Temporal and Spatial Dynamics of Phytoplankton Primary Production in Lake Taihu Derived from MODIS Data
Abstract
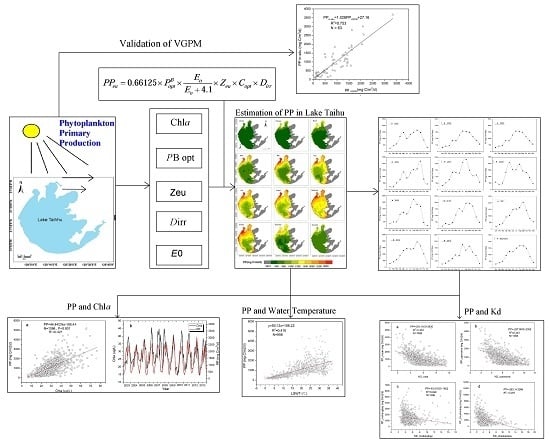
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Data
2.3. Image Data Description and Processing
2.4. Method Description
2.5. Statistical Analysis and Accuracy Assessment
3. Results
3.1. Validation of VGPM
3.2. Temporal-Spatial Distribution of Phytoplankton Primary Production
3.2.1. Annual Variation of Phytoplankton Primary Production
3.2.2. Seasonal Variation of Phytoplankton Primary Production
3.2.3. Monthly Variation of Phytoplankton Primary Production
3.2.4. Spatial Variation of Phytoplankton Primary Production
4. Discussion
4.1. Correlation between the Primary Production with Chla and Nutrient Concentrations
4.2. Correlation between the Primary Production and Lake Water Temperature
4.3. The Effects of Wind-Driven Sediment Resuspension on the Spatial Distribution of Phytoplankton Primary Production
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lakshmi, E.; Pratap, D.; Nagamani, P.V.; Rao, K.H.; Latha, T.P.; Choudhury, S.B. Time Series Analysis of Primary production along the East Coast of India Using Oceansat-2 Ocean Colour Monitor (O cm). Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2014, 40, 1049–1053. [Google Scholar] [CrossRef]
- Deines, A.M.; Bunnell, D.B.; Rogers, M.W. A review of the global relationship among freshwater fish, autotrophic activity, and regional climate. Rev. Fish Biol. Fish. 2015, 25, 323–336. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Boss, E.; Siegel, D.A.; Shea, D.M. Carbon-based ocean productivity and phytoplankton physiology from space. Glob. Biogeochem. Cycles 2005, 19, 177–202. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystem. EOS Trans. Am. Geophys. Union 2001, 21, 1–9. [Google Scholar]
- Sterner, R.W. In situ-measured primary production in Lake Superior. J. Great Lakes Res. 2010, 36, 139–149. [Google Scholar] [CrossRef]
- Smith, R.C.; Prezelin, B.B.; Bidigare, R.R.; Baker, K.S. Bio-optical modeling of photosynthetic production. Limnol. Oceanogr. 1989, 34, 1524–1544. [Google Scholar] [CrossRef]
- Li, G.; Ping, G.; Fang, W.; Qiang, L. Estimation of ocean primary production and its spatio-temporal variation mechanism for East China Sea based on VGPM model. J. Geogr. Sci. 2004, 14, 32–40. [Google Scholar] [CrossRef]
- Fahnenstiel, G.L.; Sayers, M.J.; Shuchman, R.A.; Yousef, F.; Pothoven, S.A. Lake-wide phytoplankton production and abundance in the Upper Great Lakes: 2010–2013. J. Great Lakes Res. 2016, 42, 619–629. [Google Scholar] [CrossRef]
- Morel, A.; Berthon, J.F. Surface pigments, algal biomass profiles, and potential production of the euphotic layer: Relationships reinvestigated in view of remote sensing applications. Limnol. Oceanogr. 1989, 34, 1545–1562. [Google Scholar] [CrossRef]
- Fahnenstiel, G.; Pothoven, S.; Vanderploeg, H.; Klarer, D.; Nalepa, T.; Scavia, D. Recent Changes in Primary Production and Phytoplankton in the Offshore Region of Southeastern Lake Michigan. J. Great Lakes Res. 2010, 36, 20–29. [Google Scholar] [CrossRef]
- Eppley, R.W.; Stewart, E.; Abbott, M.R.; Heyman, U. Estimating ocean primary production from satellite chlorophyll—Introduction to regional differences and statistics for the southern California bight. J. Plankton Res. 1985, 7, 227–234. [Google Scholar] [CrossRef]
- Behrenfeld, M.J.; Falkowski, P.G. A consumer’s guide to phytoplankton primary production models. Limnol. Oceanogr. 1997, 42, 1479–1491. [Google Scholar] [CrossRef]
