Predicting Rice Grain Yield Based on Dynamic Changes in Vegetation Indexes during Early to Mid-Growth Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Data Acquisition and Determination
2.3. Data Analysis
2.3.1. Calculation of Relative Accumulated Growing Degree Days (RAGDD)
2.3.2. Statistical Analyses
3. Results
3.1. Dynamic Changes in Agronomic Parameters and Spectral Indexes before Flowering
3.2. Relationship between the Spectral Indexes and Agronomic Parameters
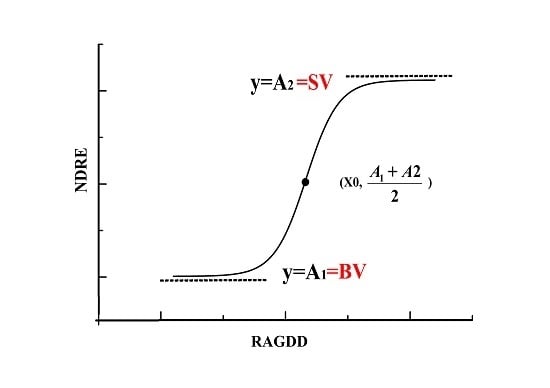
3.3. Construction of a Dynamic Model of NDRE
3.4. Testing of Each Vegetation Index-Based Dynamic Model and Parameter Analysis
3.4.1. Verification of Model Construction Correctness
3.4.2. Changes in BV and SV under Different Treatments and Their Relationship with Agronomic Parameters
3.5. Prediction Algorithm for Rice Grain Yield
4. Discussion
4.1. Spectral Indexes for Rice Growth Diagnosis
4.2. The Dynamic Model and Parameters of Rice Spectral Indexes
4.3. SV-Based Yield Prediction Algorithm
4.4. Interval System Errors between Experiments and Uncertainties of the Model
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lundström, C.; Lindblom, J.; Hansen, J.W.; Thornton, P.K.; Berentsen, P.B.M. Considering farmers’ situated knowledge of using agricultural decision support systems (AgriDSS) to Foster farming practices: The case of CropSAT. Agric. Syst. 2018, 159, 9–20. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Gong, J.; Yong, C.; Min, I.; Hui, G.; Dai, Q.; Huo, Z.; Ke, X.; Wei, H. The productive advantages and formation mechanisms of “Indica Rice to Japonica Rice”. Sci. Agric. Sinica 2013, 46, 686–704. [Google Scholar]
- He, J.; Xiao, H.; Huang, X.; Zhou, Q.; Hu, D. Effects of real-time and site-specific nitrogen management on rice yield and nitrogen use efficiency. Southwest China J. Agric. Sci. 2010, 23, 1132–1136. [Google Scholar]
- Diacono, M.; Rubino, P.; Montemurro, F. Precision nitrogen management of wheat. A review. Agron. Sustain. Dev. 2013, 33, 219–241. [Google Scholar] [CrossRef]
- Peng, S.; Buresh, R.J.; Huang, J.; Zhong, X.; Zou, Y.; Yang, J.; Wang, G.; Liu, Y.; Hu, R.; Tang, Q. Improving nitrogen fertilization in rice by Site-Specific N Management. Agron. Sustain. Dev. 2010, 30, 649–656. [Google Scholar] [CrossRef]
- Huang, J.; He, F.; Cui, K.; Buresh, R.J.; Xu, B.; Gong, W.; Peng, S. Determination of optimal nitrogen rate for rice varieties using a chlorophyll meter. Field Crops Res. 2008, 105, 70–80. [Google Scholar] [CrossRef]
- Peng, S.; Garcia, F.V.; Laza, R.C.; Sanico, A.L.; Visperas, R.M.; Cassman, K.G. Increased N-use efficiency using a chlorophyll meter on high-yielding irrigated rice. Field Crops Res. 1996, 47, 243–252. [Google Scholar] [CrossRef]
- Bijay-Singh; Varinderpal-Singh; Yadvinder-Singh; Thind, H.S.; Kumar, A.; Gupta, R.K.; Kaul, A.; Vashistha, M. Fixed-time adjustable dose site-specific fertilizer nitrogen management in transplanted irrigated rice (Oryza sativa L.) in South Asia. Field Crops Res. 2012, 126, 63–69. [Google Scholar] [CrossRef]
