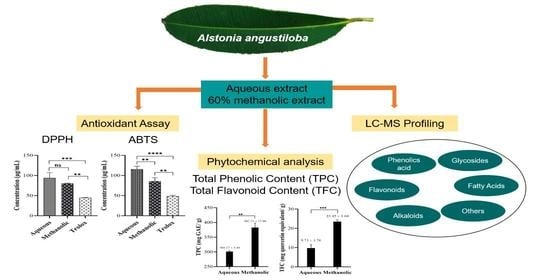
Antioxidant Activity, Total Phenolic and Flavonoid Content and LC–MS Profiling of Leaves Extracts of Alstonia angustiloba
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of Aqueous Extracts
2.3. Methanol Extraction by Soxhlet Technique
2.4. Antioxidant Activities
- (a)
- 2,2-diphenyl-1-picrylhydrazyl (DPPH)
- (b)
- 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid (ABTS)
2.5. Total Phenolic Content (TPC)
2.6. Total Flavonoid Content (TFC)
2.7. Liquid Chromatography–Mass Spectrometry (LC–MS) Analysis of A. angustiloba Leaves Extracts
2.8. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity of A. angustiloba Leaves Extracts
3.2. Total Phenolic and Flavonoid Content of A. angustiloba Leaves Extracts
3.3. Correlation between Antioxidant Activity and TPC and TFC of A. angustiloba Leaves Extracts
3.4. LC–MS Analysis of A. angustiloba Leaves Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phuyal, N.; Jha, P.K.; Raturi, P.P.; Rajbhandary, S. Total Phenolic, Flavonoid Contents, and Antioxidant Activities of Fruit, Seed, and Bark Extracts of Zanthoxylum armatum DC. Sci. World J. 2020, 2020, 8780704. [Google Scholar] [CrossRef] [PubMed]
- Rao, U.S.M.; Abdurrazak, M.; Mohd, K.S. Phytochemical screening, total flavonoid and phenolic content assays of various solvent extracts of tepal of Musa paradisiaca. Malays. J. Anal. Sci. 2016, 20, 1181–1190. [Google Scholar] [CrossRef]
- Xu, D.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Matsui, T. Nitric oxide, a janus-faced therapeutic target for diabetic microangiopathy—Friend or foe? Pharmacol. Res. 2011, 64, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Phenolic acids and flavonoids profiles of extracts from edible wild fruits and their antioxidant properties. Int. J. Food Prop. 2017, 20, 3124–3134. [Google Scholar] [CrossRef]
- Kadum, H.; Hamid, A.A.; Abas, F.; Ramli, N.S.; Mohammed, A.K.S.; Muhialdin, B.J.; Jaafar, A.H. Bioactive Compounds Responsible for Antioxidant Activity of Different Varieties of Date (Phoenix dactylifera L.) Elucidated by 1 H-NMR Based Metabolomics. Int. J. Food Prop. 2019, 22, 462–476. [Google Scholar] [CrossRef]
- Truong, D.H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef]
- Metrouh-Amir, H.; Duarte, C.M.M.; Maiza, F. Solvent effect on total phenolic contents, antioxidant, and antibacterial activities of Matricaria pubescens. Ind. Crops Prod. 2015, 67, 249–256. [Google Scholar] [CrossRef]
- Tchabo, W.; Ma, Y.; Kwaw, E.; Xiao, L.; Wu, M.; Apaliya, M.T. Impact of extraction parameters and their optimization on the nutraceuticals and antioxidant properties of aqueous extract mulberry leaf. Int. J. Food Prop. 2018, 21, 717–732. [Google Scholar] [CrossRef]
- Ng, F.S. Tropical Horticulture and Gardening; Clearwater Publications: Broomfield, CO, USA, 2005. [Google Scholar]
- Wiart, C. Medicinal Plants of Asia and the Pacific, 1st ed.; Taylor & Francis: Abingdon, UK, 2006. [Google Scholar] [CrossRef]
- Al-Adhroey, A.H.; Nor, Z.M.; Al-Mekhlafi, H.M.; Mahmud, R. Ethnobotanical study on some Malaysian anti-malarial plants: A community based survey. J. Ethnopharmacol. 2010, 132, 362–364. [Google Scholar] [CrossRef]
- Neo, L.; Yee, A.T.K.; Chong, K.Y.; Kee, C.Y.; Tan, H.T.W. The Vascular plant flora of Admiralty Forest, Singapore. Nat. Singap. 2013, 6, 61–72. [Google Scholar]
- Koyama, K.; Hirasawa, Y.; Zaima, K.; Hoe, T.C.; Chan, K.L.; Morita, H. Alstilobanines A-E, new indole alkaloids from Alstonia angustiloba. Bioorg. Med. Chem. 2008, 16, 6483–6488. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Lim, Y.Y.; Ling, S.K.; Chan, E.W.C. Caffeoylquinic acids in leaves of selected Apocynaceae species: Their isolation and content. Pharmacogn. Res. 2014, 6, 67–72. [Google Scholar] [CrossRef]
- Goh, S.H.; Lee, K.H.; Chuah, C.H.; Ong, H.C.; Madani, L.; Pereira, J.T. A phytochemical study of borneo: Selected plants from Sabah lowland forests. J. Herbs Spices Med. Plants 1997, 5, 29–52. [Google Scholar] [CrossRef]
- Ku, W.F.; Tan, S.J.; Low, Y.Y.; Komiyama, K.; Kam, T.S. Angustilobine and andranginine type indole alkaloids and an uleine-secovallesamine bisindole alkaloid from Alstonia angustiloba. Phytochemistry 2011, 72, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Rahim, N.A.; Mail, M.H.; Muhamad, M.; Sapuan, S.; Mydin, R.B.S.M.N.; Seeni, A. Investigation of antiproliferative mechanisms of Alstonia angustiloba-silver nanoparticles in skin squamous cell carcinoma (A431 cell line). J. Mol. Struct. 2022, 1250, 131814. [Google Scholar] [CrossRef]
- Wong, S.K.; Lim, Y.Y.; Abdullah, N.R.; Nordin, F.J. Assessment of antiproliferative and antiplasmodial activities of five selected Apocynaceae species. BMC Complement. Altern. Med. 2011, 11, 3. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Wong, S.K.; Chan, H.T. Apocynaceae species with antiproliferative and/or antiplasmodial properties: A review of ten genera. J. Integr. Med. 2016, 14, 269–284. [Google Scholar] [CrossRef]