- Carr, M.E.; Friedrichs, M.A.M.; Schmeltz, M.; Aita, M.N.; Antoine, D.; Arrigo, K.R.; Asanuma, I.; Aumont, O.; Barber, R.; Behrenfeld, M. A comparison of global estimates of marine primary production from ocean color. Deep Sea Res. Part II Top. Stud. Oceanogr. 2006, 53, 741–770. [Google Scholar] [CrossRef] [Green Version]
- Behrenfeld, M.J.; Falkowski, P.G. Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol. Oceanogr. 1997, 42, 1–20. [Google Scholar] [CrossRef]
- Tripathy, S.C.; Ishizaka, J.; Siswanto, E.; Shibata, T.; Mino, Y. Modification of the vertically generalized production model for the turbid waters of Ariake bay, southwestern Japan. Estuar. Coast Shelf 2012, 97, 66–77. [Google Scholar] [CrossRef]
- Kameda, T.; Ishizaka, J.; Murakami, H. Two-phytoplankton community model of primary production for ocean color satellite data. Hyperspectr. Remote Sens. Ocean. 2000, 4154, 159–165. [Google Scholar]
- Ye, H.B.; Chen, C.Q.; Sun, Z.H.; Tang, S.L.; Song, X.Y.; Yang, C.Y.; Tian, L.Q.; Liu, F.F. Estimation of the primary production in pearl river estuary using MODIS data. Estuar. Coast Shelf 2015, 38, 506–518. [Google Scholar] [CrossRef]
- Vollenweider, R.A.; Munawar, M.; Stadelmann, P. A comparative Review of Phytoplankton and Primary Production in the Laurentian Great Lakes. J. Fish. Res. Board Can. 2011, 31, 739–762. [Google Scholar] [CrossRef]
- Kemili, P.; Putri, M.R. Estimation of primary production in Banda Sea using the vertical distribution model. AIP Conf. Proc. 2014, 1589, 389–393. [Google Scholar]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Kahru, M.; Mitchell, B.G. Influence of the El Niño—La Niña cycle, on satellite-derived primary production, in the California Current. Investig. Mar. 2002, 29, 27-1–27-4. [Google Scholar] [CrossRef]
- Mcclain, C.R.; Christian, J.R.; Signorini, S.R.; Lewis, M.R.; Asanuma, I.; Turk, D.; Dupouy-Douchement, C. Satellite ocean-color observations of the tropical pacific ocean. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 2533–2560. [Google Scholar] [CrossRef]
- Gregg, W.W.; Conkright, M.E.; Ginoux, P.; O’Reilly, J.E.; Casey, N.W. Ocean primary production and climate: Global decadal changes. Geophys. Res. Lett. 2003, 30, 157–168. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Qin, B.Q.; Liu, M.L. Temporal—Spatial variations of chlorophyll a and primary production in meiliang bay, Lake Taihu, China from 1995 to 2003. J. Phytoplankton Res. 2007, 29, 707–719. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, S.; Ronghua, M.A.; Liu, M.A.; Qin, B. Spatial pattern of euphotic depth and estimation of phytoplankton primary production in Lake Taihu in autumn 2004. J. Lake Sci. 2008, 20, 380–388. [Google Scholar]
- Yin, Y.; Zhang, Y.; Shi, Z.; Liu, X. Estimation of spatial and seasonal changes in phytoplankton primary production in meiliang bay, Lake Taihu, based on the vertically generalized production model and MODIS data. Acta Ecol. Sin. 2012, 32, 3528–3537. [Google Scholar] [CrossRef]
- Cheng-Feng, L.E.; Yun-Mei, L.I.; Zha, Y.; Sun, D.Y.; Heng, L.U. Simulation of backscattering properties of Taihu Lake. Adv. Water Sci. 2009, 20, 707–713. [Google Scholar]
- Le, C.F.; Li, Y.M.; Zha, Y.; Sun, D.; Huang, C.; Lu, H. A four-band semi-analytical model for estimating chlorophyll a in highly turbid lakes: The case of Taihu Lake, China. Remote Sens. Environ. 2009, 113, 1175–1182. [Google Scholar] [CrossRef]
- Qin, B.; Xu, P.; Wu, Q.; Luo, L.; Zhang, Y. Environmental issues of Lake Taihu, China. Hydrobiologia 2007, 581, 3–14. [Google Scholar] [CrossRef]