- Yi, Q.; Zhao, S.; Zhang, X.; Yang, L.; Xiong, G.; He, P. Yield and nitrogen use efficiency as influenced by real time and site specific nitrogen management in two rice cultivars. Plant Nutr. Fertil. Sci. 2012, 18, 777–785. [Google Scholar]
- Fan, L.; Peng, X.; Liu, Y.; Song, T. Study on the site-specific nitrogen management of rice in cold area of northeastern China. Sci. Agric. Sinica 2005, 38, 1761–1766. [Google Scholar]
- Justes, E.; Mary, B.; Meynard, J.M.; Machet, J.M.; Thelierhuche, L. Determination of a critical nitrogen dilution curve for winter wheat crops. Ann. Bot. 1994, 74, 397–407. [Google Scholar] [CrossRef]
- Greenwood, D.J.; Neeteson, J.J.; Draycott, A. Quantitative relationships for the dependence of growth rate of arable crops on their nitrogen content, dry weight and aerial environment. Plant Soil 1986, 91, 281–301. [Google Scholar] [CrossRef]
- Lemaire, G.; Jeuffroy, M.H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage: Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Liu, X.; Lu, Z.; Yuan, Z.; Zhu, Y.; Cao, W. In-season estimation of rice grain yield using critical nitrogen dilution curve. Field Crops Res. 2016, 195, 1–8. [Google Scholar] [CrossRef]
- Xia, T.; Miao, Y.; Wu, D.; Shao, H.; Khosla, R.; Mi, G. Active optical sensing of spring maize for in-season diagnosis of nitrogen status based on nitrogen nutrition index. Remote Sens. 2016, 8, 605. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Z.; Ata-Ul-Karim, S.T.; Xiao, J.; Liu, Z.; Qi, A.; Ning, D.; Nan, J.; Duan, A. Rapid and nondestructive estimation of the nitrogen nutrition index in winter barley using chlorophyll measurements. Field Crops Res. 2016, 185, 59–68. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Liu, X.; Lu, Z.; Zheng, H.; Cao, W.; Zhu, Y. Estimation of nitrogen fertilizer requirement for rice crop using critical nitrogen dilution curve. Field Crops Res. 2017, 201, 32–40. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, J.P.; Lv, Y.M.; He, J.J. SPAD values and nitrogen nutrition index for the evaluation of rice nitrogen status. Plant Prod. Sci. 2014, 17, 81–92. [Google Scholar]
- Houlès, V.; Guérif, M.; Mary, B. Elaboration of a nitrogen nutrition indicator for winter wheat based on leaf area index and chlorophyll content for making nitrogen recommendations. Eur. J. Agron. 2007, 27, 1–11. [Google Scholar] [CrossRef]
- Debaeke, P.; Rouet, P.; Justes, E. Relationship between the normalized SPAD index and the nitrogen nutrition index: Application to durum wheat. J. Plant Nutr. 2006, 29, 75–92. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Zhu, Y.; Liu, X.; Cao, Q.; Tian, Y.; Cao, W. Comparison of different critical nitrogen dilution curves for nitrogen diagnosis in rice. Sci. Rep. 2017, 7, 42679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdan, K.; Andrzej, L.; Andrzej, O.; Marek, K. The effect of tillage system and forecrop on the yield and values of LAI and SPAD indices of spring wheat. Eur. J. Agron. 2010, 33, 43–51. [Google Scholar]
