, S.; Vona, V.; Di Martino, C.; Carfagna, S. Heterotrophic Cultures of Galdieria phlegrea Shift to Autotrophy in the Presence or Absence of Glycerol. J. Plant. Growth Regul. 2020. [Google Scholar] [CrossRef]
- Barone, R.; De Napoli, L.; Mayol, L.; Paolucci, M.; Volpe, M.G.; D’Elia, L.; Pollio, A.; Guida, M.; Gambino, E.; Carraturo, F.; et al. Autotrophic and Heterotrophic Growth Conditions Modify Biomolecole Production in the Microalga Galdieria sulphuraria (Cyanidiophyceae, Rhodophyta). Mar. Drugs 2020, 18, 169. [Google Scholar] [CrossRef] [Green Version]
- Bugajski, P.; Kurek, K.; Jóźwiakowski, K. Effect of wastewater temperature and concentration of organic compounds on the efficiency of ammonium nitrogen removal in a household treatment plant servicing a school building. Arch. Environ. Prot. 2019, 45, 31–37. [Google Scholar]
- Alisawi, H.A.O. Performance of wastewater treatment during variable temperature. Appl. Water Sci. 2020, 10, 89. [Google Scholar] [CrossRef] [Green Version]
- Varshney, P.; Mikulic, P.; Vonshak, A.; Beardall, J.; Wangikar, P.P. Extremophilic micro-algae and their potential contribution in biotechnology. Bioresour. Technol. 2015, 184, 363–372. [Google Scholar] [CrossRef]
- Von Sperling, M. Wastewater Characteristics, Treatment and Disposal; IWA Publishing: London, UK, 2007. [Google Scholar]
- Langenhoff, A.A.M.; Stuckey, D.C. Treatment of dilute wastewater using an anaerobic baffled reactor: Effect of low temperature. Water Res. 2000, 34, 3867–3875. [Google Scholar] [CrossRef]
- Lettinga, G.; Rebac, S.; Zeeman, G. Challenge of psychrophilic anaerobic wastewater treatment. Trends Biotechnol. 2001, 19, 363–370. [Google Scholar] [CrossRef]
- Vona, V.; Di Martino Rigano, V.; Andreoli, C.; Lobosco, O.; Caiazzo, M.; Martello, A.; Carfagna, S.; Salbitani, G.; Rigano, C. Comparative analysis of photosynthetic and respiratory parameters in the psychrophilic unicellular green alga Koliella antarctica, cultured in indoor and outdoor photo-bioreactors. Physiol. Mol. Biol. Plants 2018, 24, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, C.; Lokhrost, G.M.; Mani, A.M.; Scarabel, L.; Moro, I.; La Rocca, N.; Tognetto, L. Koliella antarctica sp. nov. (Klebsormidiales) a new marine green microalga from the Ross Sea. Algological Studies Archiv für Hydrobiologie 1998, 125, 1–8. [Google Scholar]
- Fogliano, V.; Andreoli, C.; Martello, A.; Caiazzo, M.; Lobosco, O.; Formisano, F.; Carlino, P.A.; Meca, G.; Graziani, G.; Di Martino Rigano, V.; et al. Functional ingredients produced by culture of Koliella antartica. Aquaculture 2010, 299, 115–120. [Google Scholar] [CrossRef]
- Alavi, S.; Rafieyan, S.; Yavari-Bafghi, M.; Amoozegar, M.A. Extremophiles: A Powerful Choice for Bioremediation of Toxic Oxyanions. In Microbial Bioremediation & Biodegradation; Shah, M., Ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Yu, Z.; Song, M.; Pei, H.; Han, F.; Jiang, L.; Hou, Q. The growth characteristics and biodiesel production of ten algae strains cultivated in anaerobically digested effluent from kitchen waste. Alga Res. 2017, 24, 265–275. [Google Scholar] [CrossRef]
- Sharma, J.; Kumar, V.; Kumar, S.S.; Malyan, S.K.; Mathimani, T.; Bishnoi, N.R.; Pugazhendhi, A. Microalgal consortia for municipal wastewater treatment—Lipid augmentation and fatty acid profiling for biodiesel production. J. Photochem. Photobiol. B 2020, 202, 111638. [Google Scholar] [CrossRef]
- Yang, J.; Li, X.; Hu, H.Y.; Zhang, X.; Yu, Y.; Chen, Y.S. Growth and lipid accumulation properties of a freshwater microalga, Chlorella ellipsoidea Yj1, in domestic secondary effluents. Appl. Energy 2011, 88, 3295–3299. [Google Scholar] [CrossRef]
- Li, X.; Hu, H.Y.; Yang, J. Lipid accumulation and nutrient removal properties of a newly isolated freshwater microalga, Scenedesmus sp. LX1, growing in secondary effluent. New Biotechnol. 2010, 27, 59–63. [Google Scholar]
- Meng, T.K.; Kassim, M.A.; Cheirsilp, B. Chapter 4—Mixotrophic cultivation: Biomass and biochemical biosynthesis for biofuel production. In Microalgae Cultivation for Biofuels Production; Yousuf, A., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 51–67. [Google Scholar]