- Le, C.F.; Li, Y.M.; Zha, Y.; Sun, D.Y. Specific absorption coefficient and the phytoplankton package effect in Lake Taihu, China. Hydrobiologia 2009, 619, 27–37. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, Y.; Zhou, Y.; Liu, X.; Zhu, G.; Qin, B.; Gao, G. Long-term MODIS observations of cyanobacterial dynamics in Lake Taihu: Responses to nutrient enrichment and meteorological factors. Sci. Rep. 2017. [CrossRef] [PubMed]
- Qin, B.; Zhu, G.; Gao, G.; Zhang, Y.; Wei, L.; Paerl, H.W.; Carmichael, W.W. A drinking water crisis in Lake Taihu, China: Linkage to climatic variability and lake management. Environ. Manag. 2010, 45, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, K.; Liu, X.; Zhou, Y.; Qin, B. Lake topography and wind waves determining seasonal-spatial dynamics of total suspended matter in turbid Lake Taihu, China: Assessment using long-term high-resolution MERIS data. PLoS ONE 2014, 9, e98055. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Xiaohong amp, G.U.; Yang, L. Analyses on water quality and its protection in east Lake Taihu. J. Lake Sci. 2006, 18, 91–96. [Google Scholar]
- Kauer, T.; Kutser, T.; Arst, H.; Danckaert, T.; Nõges, T. Modelling primary production in shallow well mixed lakes based on MERIS satellite data. Remote Sens. Environ. 2015, 163, 53–261. [Google Scholar] [CrossRef]
- Fahnenstiel, G.L.; Carrick, H.J. Primary production in lakes Huron and Michigan: In vitro and in situ comparisons. J. Plankton Res. 1988, 10, 1273–1283. [Google Scholar] [CrossRef]
- Gaarder, T.; Gran, H.H. Investigations of the production of plankton in the Oslo Fjord. J. Conseil—Conseil Permanent Int. l’Explor. Mer. 1927, 42, 1–48. [Google Scholar]
- Liu, G.; Ou, W.; Zhang, Y.; Wu, T.; Zhu, G.; Shi, K.; Qin, B. Validating and mapping surface water temperatures in Lake Taihu: Results from MODIS land surface temperature products. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 1–15. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, Y.; Liu, X.; Wang, M.; Qin, B. Remote sensing of diffuse attenuation coefficient of photosynthetically active radiation in Lake Taihu using MERIS data. Remote Sens. Environ. 2014, 140, 365–377. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, B. The basic characteristic and climatological calculation of the photosythetically available radiation in Taihu region. Acta Energiae Solaris Sinica 2002, 21, 118–123. [Google Scholar]
- Zhang, Y.; Sciences, C.A.O. Nanjing. Climatological calculation and characteristic analysis of global radiation over Wuxi region. Q. J. Appl. Meteorol. 2003, 14, 339–347. [Google Scholar]
- Shuchman, R.A.; Sayers, M.; Fahnenstiel, G.L.; Leshkevich, G. A model for determining satellite-derived primary production estimates for Lake Michigan. J. Great Lakes Res. 2013, 39, 46–54. [Google Scholar] [CrossRef]
- Warner, D.M.; Lesht, B.M. Relative importance of phosphorus, invasive mussels and climate for patterns in chlorophyll a and primary production in Lakes Michigan and Huron. Freshw. Biol. 2015, 60, 1029–1043. [Google Scholar] [CrossRef]
- Asano, T.; Li, G.M.; Hirai, Y.; Kim, D.C.; Ito, T. Eutrophication of lake taihu in China and post-response to the plague of algal bloom in 2007. E-Journal GEO 2011, 5, 138–153. [Google Scholar] [CrossRef]
- Hu, C.; Zhongping, L.; Ma, R.; Yu, K.; Li, D.; Shang, S. Moderate resolution imaging spectroradiometer (MODIS) observations of cyanobacteria blooms in Taihu Lake, China. Prehosp. Disaster Med. 2010, 29, 303–306. [Google Scholar] [CrossRef]
- Wang, M.; Shi, W.; Tang, J. Water property monitoring and assessment for China’s inland Lake Taihu from MODIS-Aqua measurements. Remote Sens. Environ. 2011, 115, 841–854. [Google Scholar] [CrossRef]