- Alen, Y.; Nakajima, S.; Baba, N.; Kanzaki, H.; Kawazu, K.; Nitoda, T. Antinematodal Activity of Some Tropical Rainforest Plants against the Pinewood Nematode, Bursaphelenchus xylophilus. Z. Naturforsch. Sect. C J. Biosci. 2000, 55, 295–299. [Google Scholar] [CrossRef]
- Norhayati, I.; Getha, K.; Haffiz, J.M.; Ilham, A.M.; Sahira, H.L.; Syarifah, M.M.S.; Syamil, A.M. In vitro antitrypanosomal activity of Malaysian plants. J. Trop. For. Sci. 2013, 25, 52–59. [Google Scholar]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I.; Kabbashi, N.A. Extraction and characterization of bioactive compounds in Vernonia amygdalina leaf ethanolic extract comparing Soxhlet and microwave-assisted extraction techniques. J. Taibah Univ. Sci. 2019, 13, 414–422. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.Z.; Toha, Z.M.; Muhamad, M.; Kamal, N.N.S.N.M.; Zain, N.N.M.; Arsad, H. Antioxidant effects, antiproliferative effects, and molecular docking of Clinacanthus nutans leaf extracts. Molecules 2020, 25, 2067. [Google Scholar] [CrossRef] [PubMed]
- Awang, N.; Ali, N.; Majid, F.A.A.; Hamzah, S.; Razak, S.B.A. Total flavonoids and phenolics contents of sticky and hard propolis from 10 species of Indo-Malayan stingless bees. Malays. J. Anal. Sci. 2018, 22, 877–884. [Google Scholar]
- Araujo, N.M.P.; Silvano, H.S.; Santos, F.N.d.; de Morais, D.R.; Pereira, G.A.; Pastore, G.M. LC-MS/MS screening and identification of bioactive compounds in leaves, pulp and seed from Eugenia calycina Cambess. Food Res. Int. 2020, 137, 109556. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoʇlu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.H.; Amira, N.B.N.; Asmah, R. Antioxidant analysis of different types of edible mushrooms (Agaricus bisporous and Agaricus brasiliensis). Int. Food Res. J. 2013, 20, 1095–1102. [Google Scholar]
- Olugbami, J.O.; Gbadegesin, M.A.; Odunola, O.A. In vitro free radical scavenging and antioxidant properties of ethanol extract of Terminalia glaucescens. Pharmacogn. Res. 2015, 7, 49–56. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.A.; Intipunya, P.; Rachtanapun, P. Predictive mathematical modeling for EC50 calculation of antioxidant activity and antibacterial ability of Thai bee products. J. Appl. Pharm. Sci. 2017, 7, 122–133. [Google Scholar] [CrossRef]
- Shariff, N.F.S.M.; Singgampalam, T.; Ng, C.H.; Kue, C.S. Antioxidant activity and zebrafish teratogenicity of hydroalcoholic Moringa oleifera L. leaf extracts. Br. Food J. 2020, 122, 3129–3137. [Google Scholar] [CrossRef]
- Aziz, N.A.A.; Hasham, R.; Sarmidi, M.R.; Suhaimi, S.H.; Idris, M.K.H. A review on extraction techniques and therapeutic value of polar bioactives from Asian medicinal herbs: Case study on Orthosiphon aristatus, Eurycoma longifolia and Andrographis paniculata. Saudi Pharm. J. 2021, 29, 143–165. [Google Scholar] [CrossRef]
- Muzolf-Panek, M.; Stuper-Szablewska, K. Comprehensive study on the antioxidant capacity and phenolic profiles of black seed and other spices and herbs: Effect of solvent and time of extraction. J. Food Meas. Charact. 2021, 15, 4561–4574. [Google Scholar] [CrossRef]
- Namvar, K.; Mohammadi, A.; Salehi, E.A.; Feyzi, P. Evaluation of solvent effect (methanol: Water mixture) on the phenolic content and antioxidant activities of Stachys turcomanica Trautv. Pharm. Sci. 2017, 23, 244–248. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Tan, M.; Carranza, M.; Linis, V.; Malabed, R.; Reyes, Y.; Franco, F.; Oyong, G. Antioxidant, cytotoxic, and anti-venom activity of Alstonia parvifolia Merr. Bark. Asian Pac. J. Trop. Biomed. 2021, 11, 460–468. [Google Scholar] [CrossRef]
- Akinnawo, O.O.; Anyasor, G.N.; Osilesi, O. Aqueous fraction of Alstonia boonei de Wild leaves suppressed inflammatory responses in carrageenan and formaldehyde induced arthritic rats. Biomed. Pharm. 2017, 86, 95–101. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Ali, A.; Bashmil, Y.M.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. LC-MS/MS-QTOF screening and identification of phenolic compounds from Australian grown herbs and their antioxidant potential. Antioxidants 2021, 10, 1770. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Atanu, F.O.; Idih, F.M.; Nwonuma, C.O.; Hetta, H.F.; Alamery, S.; El-Saber Batiha, G. Evaluation of antimalarial potential of extracts from Alstonia boonei and Carica papaya in Plasmodium berghei-infected mice, Evidence-Based Complement. Altern. Med. 2021, 2021, 2599191. [Google Scholar] [CrossRef]
- Ganjewala, D.; Gupta, A.K. Study on phytochemical composition, antibacterial and antioxidant properties of different parts of Alstonia scholaris Linn. Adv. Pharm. Bull. 2013, 3, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Johari, M.A.; Khong, H.Y. Total phenolic content and antioxidant and antibacterial activities of Pereskia bleo. Adv. Pharmacol. Sci. 2019, 2019, 7428593. [Google Scholar] [CrossRef] [Green Version]