- Rodrigues, D.B.; Flores, É.M.M.; Barin, J.S.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Production of carotenoids from microalgae cultivated using agroindustrial wastes. Food Res. Int. 2014, 65, 144–148. [Google Scholar] [CrossRef]



| Wastewater Type | NH3-NH4+ Concentration (mg L−1) | Ref. |
|---|---|---|
| Municipal wastewater | 27–100 | [61,62,63] |
| Domestic wastewater | 39–60 | [64,65] |
| Fish processing wastewater | 8–42 | [66] |
| Piggery wastewater | 220–2945 | [16,52,67,68] |
| Wool textile mill | 54 | [69] |
| Coal gasification | 130–280 | [57,58] |
| Paper mill | 11 | [70] |
| Soybean processing | 90 | [71] |
| Olive mill | 530 | [70] |
| Winery | 110 | [72] |
| Coke production | 60 | [73] |
| Dairy effluent | 49 | [74,75] |
| Class | Species | Strain | Wastewater Type | pH | NH4+-N (mg L−1) | NH4+-N Removal (%) | Removal Time (Days) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Chlorophyceae | Chlorella sorokiniana | UTEX 2805 | Synthetic wastewater | – | 10 | 100 | 5 | [89] |
| Chlorella sorokiniana | UTEX 1230 | Digested from cattle manure | – | 893 | 75 | 25 | [90] | |
| Chlorella sorokiniana | UTEX 2714 | Digested from cattle manure | – | 893 | 59 | 25 | [90] | |
| Chlorella sorokiniana | CS–01 | Digested from cattle manure | – | 893 | 75 | 25 | [90] | |
| Chlorella sorokiniana | – | Potato processing wastewater | 5.8 | 12 | >95 | 30 | [91] | |
| Chlorella sorokiniana | – | Secondary pig manure | 7.5 | 12 | 83 | 30 | [91] | |
| Chlorella vulgaris | FACHB–30 | Piggery wastewater | 6.3 | 220 | 50 | 7 | [52] | |
| Chlorella vulgaris | – | Landfill leachate | 7 | 760 | 70 | 30 | [80] | |
| Chlorella vulgaris | – | Domestic wastewater | 7.3–8.4 | 2.7–11 | 80–87 | 1 | [92] | |
| Chlorella vulgaris | LEM 07 | Domestic wastewater | 7.2 | 13 | – | – | [93] | |
| Chlorella minutissima | – | Domestic wastewater | 7.2 | 13 | – | – | [93] | |
| Chlorella zofingiensis | – | Piggery wastewater | 6.2 | – | 65–80 | 4 | [93] | |
| Chlamydomonas reinhardtii | – | Landfill leachate | 7 | 760 | 70 | 40 | [80] | |
| Coelastrum microporum | IFA9 | Municipal wastewater | 7.3 | – | – | – | [62] | |
| Graesiella emersonii | ATCC 13482 | Synthetic wastewater | – | 4–16 | >99 | 18 | [88] | |
| Neochloris oleoabundans | LEM 17 | Domestic wastewater | 7.2 | 13 | – | – | [93] | |
| Scenedesmus dimorphus | UTEX 1237 | Animal production operation | 7 | 45 | 95 | – | [94] | |
| Scenedesmus sp | – | Anaerobic digestate | 8 | 50–260 | 60–100 | 7 | [95] | |
| Scenedesmus sp | – | Fertilizer Plant Wastewater | 7.3 | 27 | 93 | 10 | [96] | |
| Cyanidiophyceae | Galdieria sulphuraria | CCMEE 5587.1 | Urban wastewater | 1–4 | 15–25 | 63–89 | 6 | [97] |
| Galdieria sulphuraria | CCMEE 5587.1 | Primary wastewater effluent | 2.5 | 40 | 88 | 7 | [98] | |
| Galdieria sulphuraria | 074G | Food waste hydrolysates | 2 | 500 | – | – | [99] | |
| Cyanophyceae | Nostoc sp. | – | Food–industry wastewater | 7.6–9.8 | 44 | – | – | [100] |
| Arthrospira platensis | – | Food-industry wastewater | 8.6–8.9 | 38–61 | – | – | [100] | |
| Phormidium tergestinum | – | Slaughterhouse wastewater | 9.2 | – | – | – | [101] | |
| Porphyridium purpureum | – | Food–industry wastewater | 8.6–8.9 | 43–58 | – | – | [100] | |
| Synechoccus nidulans | LEM06 | Domestic wastewater | 7.2 | 13 | – | – | [93] | |
| Thermosynechococcus | CL–1 | Anoxic swine wastewater | 7.2 | 359 | 50–100 | 0.5 | [102] | |
| Thermosynechococcus | CL–1 | Aerobic swine wastewater | 7.5 | 182 | 30–60 | 0.5 | [102] | |
| Trebouxiophyceae | Botryococcus braunii | LEM 14 | Domestic wastewater | 7.2 | 13 | – | – | [93] |
| Botryococcus braunii | UTEX LB 572 | Domestic effluent | 6.4 | 8 | 62–63 | 60 | [103] | |
| Botryococcus braunii | BL C116 | Domestic effluent | 6.4 | 8 | 61–65 | 60 | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salbitani, G.; Carfagna, S. Ammonium Utilization in Microalgae: A Sustainable Method for Wastewater Treatment. Sustainability 2021, 13, 956. https://doi.org/10.3390/su13020956
Salbitani G, Carfagna S. Ammonium Utilization in Microalgae: A Sustainable Method for Wastewater Treatment. Sustainability. 2021; 13(2):956. https://doi.org/10.3390/su13020956
Chicago/Turabian StyleSalbitani, Giovanna, and Simona Carfagna. 2021. "Ammonium Utilization in Microalgae: A Sustainable Method for Wastewater Treatment" Sustainability 13, no. 2: 956. https://doi.org/10.3390/su13020956