Assessing Ecosystem Isoprene Emissions by Hyperspectral Remote Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Study Site and Sampling Protocols
2.2. Measurements of Photosynthetic Capacity and Light-Use Efficiency
2.3. Measurements of Isoprene Emissions
2.4. Measurements of Hyperspectral Reflectance
2.5. Pigment Analyses
2.6. Statistical Analyses
3. Results
3.1. Canopy Profile of Foliar Isoprene Emissions, Light Use Efficency and Photochemical Reflectance Index
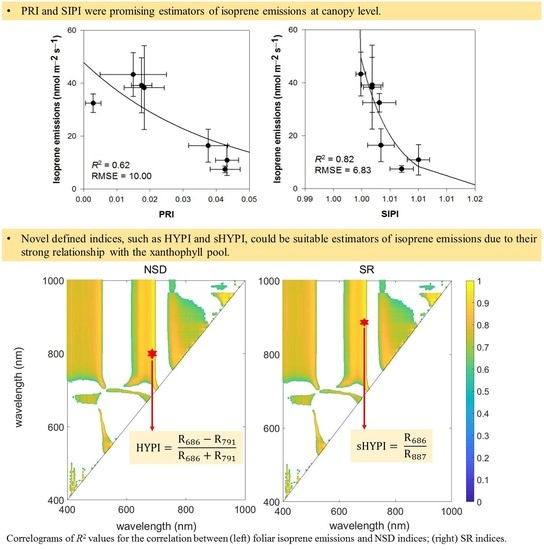
3.2. Exploring Novel Vegetation Indices to Predict Isoprene Emissions
3.3. Relationship between Foliar Pigments and Vegetation Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guenther, A.; Hewitt, C.N.; Erickson, D.; Fall, R.; Geron, C.; Graedel, T.; Harley, P.; Klinger, L.; Lerdau, M.; Mckay, W.A.; et al. A global model of natural volatile organic compound emissions. J. Geophys. Res-Atmos 1995, 100, 8873–8892. [Google Scholar] [CrossRef]
- Fiore, A.M.; Naik, V.; Spracklen, D.V.; Steiner, A.; Unger, N.; Prather, M.; Bergmann, D.; Cameron-Smith, P.J.; Cionni, I.; Collins, W.J.; et al. Global air quality and climate. Chem. Soc. Rev. 2012, 41, 6663–6683. [Google Scholar] [CrossRef] [PubMed]
- Calfapietra, C.; Fares, S.; Loreto, F. Volatile organic compounds from Italian vegetation and their interaction with ozone. Environ. Pollut. 2009, 157, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.D.; Lerdau, M.; Atkinson, R.; Baldocchi, D.; Bottenheim, J.W.; Ciccioli, P.; Lamb, B.; Geron, C.; Gu, L.; Guenther, A.; et al. Biogenic hydrocarbons in the atmospheric boundary layer: A review. Bull. Amer. Meteorol. Soc. 2000, 81, 1537–1576. [Google Scholar] [CrossRef]
- Peñuelas, J.; Marino, G.; Llusia, J.; Morfopoulos, C.; Farré-Armengol, G.; Filella, I. Photochemical reflectance index as an indirect estimator of foliar isoprenoid emissions at the ecosystem level. Nat. Commun. 2013, 4, 2604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X. The model of emissions of gases and aerosols from nature version 2.1 (MEGAN2.1): An extended and updated framework for modeling biogenic emissions. Geosci. Model Dev. 2012, 5, 1471–1492. [Google Scholar] [CrossRef] [Green Version]
- Müller, J.F.; Stavrakou, T.; Wallens, S.; De Smedt, I.; Van Roozendael, M.; Potosnak, M.J.; Rinne, J.; Munger, B.; Goldstein, A.; Guenther, A.B. Global isoprene emissions estimated using MEGAN, ECMWF analyses and a detailed canopy environment model. Atmos. Chem. Phys. 2008, 8, 1329–1341. [Google Scholar] [CrossRef] [Green Version]
- Barkley, M.P.; Palmer, P.; Kuhn, U.; Kesselmeier, J.; Chance, K.; Kurosu, T.P.; Martin, R.V.; Helmig, D.; Guenther, A. Net ecosystem fluxes of isoprene over tropical South America inferred from global ozone monitoring experiment (GOME) observations of HCHO columns. J. Geophys. Res-Atmos 2008, 113. [Google Scholar] [CrossRef]
- Gamon, J.A.; Peñuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Gamon, J.A. Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytol. 1995, 131, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Filella, I.; Porcar-Castell, A.; Munné-Bosch, S.; Bäck, J.; Garbulsky, M.F.; Peñuelas, J. PRI assessment of long-term changes in carotenoids/chlorophyll ratio and short-term changes in de-epoxidation state of the xanthophyll cycle. Int. J. Remote Sens. 2009, 30, 4443–4455. [Google Scholar] [CrossRef]
- Gamon, J.A.; Huemmrich, K.F.; Wong, C.Y.S.; Ensminger, I.; Garrity, S.; Hollinger, D.Y.; Noormets, A.; Peñuelas, J. A remotely sensed pigment index reveals photosynthetic phenology in evergreen conifers. Proc. Natl. Acad. Sci. USA 2016, 113, 13087–13092. [Google Scholar] [CrossRef] [PubMed]