- Ma, W.; Chai, F.; Xiu, P.; Xue, H.; Tian, J. Modeling the long-term variability of phytoplankton functional groups and primary production in the South China Sea. J. Oceanogr. 2013, 69, 527–544. [Google Scholar] [CrossRef]
- Pei, G.; Hong, X. Primary production of benthic algae community in the Donghu Lake. J. South-Central Univ. Natly (Nat. Sci. Ed.) 2010, 29, 28–31. [Google Scholar]
- Bergamino, N.; Horion, S.; Stenuite, S.; Cornet, Y.; Loiselle, S.; Plisnier, P.D.; Descy, J.P. Spatio-temporal dynamics of phytoplankton and primary production in lake Tanganyika using a MODIS based bio-optical time series. Remote Sens. Environ. 2010, 114, 772–780. [Google Scholar] [CrossRef]
- Ardyna, M.; Babin, M.; Gosselin, M.; Devred, E. Parameterization of vertical chlorophyll a in the Arctic ocean: Impact of the subsurface chlorophyll maximum on regional, seasonal and annual primary production estimates. Biogeosciences 2013, 10, 4383–4404. [Google Scholar] [CrossRef]
- Tan, S.; Shi, G.; Tan, S.; Shi, G. Satellite-derived primary production and its spatial and temporal variability in the China seas. J. Geogr. Sci. 2006, 16, 447–457. [Google Scholar] [CrossRef]
- Kameda, T.; Ishizaka, J. Size-fractionated primary production estimated by a Two-phytoplankton community model applicable to ocean color remote sensing. J. Oceanogr. 2005, 61, 663–672. [Google Scholar] [CrossRef]
- Deng, J.; Qin, B.; Paerl, H.W.; Zhang, Y.; Ma, J.; Chen, Y. Earlier and warmer springs increase cyanobacterial (microcystis spp.) blooms in subtropical Lake Taihu, China. Freshw. Biol. 2014, 59, 1076–1085. [Google Scholar] [CrossRef]
- Deng, J.; Qin, B.; Paerl, H.W.; Zhang, Y.; Wu, P.; Ma, J.; Chen, Y. Effects of nutrients, temperature and their interactions on spring phytoplankton community succession in Lake Taihu, China. PLoS ONE 2014, 9, e113960. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, Y.; Huang, B.; Wei, Z.B.; Miao, A.J.; Yang, L.Y. Nitrogen and phosphorus limitation of phytoplankton growth in different areas of Lake Taihu, China. J. Freshw. Ecol. 2014, 30, 113–127. [Google Scholar] [CrossRef]
- Yuan, H.Z.; Shen, J.; Liu, E.F.; Wang, J.J.; Meng, X.H. Space distribution characteristics and diversity analysis of phosphorus from overlying water and surface sediments in Taihu Lake. J. Environ. Sci. 2010, 31, 954–960. [Google Scholar]
- Zhao, X. Temporal and spatial distribution of physicochemical characteristics and nutrients in sediments of Lake Taihu. J. Lake Sci. 2007, 19, 698–704. [Google Scholar]












| Lake Taihu | Spring | Summer | Autumn | Winter | Mean |
|---|---|---|---|---|---|
| Open area | 815.52 | 1458.07 | 1296.31 | 339.72 | 1019.25 |
| Meiliang Bay | 1673.32 | 1931.07 | 1707.68 | 685.55 | 1551.39 |
| Zhushan Bay | 2394.08 | 2951.64 | 2356.00 | 860.39 | 2205.47 |
| Whole lake | 921.47 | 1529.65 | 1364.75 | 378.66 | 1094.61 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Zhang, Y.; Li, D.; Shi, K.; Zhang, Y. Temporal and Spatial Dynamics of Phytoplankton Primary Production in Lake Taihu Derived from MODIS Data. Remote Sens. 2017, 9, 195. https://doi.org/10.3390/rs9030195
Deng Y, Zhang Y, Li D, Shi K, Zhang Y. Temporal and Spatial Dynamics of Phytoplankton Primary Production in Lake Taihu Derived from MODIS Data. Remote Sensing. 2017; 9(3):195. https://doi.org/10.3390/rs9030195
Chicago/Turabian StyleDeng, Yubing, Yunlin Zhang, Deping Li, Kun Shi, and Yibo Zhang. 2017. "Temporal and Spatial Dynamics of Phytoplankton Primary Production in Lake Taihu Derived from MODIS Data" Remote Sensing 9, no. 3: 195. https://doi.org/10.3390/rs9030195