- Spitkó, T.; Nagy, Z.; Zsubori, T.; Szőke, C.; Berzy, T.; Pintér, J.; Márton, L. Connection between normalized difference vegetation index and yield in maize. Plant Soil Environ. 2016, 62, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Tubaña, B.S.; Harrell, D.L.; Walker, T.; Teboh, J.; Lofton, J.; Kanke, Y. In-season canopy reflectance-based estimation of rice yield response to nitrogen. Agron. J. 2012, 104, 1604–1611. [Google Scholar] [CrossRef]
- Liu, K.; Yazhen, L.I.; Huiwen, H.U. Estimating the effect of urease inhibitor on rice yield based on NDVI at key growth stages. Front. Agric. Sci. Eng. 2014, 1, 150–157. [Google Scholar] [CrossRef]
- Xue, L.; Li, G.; Qin, X.; Yang, L.; Zhang, H. Topdressing nitrogen recommendation for early rice with an active sensor in south China. Precis. Agric. 2014, 15, 95–110. [Google Scholar] [CrossRef]
- Evert, F.K.V.; Booij, R.; Jukema, J.N.; Berge, H.F.M.T.; Uenk, D.; Meurs, E.J.J.; Geel, W.C.A.V.; Wijnholds, K.H.; Slabbekoorn, J.J. Using crop reflectance to determine sidedress N rate in potato saves N and maintains yield. Eur. J. Agron. 2012, 43, 58–67. [Google Scholar] [CrossRef]
- Morier, T.; Cambouris, A.N.; Chokmani, K. In-season nitrogen status assessment and yield estimation using hyperspectral vegetation indices in a potato crop. Agron. J. 2015, 107, 1295–1309. [Google Scholar] [CrossRef]
- Lukina, E.V.; Freeman, K.W.; Wynn, K.J.; Thomason, W.E.; Mullen, R.W.; Stone, M.L.; Solie, J.B.; Klatt, A.R.; Johnson, G.V.; Elliott, R.L. Nitrogen fertization optimization algorithm based on in-season estimates of yield and plant nitrogen uptake. J. Plant Nutr. 2001, 24, 885–898. [Google Scholar] [CrossRef]
- Raun, W.R.; Solie, J.B.; Johnson, G.V.; Stone, M.L.; Lukina, E.V.; Thomason, W.E.; Schepers, J.S. In-season prediction of potential grain yield in winter wheat using canopy reflectance. Agron. J. 2001, 93, 131–138. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Shen, J.; Yu, W.; Yuan, F.; Cheng, S.; Huang, S.; Wang, H.; Yang, W.; Liu, F. Improving in-season estimation of rice yield potential and responsiveness to topdressing nitrogen application with Crop Circle active crop canopy sensor. Precis. Agric. 2016, 17, 136–154. [Google Scholar] [CrossRef]
- Shaver, T.M.; Khosla, R.; Westfall, D.G. Evaluation of two ground-based active crop canopy sensors in maize: Growth stage, row spacing, and sensor movement speed. Soil Sci. Soc. Am. J. 2010, 74, 2101–2108. [Google Scholar] [CrossRef]
- Li, F.; Etc, Y.M. Estimating winter wheat biomass and nitrogen status using an active crop sensor. Intell. Autom. Soft Comput. 2010, 16, 1221–1230. [Google Scholar]