- Filella, I.; Zhang, C.; Seco, R.; Potosnak, M.; Guenther, A.; Karl, T.; Gamon, J.; Pallardy, S.; Gu, L.; Kim, S.; et al. A MODIS photochemical reflectance index (PRI) as an estimator of isoprene emissions in a temperate deciduous forest. Remote Sens. 2018, 10, 557. [Google Scholar] [CrossRef]
- Harris, A.; Owen, S.M.; Sleep, D.; Pereira, M.D. Constitutive changes in pigment concentrations: Implications for estimating isoprene emissions using the photochemical reflectance index. Physiol. Plant. 2015, 156, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, L.S.; Verlinden, M.S.; Ceulemans, R. Establishment and two-year growth of a bio-energy plantation with fast-growing populus trees in flanders (Belgium): Effects of genotype and former land use. Biomass Bioener. 2012, 42, 151–163. [Google Scholar] [CrossRef]
- Verlinden, M.S.; Broeckx, L.S.; Ceulemans, R. First vs. second rotation of a poplar short rotation coppice: Above-ground biomass productivity and shoot dynamics. Biomass Bioener. 2015, 73, 174–185. [Google Scholar] [CrossRef]
- Brilli, F.; Gioli, B.; Zona, D.; Pallozzi, E.; Zenone, T.; Fratini, G.; Calfapietra, C.; Loreto, F.; Janssens, I.A.; Ceulemans, R. Simultaneous leaf- and ecosystem-level fluxes of volatile organic compounds from a poplar-based SRC plantation. Agric. For. Meteorol. 2014, 187, 22–35. [Google Scholar] [CrossRef] [Green Version]
- Zenone, T.; Hendriks, C.; Brilli, F.; Fransen, E.; Gioli, B.; Portillo-Estrada, M.; Schaap, M.; Ceulemans, R. Interaction between isoprene and ozone fluxes in a poplar plantation and its impact on air quality at the european level. Sci. Rep. 2016, 6, 32676. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Estrada, M.; Zenone, T.; Arriga, N.; Ceulemans, R. Contribution of volatile organic compound fluxes to the ecosystem carbon budget of a poplar short-rotation plantation. Glob. Chang. Biol. Bioenergy 2018, 10, 405–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verlinden, M.S.; Broeckx, L.S.; Zona, D.; Berhongaray, G.; De Groote, T.; Camino Serrano, M.; Janssens, I.A.; Ceulemans, R. Net ecosystem production and carbon balance of an SRC poplar plantation during its first rotation. Biomass Bioener. 2013, 56, 412–422. [Google Scholar] [CrossRef]
- Owen, S.M.; Peñuelas, J. Volatile isoprenoid emission potentials are correlated with essential isoprenoid concentrations in five plant species. Acta Physiol. Plant. 2013, 35, 3109–3125. [Google Scholar] [CrossRef] [Green Version]
- Owen, S.M.; Peñuelas, J. Opportunistic emissions of volatile isoprenoids. Trends Plant Sci. 2005, 10, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Thayer, S.S.; Björkman, O. Leaf xanthophyll content and composition in sun and shade determined by HPLC. Photosynth. Res. 1990, 23, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J.; Garbulsky Martin, F.; Filella, I. Photochemical reflectance index (PRI) and remote sensing of plant CO2 uptake. New Phytol. 2011, 191, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Filella, I.; Garbulsky, F.M.; Peñuelas, J. Affecting factors and recent improvements of the photochemical reflectance index (PRI) for remotely sensing foliar, canopy and ecosystemic radiation-use efficiencies. Remote Sens. 2016, 8, 677. [Google Scholar] [CrossRef]
- Morfopoulos, C.; Sperlich, D.; Peñuelas, J.; Filella, I.; Llusià, J.; Medlyn Belinda, E.; Niinemets, Ü.; Possell, M.; Sun, Z.; Prentice Iain, C. A model of plant isoprene emission based on available reducing power captures responses to atmospheric CO2. New Phytol. 2014, 203, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Filella, I.; Peñuelas, J.; Llorens, L.; Estiarte, M. Reflectance assessment of seasonal and annual changes in biomass and CO2 uptake of a mediterranean shrubland submitted to experimental warming and drought. Remote Sens. Environ. 2004, 90, 308–318. [Google Scholar] [CrossRef]