- Gou, Z.P.; Zhao, Y.L.; Zou, L.L.; Wang, Y.; Shu, S.Q.; Zhu, X.H.; Zheng, L.; Shen, Q.; Luo, Z.; Miao, J.; et al. The safety and tolerability of alkaloids from Alstonia scholaris leaves in healthy Chinese volunteers: A single-centre, randomized, double-blind, placebo-controlled phase I clinical trial. Pharm. Biol. 2021, 59, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Z.; Shang, J.; Huang, W.; Wang, B.; Wei, X.; Khan, A.; Yuan, Z.-W.; Liu, Y.-P.; Wang, Y.-F.; et al. Effects of indole alkaloids from leaf of Alstonia scholaris on post-infectious cough in mice. J. Ethnopharmacol. 2018, 218, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Yang, Z.F.; Wu, B.F.; Shang, J.H.; Liu, Y.P.; Wang, X.H.; Luo, X.D. Indole alkaloids from leaves of Alstonia scholaris (L.) R. Br. protect against emphysema in mice. J. Ethnopharmacol. 2020, 259, 112949. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Pu, S.B.; Qi, Y.; Wu, B.F.; Shang, J.H.; Liu, Y.P.; Hu, D.; Luo, X.D. Pharmacological effects of indole alkaloids from Alstonia scholaris (L.) R. Br. on pulmonary fibrosis in vivo. J. Ethnopharmacol. 2021, 267, 113506. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Han, S.; Pu, L.; Zhang, T.-C.; Zhan, X.; Fu, T.; Dai, Y.-H.; Li, Y.-X. Bioinformatics and machine learning methods to identify FN1 as a novel biomarker of aortic valve calcification. Front. Cardiovasc. Med. 2022, 9, 1–19. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]




| DPPH | ABTS | TPC | TFC | |
|---|---|---|---|---|
| DPPH | 1.000 | 0.958 *** | 0.924 ** | 0.531 |
| ABTS | 0.958 *** | 1.000 | 0.891 ** | 0.406 |
| TPC | 0.924 ** | 0.891 ** | 1.000 | 0.775 * |
| TFC | 0.531 | 0.406 | 0.775 * | 1.000 |
| DPPH | ABTS | TPC | TFC | |
|---|---|---|---|---|
| DPPH | 1.000 | 0.927 ** | 0.915 ** | 0.627 |
| ABTS | 0.927 ** | 1.000 | 0.884 ** | 0.449 |
| TPC | 0.915 ** | 0.884 ** | 1.000 | 0.808 * |
| TFC | 0.627 | 0.449 | 0.808 * | 1.000 |
| Compound | ts (min) | m/z Experimental | Teoric Mass | MS/MS Fragments | Molecular Formula | Error (ppm) |
|---|---|---|---|---|---|---|
| Phenolic acids | ||||||
| m-Coumaric acid | 8.231 | 165.0543 [M + H]+ | 164.047 | 151 | C9 H8 O3 | 1.8 |
| Gallic acid | 1.575 | 171.0286 (M + H)+ | 170.0214 | 141/151/160 | C7 H6 O5 | 0.51 |
| Aconitic acid | 0.903 | 175.0232 (M + H)+ | 174.0161 | 149/157 | C6 H6 O6 | 1.89 |
| Quinic acid | 0.668 | 193.071 (M + H)+ | 192.0637 | 174 | C7 H12 O6 | −1.84 |
| 4-(2-hydroxypropoxy)-3,5-dimethyl-Phenol | 9.113 | 197.1171 (M + H)+ | 196.1099 | - | C11 H16 O3 | 0.31 |
| 3-Methoxy-4,5-methylenedioxybenzoic acid | 0.627 | 219.026 (M + Na)+ | 196.0369 | 209 | C9 H8 O5 | 1.65 |
| 4-p-Coumaroylquinic acid | 8.007 | 339.1079 (M + H)+ | 338.1006 | - | C16 H18 O8 | −1.36 |
| Flavonoids | ||||||
| 5,7,2′,3′-Tetrahydroxyflavone | 9.59 | 287.0552 (M + H)+ | 286.0481 | 265/275 | C15 H10 O6 | −1.29 |
| ent-Fisetinidol-4beta-ol | 7.327 | 291.087 (M + H)+ | 290.0797 | 262 | C15 H14 O6 | −2.14 |
| 3,5,7,2′,5′-Pentahydroxyflavone | 9.326 | 303.0505 (M + H)+ | 302.0431 | 273/289 | C15 H10 O7 | −1.5 |
| 2′,4′,6′-Trihydroxy-3′-prenyldihydrochalcone | 8.976 | 327.1594 (M + H)+ | 326.1519 | 303 | C20 H22 O4 | −0.15 |
| Isovitexin | 9.755 | 433.1138 (M + H)+ | 432.1066 | - | C21 H20 O10 | −2.29 |
| 6-C-Galactosylisoscutellarein | 9.185 | 449.1086 (M + H)+ | 448.1016 | 434 | C21 H20 O11 | −2.21 |
| 6-Hydroxyluteolin 5-rhamnoside | 9.325 | 449.1091 (M + H)+ | 448.1017 | - | C21 H20 O11 | −2.59 |
| 8-Hydroxyluteolin 8-glucoside | 8.89 | 465.1035 (M + H)+ | 464.096 | 341 | C21 H20 O12 | −1.13 |
| Apigenin 7-(2″-E-p-coumaroylglucoside | 6.935 | 579.1509 (M + H)+ | 578.1435 | - | C30 H26 O12 | −1.81 |
| Isovitexin 7-O-rhamnoside | 9.591 | 579.1716 (M + H)+ | 578.1642 | 327 | C27 H30 O14 | −1.12 |
| Luteolin 7-rhamnosyl(1->6)galactoside | 9.212 | 595.1669 (M + H)+ | 594.1596 | 449 | C27 H30 O15 | −1.98 |
| Robinetin 3-rutinoside | 8.729 | 611.16 (M + H)+ | 610.1527 | 341 | C27 H30 O16 | 1.11 |
| Robinetinidol-(4alpha->8)-catechin-(6->4alpha)-robinetinidol | 7.545 | 867.2129 (M + H)+ | 866.2055 | 420 | C45 H38 O18 | 0.38 |
| Alkaloids | ||||||
| Gentiatibetine | 1.821 | 166.0859 (M + H)+ | 165.0787 | 143/151 | C9 H11 N O2 | 1.97 |
| Fagomine | 0.871 | 170.0795 (M + Na)+ | 147.0903 | 147/163 | C6 H13 N O3 | −5.01 |
| Boschniakine | 0.697 | 184.0726 (M + Na)+ | 161.0833 | 163/174 | C10 H11 N O | 4.53 |
| Sarpagine | 8.783 | 311.1752 (M + H)+ | 310.1679 | 289/303 | C19 H22 N2 O2 | 0.71 |
| Quinidine | 9.747 | 325.1922 (M + H)+ | 324.1849 | 305/317 | C20 H24 N2 O2 | −3.38 |
| Yohimbic Acid | 8.782 | 341.1866 (M + H)+ | 340.1792 | - | C20 H24 N2 O3 | −1.52 |