- Lofton, J.; Tubana, B.S.; Kanke, Y.; Teboh, J.; Viator, H.; Dalen, M. Estimating sugarcane yield potential using an in-season determination of normalized difference vegetative index. Sensors 2012, 12, 7529. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Miao, Y.; Jiang, R.; Khosla, R.; Gnyp, M.L.; Bareth, G. Evaluating different active crop canopy sensors for estimating rice yield potential. In Proceedings of the Second International Conference on Agro-Geoinformatics, Fairfax, VA, USA, 12–16 August 2013; pp. 538–542. [Google Scholar]
- Thompson, L.J.; Ferguson, R.B.; Kitchen, N.; Frazen, D.W.; Mamo, M.; Yang, H.; Schepers, J.S. Model and sensor-based recommendation approaches for in-season nitrogen management in corn. Agron. J. 2015, 107, 2020–2030. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Wang, H.; Huang, S.; Cheng, S.; Khosla, R.; Jiang, R. Non-destructive estimation of rice plant nitrogen status with Crop Circle multispectral active canopy sensor. Field Crops Res. 2013, 154, 133–144. [Google Scholar] [CrossRef]
- Russelle, M.P.; Wilhelm, W.W.; Olson, R.A.; Power, J.F. Growth analysis based on degree days. Crop Sci. 1984, 24, 28–32. [Google Scholar] [CrossRef]
- Su, L.; Wang, Q.; Bai, Y. An analysis of yearly trends in growing degree days and the relationship between growing degree day values and reference evapotranspiration in Turpan area, China. Theor. Appl. Climatol. 2013, 113, 711–724. [Google Scholar] [CrossRef]
- Liu, X.; Ferguson, R.B.; Zheng, H.; Cao, Q.; Tian, Y.; Cao, W.; Zhu, Y. Using an active-optical sensor to develop an optimal NDVI dynamic model for high-yield rice Production (Yangtze, China). Sensors 2017, 17, 672. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Yang, G.; Feng, H. Variations in crop variables within wheat canopies and responses of canopy spectral characteristics and derived vegetation indices to different vertical leaf layers and spikes. Remote Sens. Environ. 2015, 169, 358–374. [Google Scholar] [CrossRef]
- Kanke, Y.; Raun, W.; Solie, J.; Stone, M.; Taylor, R. Red edge as a potential index for detecting differences in plant nitrogen status in winter wheat. J. Plant Nutr. 2012, 35, 1526–1541. [Google Scholar] [CrossRef]
- Prasad, B.; Carver, B.F.; Stone, M.L.; Babar, M.A.; Raun, W.R.; Klatt, A.R. Genetic analysis of indirect selection for winter wheat grain yield using spectral reflectance indices. Crop Sci. 2007, 47, 1416–1425. [Google Scholar] [CrossRef]
- Gutierrez, M.; Reynolds, M.P.; Raun, W.R.; Stone, M.L.; Klatt, A.R. Spectral water indices for assessing yield in elite bread wheat genotypes under well-irrigated, water-stressed, and high-temperature conditions. Crop Sci. 2010, 50, 197–214. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, Y.; Huang, S.; Gao, L.; Ma, X.; Zhao, G.; Jiang, R.; Chen, X.; Zhang, F.; Yu, K. Active canopy sensor-based precision N management strategy for rice. Agron. Sustain. Dev. 2012, 32, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Nguyrobertson, A.; Gitelson, A.; Peng, Y.; Viña, A.; Arkebauer, T.; Rundquist, D. Green leaf area index estimation in maize and soybean: Combining vegetation indices to achieve maximal sensitivity. Agron. J. 2012, 104, 1336. [Google Scholar] [CrossRef]