- Rahimzadeh-Bajgiran, P.; Munehiro, M.; Omasa, K. Relationships between the photochemical reflectance index (PRI) and chlorophyll fluorescence parameters and plant pigment indices at different leaf growth stages. Photosynth. Res. 2012, 113, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Porcar-Castell, A.; Garcia-Plazaola, J.I.; Nichol, C.J.; Kolari, P.; Olascoaga, B.; Kuusinen, N.; Fernández-Marín, B.; Pulkkinen, M.; Juurola, E.; Nikinmaa, E. Physiology of the seasonal relationship between the photochemical reflectance index and photosynthetic light use efficiency. Oecologia 2012, 170, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Ollinger, S.V. Sources of variability in canopy reflectance and the convergent properties of plants. New Phytol. 2010, 189, 375–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, W.K.; Bell, D.T.; Shepherd, K.A. Associations between leaf structure, orientation, and sunlight exposure in five Western Australian communities. Am. J. Bot. 1998, 85, 56–63. [Google Scholar] [CrossRef]




| Predictor | Univariate Analysis | SBRA | ||
|---|---|---|---|---|
| Variable Type | R2 (p) | Post-Hoc (p) | R2 (p) | |
| PRI | continuous | 0.78 (0.008) | X | |
| PAR | continuous | 0.94 (<0.001) | X | |
| Leaf position | categorical | (0.002) | ||
| Stepwise backward regression | 0.95 (0.002) | |||
| Model | ||||
| Log (Isoprene Emissions) | PRI | SIPI | HYPI | sHYPI | Car | Chl | Car/Chl | N/Chl | V/Chl | A/Chl | Z/Chl | L/Chl | b/Chl | VAZ/Chl | VAZ | EPS | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log (isoprene emissions) | 1 | ||||||||||||||||
| PRI | −0.88 | 1 | |||||||||||||||
| SIPI | −0.87 | 0.75 | 1 | ||||||||||||||
| HYPI | −0.96 | 0.97 | 0.83 | 1 | |||||||||||||
| sHYPI | −0.96 | 0.97 | 0.83 | 1.00 | 1 | ||||||||||||
| Car | −0.11 | 0.27 | 0.37 | 0.23 | 0.23 | 1 | |||||||||||
| Chl | −0.29 | 0.47 | 0.47 | 0.42 | 0.42 | 0.97 | 1 | ||||||||||
| Car/Chl | 0.40 | −0.39 | −0.03 | −0.40 | −0.39 | 0.73 | 0.54 | 1 | |||||||||
| N/Chl | −0.80 | 0.91 | 0.82 | 0.91 | 0.91 | 0.58 | 0.72 | −0.05 | 1 | ||||||||
| V/Chl | 0.86 | −0.80 | −0.89 | −0.85 | −0.85 | −0.38 | −0.53 | 0.19 | −0.81 | 1 | |||||||
| A/Chl | 0.92 | −0.94 | −0.76 | −0.93 | −0.93 | −0.06 | −0.25 | 0.44 | −0.80 | 0.70 | 1 | ||||||
| Z/Chl | 0.68 | −0.63 | −0.27 | −0.66 | −0.65 | 0.49 | 0.29 | 0.86 | −0.32 | 0.35 | 0.73 | 1 | |||||
| L/Chl | −0.03 | 0.01 | 0.26 | −0.01 | −0.01 | 0.63 | 0.53 | 0.79 | 0.22 | 0.01 | −0.09 | 0.42 | 1 | ||||
| b/Chl | 0.40 | −0.52 | −0.03 | −0.49 | −0.48 | 0.57 | 0.38 | 0.92 | −0.22 | 0.12 | 0.55 | 0.85 | 0.64 | 1 | |||
| VAZ/Chl | 0.97 | −0.93 | −0.86 | −0.97 | −0.97 | −0.16 | −0.36 | 0.43 | −0.83 | 0.91 | 0.92 | 0.66 | 0.05 | 0.43 | 1 | ||
| VAZ | 0.85 | −0.67 | −0.68 | −0.75 | −0.75 | 0.38 | 0.21 | 0.69 | −0.45 | 0.65 | 0.81 | 0.79 | 0.27 | 0.58 | 0.83 | 1 | |
| EPS | −0.65 | 0.60 | 0.22 | 0.61 | 0.61 | −0.43 | −0.25 | −0.74 | 0.30 | −0.25 | −0.74 | −0.97 | −0.21 | −0.78 | −0.60 | −0.71 | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balzarolo, M.; Peñuelas, J.; Filella, I.; Portillo-Estrada, M.; Ceulemans, R. Assessing Ecosystem Isoprene Emissions by Hyperspectral Remote Sensing. Remote Sens. 2018, 10, 1086. https://doi.org/10.3390/rs10071086
Balzarolo M, Peñuelas J, Filella I, Portillo-Estrada M, Ceulemans R. Assessing Ecosystem Isoprene Emissions by Hyperspectral Remote Sensing. Remote Sensing. 2018; 10(7):1086. https://doi.org/10.3390/rs10071086
Chicago/Turabian StyleBalzarolo, Manuela, Josep Peñuelas, Iolanda Filella, Miguel Portillo-Estrada, and Reinhart Ceulemans. 2018. "Assessing Ecosystem Isoprene Emissions by Hyperspectral Remote Sensing" Remote Sensing 10, no. 7: 1086. https://doi.org/10.3390/rs10071086