| 14β-Hydroxyyohimbine | 9.861 | 371.1969 (M + H)+ | 370.1897 | 341/352 | C21 H26 N2 O4 | −1.14 |
| Glycosides | ||||||
| Scopolin | 7.304 | 355.103 (M + H)+ | 354.0958 | 327/337 | C16 H18 O9 | −2.08 |
| Blumenol C glucoside | 9.221 | 373.2229 (M + H)+ | 372.2159 | 355 | C19 H32 O7 | −3.06 |
| Dihydroferulic acid 4-O-glucuronide | 8.2 | 390.1408 (M + NH4)+ | 372.1075 | 351/373 | C16 H20 O10 | −4.87 |
| (1RS,2RS)-Guaiacylglycerol 1-glucoside | 6.886 | 394.1717 (M + NH4)+ | 376.1377 | - | C16 H24 O10 | −1.94 |
| Benzyl O-[arabinofuranosyl-(1->6)-glucoside] | 7.644 | 420.1869 (M + NH4)+ | 402.1526 | 390/402 | C18 H26 O10 | −0.08 |
| Lucuminic acid | 8.104 | 464.1773 (M + NH4)+ | 446.1434 | 341 | C19 H26 O12 | −2.07 |
| Eugenol O-[a-L-Arabinofuranosyl-(1->6)-b-D-glucopyranoside] | 9.306 | 476.2131 (M + NH4)+ | 458.1791 | 449 | C21 H30 O11 | −0.55 |
| Mascaroside | 8.977 | 542.2601 (M + NH4)+ | 524.2261 | 465 | C26 H36 O11 | −0.58 |
| Prupaside | 8.581 | 570.2548 (M + NH4)+ | 552.2214 | 540 | C27 H36 O12 | −1.26 |
| (7′R)-(+)-Lyoniresinol 9′-glucoside | 8.404 | 600.2655 (M + NH4)+ | 582.2315 | 570/579 | C28 H38 O13 | −0.41 |
| Fatty acids | ||||||
| 8S-hydroxy-2E-Decene-4,6-diynoic acid | 6.888 | 179.0702 (M + H)+ | 178.063 | 153/167 | C10 H10 O3 | −0.31 |
| 10-Tridecynoic acid | 10.447 | 211.1692 (M + H)+ | 210.1619 | - | C13 H22 O2 | 0.59 |
| 9-keto palmitic acid | 11.757 | 271.2273 (M + H)+ | 270.2198 | 253 | C16 H30 O3 | −1.22 |
| 9,16-dihydroxy-palmitic acid | 11.758 | 289.2386 (M + H)+ | 288.2306 | 271 | C16 H32 O4 | −1.91 |
| Others | ||||||
| Isoamyl nitrite | 0.673 | 118.0864 [M + H]+ | 117.079 | 104 | C5 H11 N O2 | −0.52 |
| Pyroglutamic acid | 0.923 | 130.0498 [M + H]+ | 129.0425 | - | C5 H7 N O3 | 0.4 |
| Piperonal | 7.341 | 151.0388 [M + H]+ | 150.0316 | 121/139 | C8 H6 O3 | 0.83 |
| 3-Hydroxycoumarin | 7.303 | 163.039 [M + H]+ | 162.0319 | 139/151 | C9 H6 O3 | −1.38 |
| 2-Propenyl propyl disulfide | 1.08 | 166.0723 (M + NH4)+ | 148.0386 | 121/149 | C6 H12 S2 | −3.48 |
| 3-tert-Butyl-5-methylcatechol | 12.155 | 181.1221 (M + H)+ | 180.1148 | 158 | C11 H16 O2 | 1.36 |
| N-Hydroxy-L-phenylalanine | 1.015 | 182.0809 (M + H)+ | 181.0737 | 166 | C9 H11 N O3 | 1.32 |
| 3,4-Dehydro-6-hydroxymellein | 7.343 | 193.0492 (M + H)+ | 192.042 | 163/171 | C10 H8 O4 | 1.33 |
| 2,3-Dihydroxy-p-cumate | 6.889 | 197.0808 (M + H)+ | 196.0736 | 167/179 | C10 H12 O4 | −0.43 |
| N17-Dimethylindole-3-carboxaldehyde | 7.882 | 197.0813 (M + Na)+ | 174.0921 | 179 | C11 H12 N O | −1.16 |
| 2-Phenylethyl 3-methylbutanoate | 7.774 | 207.1376 (M + H)+ | 206.1304 | 179/197 | C13 H18 O2 | 1.4 |
| (5alpha,8beta,9beta)-5,9-Epoxy-3,6-megastigmadien-8-ol | 10.261 | 209.1538 (M + H)+ | 208.1464 | 183/195 | C13 H20 O2 | −0.36 |
| Vanilpyruvic acid | 7.342 | 211.0602 (M + H)+ | 210.0529 | 193 | C10 H10 O5 | −0.6 |
| 6-(2-Methoxyvinyl)benzo[1,3]dioxole-5-carboxylic acid | 9.014 | 223.0601 (M + H)+ | 222.053 | 197/209/219 | C11 H10 O5 | −0.87 |
| Haematommic Acid, Ethyl Ester, | 8.231 | 225.0757 (M + H)+ | 224.0686 | 197/211 | C11 H12 O5 | −0.42 |
| 2-Hydroxy-3-carboxy-6-oxo-7-methylocta-2,4-dienoate | 7.344 | 229.0714 (M + H)+ | 228.0641 | 211 | C10 H12 O6 | 4 |
| Depdecin | 7.844 | 229.1073 (M + H)+ | 228.1002 | 207 | C11 H16 O5 | −1.75 |
| Quebrachitol | 0.635 | 233.0422 (M + K)+ | 194.0789 | 209/226 | C7 H14 O6 | 0.7 |
| Elenaic acid | 8.232 | 243.0864 (M + H)+ | 242.0791 | 225 | C11 H14 O6 | −0.34 |
| (+)-cis-5,6-Dihydro-5-hydroxy-4-methoxy-6-(2-phenylethyl)-2H-pyran-2-one | 8.82 | 249.112 (M + H)+ | 248.1048 | 219/237 | C14 H16 O4 | 0.38 |
| Pyriculol | 8.403 | 249.1124 (M + H)+ | 248.1051 | 219 | C14 H16 O4 | −0.81 |
| D-1-[(3-Carboxypropyl)amino]-1-deoxyfructose | 0.645 | 266.1238 (M + H)+ | 265.1162 | 239/247/258 | C10 H19 N O7 | −0.24 |
| Acetyltryptophanamide | 1.021 | 268.106 (M + Na)+ | 245.1168 | - | C13 H15 N3 O2 | −1.6 |
| Modafinil | 0.869 | 274.0902 (M + H)+ | 273.0834 | 245/256 | C15 H15 N O2 S | −3.92 |
| Ilicifolinoside A | 1.29 | 282.1546 (M + NH4)+ | 264.1206 | 253/270 | C11 H20 O7 | 1 |
| Oxaprozin | 7.816 | 311.139 (M + NH4)+ | 293.1052 | 289 | C18 H15 N O3 | 0.13 |
| Fluoxetine | 9.646 | 327.1685 (M + NH4)+ | 309.1347 | 309/317 | C17 H18 F3 N O | −2.13 |
| Epitestosterone | 8.194 | 327.1709 (M + K)+ | 288.2081 | - | C19 H28 O2 | 2.74 |
| Compound V(S) | 8.415 | 329.1869 (M + H)+ | 328.1796 | 303/311 | C19 H24 N2 O3 | −2.66 |
| N′-Hydroxyneosaxitoxin | 0.864 | 332.1314 (M + H)+ | 331.1238 | 314/322 | C10 H17 N7 O6 | 0.75 |
| p,γ-Dihydroxyphenylbutazone | 4.044 | 341.1499 (M + H)+ | 340.1427 | 314/325 | C19 H20 N2 O4 | −1.16 |
| 6′-Hydroxyhydrodolasetron; MDL 73492 | 8.563 | 343.1659 (M + H)+ | 342.1586 | 325/335 | C19 H22 N2 O4 | −1.8 |
| b-D-Glucopyranosiduronic acid | 7.234 | 344.1343 (M + H)+ | 343.1271 | 319/327 | C15 H21 N O8 | −1.17 |