- Gnyp, M.L.; Miao, Y.; Yuan, F.; Ustin, S.L.; Yu, K.; Yao, Y.; Huang, S.; Bareth, G. Hyperspectral canopy sensing of paddy rice aboveground biomass at different growth stages. Field Crops Res. 2014, 155, 42–55. [Google Scholar] [CrossRef]
- Beck, P.S.A.; Atzberger, C.; Høgda, K.A.; Johansen, B.; Skidmore, A.K. Improved monitoring of vegetation dynamics at very high latitudes: A new method using MODIS NDVI. Remote Sens. Environ. 2006, 100, 321–334. [Google Scholar] [CrossRef]
- Ferencz, Cs.; Bognár, P.; Lichtenberger, J.; Hamar, D.; Tarcsai, Gy.; Timár, G.; Molnár, G.; Pásztor, Sz.; Steinbach, P.; Székely, B.; et al. Crop yield estimation by satellite remote sensing. Int. J. Remote Sens. 2004, 25, 4113–4149. [Google Scholar] [CrossRef]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef] [PubMed]
- Vaesen, K.; Gilliams, S.; Nackaerts, K.; Coppin, P. Ground-measured spectral signatures as indicators of ground cover and leaf area index: The case of paddy rice. Field Crops Res. 2001, 69, 13–25. [Google Scholar] [CrossRef]
- Vinciková, H.; Hanuš, J.; Pechar, L. Spectral reflectance is a reliable water-quality estimator for small, highly turbid wetlands. Wetl. Ecol. Manag. 2015, 23, 933–946. [Google Scholar] [CrossRef]
- Pradhan, S.; Bandhypadyay, K.K.; Sahoo, R.N.; Sehgal, V.K.; Singh, R.; Joshi, D.K.; Gupta, V.K. Prediction of wheat (Triticum astivum) grain biomass yield under different irrigation and nitrogen management practices using canopy reflectance spectra model. Indian J. Agric. Sci. 2013, 83, 1136–1143. [Google Scholar]
- Amaral, L.R.; Molin, J.P.; Portz, G.; Finazzi, F.B.; Cortinove, L. Comparison of crop canopy reflectance sensors used to identify sugarcane biomass and nitrogen status. Precis. Agric. 2015, 16, 15–28. [Google Scholar] [CrossRef]
- Bonfil, D.J. Wheat phenomics in the field by RapidScan: NDVI vs. NDRE. Israel J. Plant Sci. 2017, 64, 41–45. [Google Scholar] [CrossRef]
- Kanke, Y.; Tubaña, B.; Dalen, M.; Harrell, D. Evaluation of red and red-edge reflectance-based vegetation indices for rice biomass and grain yield prediction models in paddy fields. Precis. Agric. 2016, 17, 507–530. [Google Scholar] [CrossRef]
- Ali, A.M.; Thind, H.S.; Sharma, S.; Varinderpal-Singh. Prediction of dry direct-seeded rice yields using chlorophyll meter, leaf color chart and GreenSeeker optical sensor in northwestern India. Field Crops Res. 2014, 161, 11–15. [Google Scholar] [CrossRef]
- Cowley, R.B.; Luckett, D.J.; Moroni, J.S.; Diffey, S. Use of remote sensing to determine the relationship of early vigour to grain yield in canola (Brassica napus L.) germplasm. Crop Pasture Sci. 2014, 65, 1288–1299. [Google Scholar] [CrossRef]