| 2-(4-Allyl-2-methoxyphenoxy)-1-(4-hydroxy-3-methoxyphenyl)-1-propanol | 9.671 | 345.1697 (M + H)+ | 344.1625 | 317/327/335 | C20 H24 O5 | −0.45 |
| 5-(6-Hydroxy-3,7-dimethyl-2,7-octadienyloxy)-7-methoxycoumarin | 8.976 | 345.1701 (M + H)+ | 344.1625 | 327 | C20 H24 O5 | −0.42 |
| URB937 | 4.487 | 355.1655 (M + H)+ | 354.1578 | 325/343 | C20 H22 N2 O4 | 0.38 |
| Methyl-2-alpha-L-fucopyranosyl-beta-D-galactoside | 1.309 | 358.171 (M + NH4)+ | 340.1364 | 328/348 | C13 H24 O10 | 1.47 |
| 202-791 | 7.883 | 359.1349 (M + H)+ | 358.1285 | - | C17 H18 N4 O5 | −2.24 |
| Hydroxyisonobilin | 8.976 | 363.1806 (M + H)+ | 362.1738 | 333/345/355 | C20 H26 O6 | −2.49 |
| 5-Megastigmen-7-yne-3,9-diol 9-glucoside | 9.003 | 371.2067 (M + H)+ | 370.1994 | 345/355 | C19 H30 O7 | −0.62 |
| Marshmine | 7.412 | 373.1767 (M + NH4)+ | 355.1426 | 343/355 | C20 H21 N O5 | −1.73 |
| (-)-11-nor-9-carboxy-Δ9-THC | 7.381 | 383.1607 (M + K)+ | 344.1982 | 355/373 | C21 H28 O4 | 1.64 |
| Lochnerinine | 9.582 | 383.1967 (M + H)+ | 382.1892 | 352/363/371 | C22 H26 N2 O4 | 0.04 |
| Monotropein | 7.342 | 408.1508 (M + NH4)+ | 390.1169 | - | C16 H22 O11 | −1.64 |
| Todatriol glucoside | 6.91 | 408.1872 (M + NH4)+ | 390.1531 | 377/386/394 | C17 H26 O10 | −1.4 |
| Dicaffeoylputrescine | 7.271 | 413.1714 (M + H)+ | 412.1639 | 383/391/408 | C22 H24 N2 O6 | −1.03 |
| Gardenoside | 8.232 | 422.1664 (M + NH4)+ | 404.1326 | 243 | C17 H24 O11 | −1.8 |
| Ganoderol A | 18.782 | 439.3566 (M + H)+ | 438.3492 | 411 | C30 H46 O2 | 1.4 |
| Gln Tyr Tyr | 9.281 | 490.2294 (M + NH4)+ | 472.1955 | 476 | C23 H28 N4 O7 | 0.68 |
| Trilobolide | 8.331 | 561.2093 (M + K)+ | 522.2464 | 534 | C27 H38 O10 | 0.09 |
| Coproporphyrin | 8.51 | 699.2768 (M + K)+ | 660.3137 | 243 | C36 H44 N4 O8 | 3.35 |
| Betulinic Acid | 18.791 | 935.7075 (2M + Na)+ | 456.3596 | 479/758 | C30 H48 O3 | 1.74 |
| Compound | ts (min) | m/z Experimental | Teoric Mass | MS/MS Fragments | Molecular Formula | Error (ppm) |
|---|---|---|---|---|---|---|
| Phenolic acids | ||||||
| 3,4-Dihydroxybenzoic acid | 3.227 | 153.0197 (M − H)− | 154.027 | - | C7 H6 O4 | −2.54 |
| 2,4,6-Trihydroxybenzoic acid | 1.574 | 169.0146 (M − H)− | 170.0218 | - | C7 H6 O5 | −1.84 |
| Cis-5-Caffeoylquinic acid | 7.296 | 353.0893 (M − H)− | 354.0966 | - | C16 H18 O9 | −4.2 |
| Flavonoids | ||||||
| 6-Hydroxyluteolin 5-rhamnoside | 9.323 | 447.0959 (M − H)− | 448.1026 | - | C21 H20 O11 | −4.48 |
| Apigenin 7-(2″-E-p-coumaroylglucoside | 7.091 | 577.1382 (M − H)− | 578.1452 | - | C30 H26 O12 | −4.87 |
| Robinetin 3-rutinoside | 8.73 | 609.1486 (M − H)− | 610.1559 | 581/593 | C27 H30 O16 | −4.1 |
| Robinetinidol-(4alpha->8)-catechin-(6->4alpha)-robinetinidol | 7.54 | 865.2001 (M − H)− | 866.2069 | 576/720 | C45 H38 O18 | −1.22 |
| Glycosides | ||||||
| (7′R)-(+)-Lyoniresinol 9′-glucoside | 8.411 | 581.2264 (M − H)− | 582.2328 | - | C28 H38 O13 | −2.74 |
| Fatty acids | ||||||
| 11-hydroperoxy-12,13-epoxy-9-octadecenoic acid | 11.016 | 327.2192 (M − H)− | 328.2264 | 309 | C18 H32 O5 | −4.48 |
| Others | ||||||
| Oxaloglutarate | 0.759 | 203.0187 (M − H)− | 204.026 | 179/191 | C7 H8 O7 | 4.72 |
| 9-Aminoacridine | 0.635 | 229.053 (M + Cl)− | 194.0836 | 203/209/215/223 | C13 H10 N2 | 3.89 |
| Asp Trp Gly | 6.886 | 375.1322 (M − H)− | 376.1391 | - | C17 H20 N4 O6 | −2.15 |
| Acetyl-maltose | 0.67 | 383.1207 (M − H)− | 384.1278 | 357/365 | C14 H24 O12 | −2.73 |
| Trp Asp Glu | 7.64 | 447.1531 (M − H)− | 448.1608 | 416/429 | C20 H24 N4 O8 | −3.04 |
| 1,2,3,4-Tetragalloyl-alpha-D-glucose | 8.688 | 787.1018 (M − H)− | 788.1091 | 463/609/720 | C34 H28 O22 | −2.41 |
| Compound | ts (min) | m/z Experimental | Teoric Mass | MS/MS Fragments | Molecular Formula | Error (ppm) |
|---|---|---|---|---|---|---|
| Phenolic acids | ||||||
| m-Coumaric acid | 8.232 | 165.0542 (M + H)+ | 164.0472 | 137/151 | C9 H8 O3 | 1.13 |
| 4-(2-hydroxypropoxy)-3,5-dimethyl-Phenol | 9.109 | 197.1171 (M + H)+ | 196.1099 | - | C11 H16 O3 | 0.14 |
| Haematommic Acid | 0.619 | 219.0271 (M + Na)+ | 196.038 | 195/209 | C9 H8 O5 | −4.29 |
| Sphagnum acid | 9.011 | 223.0599 (M + H)+ | 222.0526 | 197/209 | C11 H10 O5 | 0.86 |
| cis-Sinapic acid | 8.231 | 225.0756 (M + H)+ | 224.0684 | 197/211 | C11 H12 O5 | 0.54 |
| 1-O-Caffeoylquinic acid | 7.304 | 355.1029 (M + H)+ | 354.0957 | 327/343 | C16 H18 O9 | −1.71 |
| Flavonoids | ||||||
| 5,7,2′,3′-Tetrahydroxyflavone | 9.75 | 287.0552 (M + H)+ | 286.0479 | 273 | C15 H10 O6 | −0.39 |
| Oritin-4beta-ol | 7.319 | 291.0866 (M + H)+ | 290.0794 | 262 | C15 H14 O6 | −1.13 |
| 3,5,7,2′,5′-Pentahydroxyflavone | 9.325 | 303.0501 (M + H)+ | 302.0428 | 287/295 | C15 H10 O7 | −0.37 |
| 2′,4′,6′-Trihydroxy-3′-prenyldihydrochalcone | 8.975 | 327.1597 (M + H)+ | 326.1524 | 303 | C20 H22 O4 | −1.96 |
| Isovitexin | 9.752 | 433.1135 (M + H)+ | 432.1063 | 325 | C21 H20 O10 | −1.45 |
| 6-C-Galactosylisoscutellarein | 9.178 | 449.1081 (M + H)+ | 448.1011 | 436 | C21 H20 O11 | −1.29 |
| 6-Hydroxyluteolin 5-rhamnoside | 9.325 | 449.1086 (M + H)+ | 448.1012 | - | C21 H20 O11 | −1.39 |
| 5,6,7,3′,4′-Pentahydroxy-8-methoxyflavone 7-apioside | 8.887 | 465.1037 (M + H)+ | 464.0965 | 341 | C21 H20 O12 | −2.18 |
| 2′,4′,6′,3-Tetrahydroxy-3′-geranyl-6″,6″-dimethylpyrano[2″,3″:4,5]dihydrochalcone | 7.69 | 515.2392 (M + Na)+ | 492.2498 | 484/497/505 | C30 H36 O6 | 2.83 |
| Apigenin 7-(2″-E-p-coumaroylglucoside | 6.93 | 579.1498 (M + H)+ | 578.1426 | 394 | C30 H26 O12 | −0.22 |
| Isovitexin 7-O-rhamnoside | 9.588 | 579.1717 (M + H)+ | 578.1643 | 383 | C27 H30 O14 | −1.22 |
| Luteolin 7-rhamnosyl(1->6)galactoside | 9.213 | 595.1658 (M + H)+ | 594.1584 | 355 | C27 H30 O15 | 0.11 |
| Robinetin 3-rutinoside | 8.73 | 611.1607 (M + H)+ | 610.1537 | 595 | C27 H30 O16 | −0.5 |
| Alkaloids | ||||||
| Caffeine | 7.349 | 195.0878 (M + H)+ | 194.0805 | 171/185 | C8 H10 N4 O2 | −0.4 |
| O-Desmethylquinidine | 8.76 | 311.1753 (M + H)+ | 310.168 | 153/193/249 | C19 H22 N2 O2 | 0.48 |
| Sarpagine | 9.879 | 311.1754 (M + H)+ | 310.1682 | 287/299 | C19 H22 N2 O2 | −0.23 |
| Benzosimuline | 9.567 | 323.1755 (M + NH4)+ | 305.1416 | 293/303/317 | C20 H19 N O2 | −0.01 |
| Affinine | 9.732 | 325.191 (M + H)+ | 324.1836 | 297/309/317 | C20 H24 N2 O2 | 0.42 |
| Caribine | 9.628 | 327.1701 (M + H)+ | 326.1628 | 303/317 | C19 H22 N2 O3 | 0.77 |
| Vinorine | 8.589 | 335.1757 (M + H)+ | 334.1687 | 311/329/341 | C21 H22 N2 O2 | −1.62 |
| Akuammicine | 9.416 | 323.1754 (M + H)+ | 322.1681 | 303 | C20 H22 N2 O2 | 0.24 |
| Tabersonine | 9.626 | 337.1914 (M + H)+ | 336.1841 | 309/317/327 | C21 H24 N2 O2 | −0.91 |
| Yohimbic Acid | 8.143 | 341.1864 (M + H)+ | 340.1791 | 329 | C20 H24 N2 O3 | −1.25 |
| 3-Hydroxyquinidine | 8.76 | 341.1864 (M + H)+ | 340.1791 | - | C20 H24 N2 O3 | −1.1 |
| Rauwolscine | 9.52 | 355.2018 (M + H)+ | 354.1944 | 327/337 | C21 H26 N2 O3 | −0.26 |
| Papaverine | 8.016 | 357.181 (M + NH4)+ | 339.1463 | 327/341/351 | C20 H21 N O4 | 2.29 |
| 11-Methoxy-vinorine | 9.056 | 365.1863 (M + H)+ | 364.1796 | 337/345/355 | C22 H24 N2 O3 | −2.39 |
| 14β-Hydroxyyohimbine | 9.846 | 371.1969 (M + H)+ | 370.1892 | 343/352 | C21 H26 N2 O4 | 0.06 |
| Glycosides | ||||||
| Ethyl beta-D-glucopyranoside | 0.696 | 209.1021 (M + H)+ | 208.0949 | - | C8 H16 O6 | −0.95 |
| Blumenol C glucoside | 9.222 | 373.2213 (M + H)+ | 372.2141 | 355 | C19 H32 O7 | 1.95 |
| (1RS,2RS)-Guaiacylglycerol 1-glucoside | 6.884 | 394.1711 (M + NH4)+ | 376.1373 | - | C16 H24 O10 | −0.89 |
| Benzyl O-[arabinofuranosyl-(1->6)-glucoside] | 7.64 | 420.1869 (M + NH4)+ | 402.153 | 392/402 | C18 H26 O10 | −0.98 |
| Lucuminic acid | 8.102 | 464.1766 (M + NH4)+ | 446.1429 | 422/448 | C19 H26 O12 | −0.98 |
| Fatty acids | ||||||
| 2-Dehydro-3-deoxy-D-xylonate | 1.076 | 166.0716 (M + NH4)+ | 148.0377 | - | C5 H8 O5 | −3.64 |
| 10-Tridecynoic acid | 10.444 | 211.1688 (M + H)+ | 210.1612 | - | C13 H22 O2 | 3.5 |
| Palmitic amide | 19.089 | 256.2628 (M + H)+ | 255.2554 | - | C16 H33 N O | 3.03 |
| 9-keto palmitic acid | 11.754 | 271.2266 (M + H)+ | 270.2192 | 253 | C16 H30 O3 | 1.21 |
| 9,16-dihydroxy-palmitic acid | 11.753 | 289.2378 (M + H)+ | 288.2303 | 271 | C16 H32 O4 | −0.78 |
| 2-Hydroxyhexadecanoic acid | 12.276 | 290.2692 (M + NH4)+ | 272.2354 | 274 | C16 H32 O3 | −0.88 |
| 13-methyl-octadecanoic acid | 13.93 | 316.3206 (M + NH4)+ | 298.2867 | 295 | C19 H38 O2 | 1.71 |
| 11-hydroperoxy-12,13-epoxy-9-octadecenoic acid | 11.006 | 346.2588 (M + NH4)+ | 328.2248 | 323/337 | C18 H32 O5 | 0.52 |
| Others | ||||||
| 2-Amino-3-methyl-1-butanol | 0.626 | 104.1071 (M + H)+ | 103.0998 | - | C5 H13 N O | −0.93 |
| Valine | 0.668 | 118.086 (M + H)+ | 117.0785 | 104 | C5 H11 N O2 | 4.16 |
| Pyroglutamic acid | 0.924 | 130.0496 (M + H)+ | 129.0424 | 121 | C5 H7 N O3 | 1.22 |
| 2,3,5-Trihydroxytoluene | 0.697 | 141.0543 (M + H)+ | 140.047 | 121 | C7 H8 O3 | 2.16 |
| Vinylacetylglycine | 1.835 | 144.0656 (M + H)+ | 143.0585 | 121 | C6 H9 N O3 | −1.66 |
| Methylitaconate | 2.86 | 145.0499 (M + H)+ | 144.0426 | 121/133 | C6 H8 O4 | −2.65 |
| 3-Hydroxy-3-methyl-glutaric acid | 2.572 | 163.06 (M + H)+ | 162.0528 | 133/143 | C6 H10 O5 | 0.11 |
| (2R,3S)-2,3-Dimethylmalate | 2.86 | 163.0601 (M + H)+ | 162.0528 | - | C6 H10 O5 | −0.08 |
| 3-tert-Butyl-5-methylcatechol | 12.149 | 181.1222 (M + H)+ | 180.1149 | - | C11 H16 O2 | 0.89 |
| 3,4-Dehydro-6-hydroxymellein | 8.869 | 193.0493 (M + H)+ | 192.0424 | - | C10 H8 O4 | −0.55 |
| Valiolone | 0.667 | 193.0703 (M + H)+ | 192.0632 | 163/173 | C7 H12 O6 | 0.91 |
| Quebrachitol | 0.648 | 195.0865 (M + H)+ | 194.0792 | - | C7 H14 O6 | −0.6 |
| (5alpha,8beta,9beta)-5,9-Epoxy-3,6-megastigmadien-8-ol | 10.257 | 209.1532 (M + H)+ | 208.1458 | - | C13 H20 O2 | 2.46 |
| 5-Hydroxyferulate | 7.337 | 211.0599 (M + H)+ | 210.0526 | 185/195 | C10 H10 O5 | 1.14 |