- Schmidt, J.; Beegle, D.; Zhu, Q.; Sripada, R. Improving in-season nitrogen recommendations for maize using an active sensor. Field Crops Res. 2011, 120, 94–101. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, L.; Zhang, J.; Pan, Y.; Zhu, W.; Hu, T. Division of winter wheat yield estimation by remote sensing based on MODIS EVI time series data and spectral angle clustering. Spectrosc. Spectr. Anal. 2012, 32, 1899–1904. [Google Scholar]
- Verrelst, J.; Muñoz, J.; Alonso, L.; Delegido, J.; Rivera, J.P.; Camps-Valls, G.; Moreno, J. Machine learning regression algorithms for biophysical parameter retrieval: Opportunities for sentinel-2 and -3. Remote Sens. Environ. 2012, 118, 127–139. [Google Scholar] [CrossRef]
- Sánchez, B.; Rasmussen, A.; Porter, J.R. Temperatures and the growth and development of maize and rice: A review. Glob. Chang. Biol. 2014, 20, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Clyde, M.; George, E.I. Model Uncertainty. Stat. Sci. 2004, 19, 81–94. [Google Scholar]
- Hansen, L.P.; Sargent, T.J. Robust Control and Model Uncertainty. Am. Econ. Rev. 2001, 91, 60–66. [Google Scholar] [CrossRef]













| Author | Eco-site | Variety | Threshold |
|---|---|---|---|
| Huang et al. [6] | Hubei, China | Shanyou63, Liangyoupei9 | 36, 38 |
| Singh et al. [7] | Indo-Gangetic plains, India | PR118, PAU201, etc. | 37.5 |
| Qiong et al. [8] | Hubei, China | Peiliangyou3076, Yangliangyou6 | 39–41 |
| He et al. [3] | Hubei, China | Liangyoupei9, Shanyou63 | 38, 39, 35–37 |
| Fan et al. [10] | Helongjiang, China | Songjing98-128 | 38–40 |
| Experiment | Location | Transplanting and Harvest Date | Cultivar | Treatment | Nitrogen fertilizer (N, kg·ha−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Basal and tillering fertilizer | Panicle fertilizer | Total fertilizer | |||||||
| Exp. 1 2015 | Rugao 118.26°E, 33.37°N | 14 June 25 Oct. | WYJ24 | N0T0 | 0 | (N0) | 0 | (T0) | 0 |
| N5T0 | 120 | (N5) | 0 | (T0) | 120 | ||||
| N5T3 | 120 | (N5) | 80 | (T3) | 200 | ||||
| N7T0 | 180 | (N7) | 0 | (T0) | 180 | ||||
| N7T6 | 180 | (N7) | 120 | (T6) | 300 | ||||
| N8T0 | 240 | (N8) | 0 | (T0) | 240 | ||||
| N8T7 | 240 | (N8) | 160 | (T7) | 400 | ||||
| Exp. 2 2015 | SiHong 118.26°E, 33.37°N | 14 June 25 Oct. | WYJ24, NJ4, LJ7 | N0T0 | 0 | (N0) | 0 | (T0) | 0 |
| N1T4 | 36 | (N1) | 84 | (T4) | 120 | ||||
| N3T8 | 72 | (N3) | 168 | (T8) | 240 | ||||
| N4T9 | 108 | (N4) | 252 | (T9) | 360 | ||||
| Exp. 3 2016 | RuGao 120.76°E, 32.27°N | 25 June 26 Oct. | WYJ24 | N0T0 | 0 | (N0) | 0 | (T0) | 0 |
| N2T0 | 60 | (N2) | 0 | (T0) | 60 | ||||
| N2T1 | 60 | (N2) | 40 | (T1) | 100 | ||||
| N2T3 | 60 | (N2) | 80 | (T3) | 140 | ||||
| N6T0 | 150 | (N6) | 0 | (T0) | 150 | ||||
| N6T2 | 150 | (N6) | 50 | (T2) | 200 | ||||
| N6T5 | 150 | (N6) | 100 | (T5) | 250 | ||||
| N8T0 | 240 | (N8) | 0 | (T0) | 240 | ||||
| N8T3 | 240 | (N8) | 80 | (T3) | 320 | ||||
| N8T7 | 240 | (N8) | 160 | (T7) | 400 | ||||
| NJ4 | N0T0 | 0 | (N0) | 0 | (T0) | 0 | |||
| N2T1 | 60 | (N2) | 40 | (T1) | 100 | ||||
| N6T5 | 150 | (N6) | 100 | (T5) | 250 | ||||
| N8T7 | 240 | (N8) | 160 | (T7) | 400 | ||||