| 2-Hydroxy-3-carboxy-6-oxo-7-methylocta-2,4-dienoate | 7.34 | 229.0713 (M + H)+ | 228.064 | 211 | C10 H12 O6 | −2.64 |
| Depdecin | 7.841 | 229.107 (M + H)+ | 228.0998 | 207 | C11 H16 O5 | −0.13 |
| Xestoaminol C | 12.175 | 230.248 (M + H)+ | 229.2407 | 203/211/219 | C14 H31 N O | −0.59 |
| 1-O-Methyl-myo-inositol | 0.634 | 233.0429 (M + K)+ | 194.0798 | 209/217/226 | C7 H14 O6 | −4.01 |
| Elenaic acid | 8.231 | 243.0863 (M + H)+ | 242.079 | 225 | C11 H14 O6 | 0.23 |
| C16 Sphinganine | 12.112 | 274.2745 (M + H)+ | 273.2672 | 244/255 | C16 H35 N O2 | −1.57 |
| C17 Sphinganine | 12.711 | 288.2897 (M + H)+ | 287.2824 | 272 | C17 H37 N O2 | 0.19 |
| 2-(beta-D-Glucosyl)-sn-glycerol | 0.655 | 293.0635 (M + K)+ | 254.1011 | 247/266/280 | C9 H18 O8 | −3.86 |
| 2,9-Dimethyl-2,9-diazatricyclo[10.2.2.25,8]octadeca-5,7,12,14,15,17-hexaene-3,10-diol, 9CI | 9.912 | 299.1757 (M + H)+ | 298.1684 | 269/283 | C18 H22 N2 O2 | −0.93 |
| Phytosphingosine | 12.203 | 318.3005 (M + H)+ | 317.2933 | 290 | C18 H39 N O3 | −0.99 |
| Compound V(S) | 8.401 | 329.1863 (M + H)+ | 328.1789 | - | C19 H24 N2 O3 | −0.77 |
| URB597 | 8.535 | 339.1702 (M + H)+ | 338.163 | 335/309 | C20 H22 N2 O3 | 0.22 |
| Epicainide | 9.994 | 339.2069 (M + H)+ | 338.1987 | 311/325 | C21 H26 N2 O2 | 2.05 |
| Phenisopham | 8.554 | 343.1653 (M + H)+ | 342.158 | 335 | C19 H22 N2 O4 | −0.02 |
| 5-(6-Hydroxy-3,7-dimethyl-2,7-octadienyloxy)-7-methoxycoumarin | 8.975 | 345.1704 (M + H)+ | 344.1628 | 327 | C20 H24 O5 | −1.13 |
| 2-Pyrrolidinone, 4-(2-morpholinoethyl)-3,3-diphenyl | 8.16 | 351.2072 (M + H)+ | 350.1998 | 329/341 | C22 H26 N2 O2 | −1.18 |
| URB937 | 9.114 | 355.1662 (M + H)+ | 354.1588 | 337 | C20 H22 N2 O4 | −2.31 |
| Methyl-2-alpha-L-fucopyranosyl-beta-D-galactoside | 1.307 | 358.1708 (M + NH4)+ | 340.1367 | 328/341 | C13 H24 O10 | 0.67 |
| 202-791 | 7.879 | 359.1352 (M + H)+ | 358.1285 | - | C17 H18 N4 O5 | −2.06 |
| Marshmine | 7.393 | 373.1763 (M + NH4)+ | 355.1425 | 343/355 | C20 H21 N O5 | −1.38 |
| Akuammine | 9.564 | 383.1971 (M + H)+ | 382.1897 | - | C22 H26 N2 O4 | −1.14 |
| N-stearoyl valine | 20.36 | 384.3477 (M + H)+ | 383.3403 | - | C23 H45 N O3 | −0.89 |
| 3α,12α-Dihydroxy-5β-chol-8(14)-en-24-oic Acid | 21.097 | 391.2847 (M + H)+ | 390.2774 | 371 | C24 H38 O4 | −1.12 |
| Gardenoside | 8.231 | 405.1392 (M + H)+ | 404.1324 | 243 | C17 H24 O11 | −1.23 |
| Monotropein | 7.339 | 408.1503 (M + NH4)+ | 390.1165 | - | C16 H22 O11 | −0.71 |
| Gln Tyr Tyr | 9.279 | 490.2294 (M + NH4)+ | 472.1954 | 479 | C23 H28 N4 O7 | 0.89 |
| Mascaroside | 8.976 | 542.26 (M + NH4)+ | 524.2261 | 365 | C26 H36 O11 | −0.66 |
| Pheophorbide a | 19.793 | 593.2762 (M + H)+ | 592.2688 | 565 | C35 H36 N4 O5 | −0.46 |
| Coproporphyrin | 8.288 | 699.2765 (M + K)+ | 660.3135 | 329 | C36 H44 N4 O8 | 3.6 |
| Compound | ts (min) | m/z Experimental | Teoric Mass | MS/MS Fragments | Molecular Formula | Error (ppm) |
|---|---|---|---|---|---|---|
| Phenolic acids | ||||||
| 3,4-Dihydroxybenzoic acid | 3.203 | 153.0196 (M − H)− | 154.0269 | - | C7 H6 O4 | −1.59 |
| 2,4,6-Trihydroxybenzoic acid | 1.568 | 169.0148 (M − H)− | 170.0221 | - | C7 H6 O5 | −3.24 |
| 1,2-Digalloyl-beta-D-glucopyranose | 7.304 | 483.0794 (M − H)− | 484.0867 | 289/353/389 | C20 H20 O14 | −2.78 |
| 1,3,4-Trigalloyl-beta-D-glucopyranose | 7.992 | 635.0907 (M − H)− | 636.0979 | 393/513/577 | C27 H24 O18 | −2.57 |
| Flavonoids | ||||||
| 4,2′,3′,4′-Tetrahydroxychalcone | 11.28 | 271.0623 (M − H)− | 272.0695 | 241/248/259 | C15 H12 O5 | −3.68 |
| 2,6,3′,4′-Tetrahydroxy-2-benzylcoumaranone | 9.814 | 287.0568 (M − H)− | 288.0641 | 277 | C15 H12 O6 | −2.61 |
| Epifisetinidol-4alpha-ol | 7.303 | 289.0725 (M − H)− | 290.0797 | - | C15 H14 O6 | −2.39 |
| Isovitexin | 9.76 | 431.0999 (M − H)− | 432.1073 | - | C21 H20 O10 | −3.74 |
| Ent-afzelechin-7-O-beta-D-glucopyranoside | 9.625 | 435.1311 (M − H)− | 436.1378 | 431 | C21 H24 O10 | −1.93 |
| 6-Hydroxyluteolin 5-rhamnoside | 9.331 | 447.0952 (M − H)− | 448.1019 | - | C21 H20 O11 | −2.92 |
| Robinetin 7-glucoside | 8.885 | 463.0903 (M − H)− | 464.0976 | - | C21 H20 O12 | −4.59 |
| Apigenin 7-(3″-p-coumaroylglucoside) | 6.921 | 577.1375 (M − H)− | 578.1446 | 375 | C30 H26 O12 | −3.84 |
| Isovitexin 7-O-rhamnoside | 9.599 | 577.1579 (M − H)− | 578.1647 | - | C27 H30 O14 | −2 |
| Luteolin 7-rhamnosyl(1->6)galactoside | 9.22 | 593.153 (M − H)− | 594.1597 | 447 | C27 H30 O15 | −2.11 |
| 8-C-Glucosyldiosmetin 4″-O-rhamnopyranoside | 9.651 | 607.1682 (M − H)− | 608.1759 | 431 | C28 H32 O15 | −2.98 |
| Robinetin 3-rutinoside | 8.735 | 609.1478 (M − H)− | 610.1551 | 463 | C27 H30 O16 | −2.76 |
| Robinetinidol-(4alpha->8)-catechin-(6->4alpha)-robinetinidol | 7.546 | 865.1987 (M − H)− | 866.2058 | 447/728 | C45 H38 O18 | 0.01 |
| Alkaloids | ||||||
| 1-Methylxanthine | 0.645 | 165.0418 (M − H)− | 166.0491 | 147 | C6 H6 N4 O2 | −0.41 |
| Enprofylline | 0.639 | 229.0502 (M + Cl)− | 194.0809 | 207/215 | C8 H10 N4 O2 | −2.89 |
| 1,3,7-Trimethyluric acid | 0.639 | 245.0453 (M + Cl)− | 210.0764 | 191/215/229 | C8 H10 N4 O3 | −5.36 |
| O-Desmethylquinidine | 8.76 | 309.1613 (M − H)− | 310.1684 | - | C19 H22 N2 O2 | −0.88 |
| Glycosides | ||||||
| Inosine | 0.656 | 267.074 (M − H)− | 268.0813 | 245 | C10 H12 N4 O5 | −1.99 |
| Isobiflorin | 7.293 | 353.0883 (M − H)− | 354.0955 | - | C16 H18 O9 | −1.07 |
| Ethyl 7-epi-12-hydroxyjasmonate glucoside | 9.026 | 415.1987 (M − H)− | 416.2058 | 389/405 | C20 H32 O9 | −2.89 |
| Dihydroferulic acid 4-O-glucuronide | 8.202 | 371.1 (M − H)− | 372.1072 | - | C16 H20 O10 | −4.17 |
| Catalposide | 9.624 | 481.1363 (M − H)− | 482.1435 | 461/471 | C22 H26 O12 | −2.2 |
| Prupaside | 8.591 | 551.2126 (M − H)− | 552.22 | - | C27 H36 O12 | 1.31 |
| (7′R)-(+)-Lyoniresinol 9′-glucoside | 8.419 | 581.2255 (M − H)− | 582.232 | - | C28 H38 O13 | −1.32 |
| 1-Octen-3-yl glucoside | 7.48 | 289.1667 (M − H)− | 290.1739 | 279 | C14 H26 O6 | −3.41 |
| Fatty acids | ||||||
| 9,16-dihydroxy-palmitic acid | 11.768 | 287.224 (M − H)− | 288.2312 | 264 | C16 H32 O4 | −4.08 |
| 11-hydroperoxy-12,13-epoxy-9-octadecenoic acid | 11.029 | 327.2189 (M − H)− | 328.2261 | - | C18 H32 O5 | −3.58 |
| 5,8,12-trihydroxy-9-octadecenoic acid | 11.437 | 329.2346 (M − H)− | 330.2418 | 301/315 | C18 H34 O5 | −3.5 |
| Others | ||||||
| N-Acryloylglycine | 0.924 | 128.0356 (M − H)− | 129.0426 | 112 | C5 H7 N O3 | −0.41 |
| Glutaconic acid | 2.572 | 129.0195 (M − H)− | 130.0268 | 112/119 | C5 H6 O4 | −1.33 |
| 3-Hydroxypicolinic acid | 9.789 | 138.0198 (M − H)− | 139.0271 | 112/119 | C6 H5 N O3 | −0.99 |
| 2-Propenyl propyl disulfide | 1.077 | 147.0308 (M − H)− | 148.0382 | 133 | C6 H12 S2 | −1.32 |
| 3,4-Dihydroxymandelaldehyde | 8.496 | 167.0347 (M − H)− | 168.042 | - | C8 H8 O4 | 1.52 |
| 4-O-Methyl-gallate | 6.591 | 183.0301 (M − H)− | 184.0374 | - | C8 H8 O5 | −1.1 |
| Nonic Acid | 9.518 | 187.0978 (M − H)− | 188.105 | 173 | C9 H16 O4 | −0.99 |
| Glu His | 0.867 | 283.1046 (M − H)− | 284.1117 | - | C11 H16 N4 O5 | 1.18 |
| Dyphylline | 0.647 | 289.0708 (M + Cl)− | 254.1016 | 267/281 | C10 H14 N4 O4 | −0.42 |
| Gingerol | 13.127 | 293.1764 (M − H)− | 294.1836 | - | C17 H26 O4 | −1.78 |
| 1-Pyrenylsulfate | 6.603 | 297.0238 (M − H)− | 298.0312 | 289 | C16 H10 O4 S | −4.07 |
| Histidinyl-Glutamate | 0.644 | 319.0819 (M + Cl)− | 284.1129 | 289/305 | C11 H16 N4 O5 | −2.88 |
| beta-Glucogallin | 1.125 | 331.0687 (M − H)− | 332.0758 | - | C13 H16 O10 | −4.35 |
| Hydroxyisonobilin | 9.678 | 361.1671 (M − H)− | 362.1743 | - | C20 H26 O6 | −3.87 |
| 2′,3′,5′-triacetyl-5-Azacytidine | 0.691 | 369.1065 (M − H)− | 370.1137 | 339/349/365 | C14 H18 N4 O8 | −3.41 |
| Trp Asp Gly | 6.883 | 375.1306 (M − H)− | 376.1383 | - | C17 H20 N4 O6 | 0.09 |
| Monotropein | 7.335 | 389.1107 (M − H)− | 390.1175 | - | C16 H22 O11 | −3.38 |
| Gardenoside | 8.234 | 403.1263 (M − H)− | 404.1333 | 377 | C17 H24 O11 | −3.68 |
| Trp Gly Phe | 9.677 | 407.173 (M − H)− | 408.1805 | 380/397 | C22 H24 N4 O4 | −1.79 |
| Trp Thr Ile | 9.232 | 417.2144 (M − H)− | 418.2216 | 405 | C21 H30 N4 O5 | 0.06 |
| Val Trp Glu | 7.778 | 431.1936 (M − H)− | 432.2012 | 403/420 | C21 H28 N4 O6 | −0.84 |
| Trp Asp Glu | 7.643 | 447.1523 (M − H)− | 448.1602 | 419/429 | C20 H24 N4 O8 | −1.67 |
| 1,2,3,4,6-Pentakis-O-galloyl-beta-D-glucose | 8.998 | 469.0542 (M − 2H) − 2 | 940.1227 | 463 | C41 H32 O26 | −4.82 |
| 3-(4-Hydroxy-3-methoxyphenyl)-1,2-propanediol 2-O-(galloyl-glucoside) | 7.708 | 511.1472 (M − H)− | 512.1547 | 501 | C23 H28 O13 | −3.37 |
| Gibberellin A38 glucosyl ester | 8.467 | 523.2198 (M − H)− | 524.2271 | 509 | C26 H36 O11 | −2.53 |
| Mascaroside | 8.984 | 523.2211 (M − H)− | 524.2274 | - | C26 H36 O11 | −3.05 |
| Citrusin B | 8.537 | 567.2072 (M − H)− | 568.2142 | 551 | C27 H36 O13 | 2.52 |
| 1,2,3,4-Tetragalloyl-alpha-D-glucose | 8.683 | 787.1015 (M − H)− | 788.1087 | 463/609 | C34 H28 O22 | −1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahim, N.A.; Roslan, M.N.F.; Muhamad, M.; Seeni, A. Antioxidant Activity, Total Phenolic and Flavonoid Content and LC–MS Profiling of Leaves Extracts of Alstonia angustiloba. Separations 2022, 9, 234. https://doi.org/10.3390/separations9090234
Rahim NA, Roslan MNF, Muhamad M, Seeni A. Antioxidant Activity, Total Phenolic and Flavonoid Content and LC–MS Profiling of Leaves Extracts of Alstonia angustiloba. Separations. 2022; 9(9):234. https://doi.org/10.3390/separations9090234
Chicago/Turabian StyleRahim, Nurhidayah Ab., Muhammad Nabil Fikri Roslan, Musthahimah Muhamad, and Azman Seeni. 2022. "Antioxidant Activity, Total Phenolic and Flavonoid Content and LC–MS Profiling of Leaves Extracts of Alstonia angustiloba" Separations 9, no. 9: 234. https://doi.org/10.3390/separations9090234