| Exp. 4 2016 | SiHong 118.26°E, 33.37°N | 18 June 22 Oct. | WYJ24, NJ4, LJ7 | N0T0 | 0 | (N0) | 0 | (T0) | 0 |
| N1T4 | 36 | (N1) | 84 | (T4) | 120 | ||||
| N3T8 | 72 | (N3) | 168 | (T8) | 240 | ||||
| N4T9 | 108 | (N4) | 252 | (T9) | 360 | ||||
| Spectral Index | Equation | Sensitivity Indicator | Reference |
|---|---|---|---|
| Normalized difference vegetation index (NDVI) | Grain yield, HI, N status, RUE, LAI, Biomass, Grain protein | [36,42] | |
| Normalized difference red edge (NDRE) | LAI, Biomass, N status | [6,33] | |
| Ratio vegetation index (RVI) | Grain yield, Biomass, LAI, Grain protein, N status | [37,42] | |
| Red-edge vegetation index (RERVI) | LAI, Biomass, N status | [37] |
| Agronomic Parameter | Spectral Index | Mid-Tillering | Stem Elongation | Panicle Initiation | Booting | Pre-Flowering | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | ||
| LAI | NDRE | 0.37 | 0.70 | 0.67 | 0.70 | 0.51 | 0.72 | 0.69 | 0.71 | 0.65 | 0.67 | 0.77 | 0.75 | 0.70 | 0.75 | 0.83 | 0.79 | 0.67 | 0.64 | 0.74 | 0.78 |
| NDVI | 0.21 | 0.54 | 0.50 | 0.56 | 0.48 | 0.69 | 0.64 | 0.65 | 0.58 | 0.69 | 0.74 | 0.64 | 0.67 | 0.66 | 0.74 | 0.68 | 0.44 | 0.66 | 0.63 | 0.69 | |
| RERVI | 0.37 | 0.69 | 0.66 | 0.70 | 0.57 | 0.71 | 0.71 | 0.72 | 0.66 | 0.70 | 0.80 | 0.77 | 0.71 | 0.77 | 0.85 | 0.81 | 0.76 | 0.61 | 0.78 | 0.81 | |
| RVI | 0.15 | 0.50 | 0.43 | 0.44 | 0.46 | 0.70 | 0.62 | 0.58 | 0.63 | 0.74 | 0.78 | 0.66 | 0.66 | 0.74 | 0.77 | 0.67 | 0.61 | 0.57 | 0.67 | 0.70 | |
| PNC | NDRE | 0.49 | 0.55 | 0.46 | 0.13 | 0.31 | 0.74 | 0.46 | 0.19 | 0.27 | 0.70 | 0.52 | 0.10 | 0.34 | 0.77 | 0.60 | 0.37 | <0.01 | 0.26 | 0.28 | 0.59 |
| NDVI | 0.30 | 0.35 | 0.29 | 0.07 | 0.21 | 0.63 | 0.39 | 0.23 | 0.25 | 0.58 | 0.46 | 0.16 | 0.32 | 0.57 | 0.49 | 0.35 | <0.01 | 0.20 | 0.23 | 0.53 | |
| RERVI | 0.50 | 0.57 | 0.47 | 0.14 | 0.19 | 0.74 | 0.40 | 0.16 | 0.23 | 0.73 | 0.55 | 0.17 | 0.43 | 0.76 | 0.62 | 0.39 | <0.01 | 0.24 | 0.27 | 0.58 | |
| RVI | 0.21 | 0.33 | 0.26 | 0.10 | 0.31 | 0.71 | 0.42 | 0.11 | 0.32 | 0.62 | 0.48 | 0.10 | 0.35 | 0.66 | 0.56 | 0.39 | 0.01 | 0.16 | 0.22 | 0.51 | |
| PNA | NDRE | 0.52 | 0.65 | 0.66 | 0.59 | 0.56 | 0.64 | 0.68 | 0.71 | 0.58 | 0.60 | 0.70 | 0.70 | 0.65 | 0.49 | 0.67 | 0.74 | 0.63 | 0.51 | 0.67 | 0.76 |
| NDVI | 0.33 | 0.55 | 0.53 | 0.53 | 0.48 | 0.70 | 0.63 | 0.59 | 0.56 | 0.54 | 0.64 | 0.64 | 0.61 | 0.53 | 0.63 | 0.60 | 0.40 | 0.56 | 0.56 | 0.62 | |
| RERVI | 0.52 | 0.62 | 0.68 | 0.70 | 0.59 | 0.62 | 0.68 | 0.72 | 0.63 | 0.62 | 0.73 | 0.72 | 0.77 | 0.51 | 0.72 | 0.77 | 0.74 | 0.50 | 0.72 | 0.79 | |
| RVI | 0.26 | 0.54 | 0.54 | 0.63 | 0.55 | 0.63 | 0.64 | 0.60 | 0.61 | 0.58 | 0.69 | 0.69 | 0.72 | 0.67 | 0.77 | 0.72 | 0.73 | 0.51 | 0.69 | 0.70 | |
| LNA | NDRE | 0.53 | 0.67 | 0.71 | 0.72 | 0.64 | 0.75 | 0.75 | 0.71 | 0.62 | 0.43 | 0.62 | 0.74 | 0.70 | 0.69 | 0.74 | 0.64 | 0.63 | 0.61 | 0.72 | 0.79 |
| NDVI | 0.34 | 0.47 | 0.51 | 0.57 | 0.54 | 0.72 | 0.70 | 0.69 | 0.55 | 0.41 | 0.56 | 0.64 | 0.62 | 0.62 | 0.65 | 0.55 | 0.60 | 0.64 | 0.67 | 0.65 | |
| RERVI | 0.52 | 0.67 | 0.71 | 0.73 | 0.61 | 0.75 | 0.74 | 0.70 | 0.53 | 0.46 | 0.60 | 0.71 | 0.63 | 0.70 | 0.74 | 0.66 | 0.63 | 0.58 | 0.71 | 0.78 | |
| RVI | 0.28 | 0.46 | 0.49 | 0.57 | 0.57 | 0.74 | 0.69 | 0.62 | 0.51 | 0.46 | 0.57 | 0.62 | 0.64 | 0.74 | 0.75 | 0.64 | 0.40 | 0.55 | 0.54 | 0.59 | |
| Growing stage | LAI | PNC | PNA | LNA |
|---|---|---|---|---|
| Tillering | 0.82 | 0.16 | 0.73 | 0.76 |
| Stem elongation | 0.72 | 0.23 | 0.70 | 0.67 |
| Panicle initiation | 0.73 | 0.09 | 0.56 | 0.74 |
| Booting | 0.76 | 0.42 | 0.75 | 0.65 |
| NDRE Exp. 1 | NDRE Exp. 3 | NDVI Exp. 1 | NDVI Exp. 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | R2 | RRMSE (%) | p-Value of Residual’s Normality | Treatments | R2 | RRMSE (%) | p-Value of Residual’s Normality | Treatments | R2 | RRMSE (%) | p-Value of Residual’s Normality | Treatments | R2 | RRMSE (%) | p-Value of Residual’s Normality |
| N0T0 | 0.94 | 4.83 | 0.89 | N0T0 | 0.94 | 2.43 | 0.9 | N0T0 | 0.82 | 14.5 | 0.64 | N0T0 | 0.87 | 21.0 | 0.72 |
| N5T0 | 0.94 | 2.52 | 0.65 | N2T0 | 0.9 | 1.55 | 0.69 | N5T0 | 0.9 | 13.0 | 0.96 | N2T0 | - | - | - |
| N5T3 | 0.97 | 2.26 | 0.62 | N2T1 | 0.93 | 2.17 | 0.79 | N5T3 | 0.92 | 13.5 | 0.81 | N2T1 | 0.89 | 19.0 | 0.9 |
| N7T0 | 0.94 | 2.35 | 0.39 | N6T0 | 0.95 | 1.07 | 0.49 | N7T0 | 0.91 | 15.0 | 0.89 | N6T0 | 0.91 | 17.0 | 0.84 |
| N7T6 | 0.94 | 3.68 | 0.74 | N6T5 | 0.96 | 1.9 | 0.51 | N7T6 | 0.93 | 17.0 | 0.9 | N6T5 | 0.92 | 16.0 | 0.97 |
| N8T0 | 0.96 | 2.34 | 0.5 | N8T0 | 0.9 | 1.93 | 0.29 | N8T0 | 0.94 | 11.0 | 0.95 | N8T0 | 0.83 | 15.0 | 0.73 |
| N8T7 | 0.97 | 2.44 | 0.56 | N8T7 | 0.91 | 2.59 | 0.44 | N8T7 | 0.95 | 7.5 | 0.79 | N8T7 | 0.9 | 16.0 | 0.81 |
| Experiment | Exp. 1 | ||||||
| Treatment | N0T0 | N5T0 | N7T0 | N5T3 | N8T0 | N7T6 | N8T3 |
| (kg∙ha−1) | 0 | 120 | 180 | 200 | 240 | 300 | 320 |
| RAGDD | 0.366 | 0.374 | 0.351 | 0.357 | 0.303 | 0.345 | 0.314 |
| Exp. 3 | |||||||
| Treatment | N0T0 | N2T0 | N2T1 | N6T0 | N6T2 | N8T0 | N8T3 |
| (kg∙ha−1) | 0 | 60 | 100 | 150 | 200 | 240 | 320 |
| RAGDD | 0.516 | 0.55 | 0.524 | 0.559 | 0.538 | 0.494 | 0.542 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Ge, X.; Shen, P.; Li, W.; Liu, X.; Cao, Q.; Zhu, Y.; Cao, W.; Tian, Y. Predicting Rice Grain Yield Based on Dynamic Changes in Vegetation Indexes during Early to Mid-Growth Stages. Remote Sens. 2019, 11, 387. https://doi.org/10.3390/rs11040387
Zhang K, Ge X, Shen P, Li W, Liu X, Cao Q, Zhu Y, Cao W, Tian Y. Predicting Rice Grain Yield Based on Dynamic Changes in Vegetation Indexes during Early to Mid-Growth Stages. Remote Sensing. 2019; 11(4):387. https://doi.org/10.3390/rs11040387
Chicago/Turabian StyleZhang, Ke, Xiaokang Ge, Pengcheng Shen, Wanyu Li, Xiaojun Liu, Qiang Cao, Yan Zhu, Weixing Cao, and Yongchao Tian. 2019. "Predicting Rice Grain Yield Based on Dynamic Changes in Vegetation Indexes during Early to Mid-Growth Stages" Remote Sensing 11, no. 4: 387. https://doi.org/10.3390/rs11040387