In-Situ Characterization by High-Energy X-ray Diffraction of the Phase Transformations Leading to Transformation-Induced Plasticity-Aided Bainitic Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results
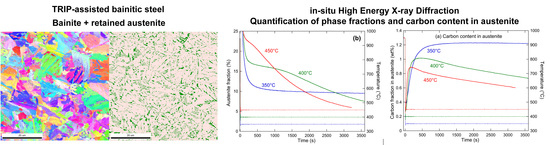
3.1. Initial Microstructure
3.2. Austenitization
3.3. Bainitic Transformation
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Schmitt, J.-H.; Iung, T. New developments of advanced high-strength steels for automotive applications. C. R. Phys. 2018, 19, 641–656. [Google Scholar] [CrossRef]
- Grajcar, A.; Kuziak, R.; Zalecki, W. Third generation of AHSS with increased fraction of retained austenite for the automotive industry. Arch. Civ. Mech. Eng. 2012, 12, 334–341. [Google Scholar] [CrossRef]
- Matlock, D.K.; Speer, J.G.; De Moor, E.; Gibbs, P.J. Recent developments in advanced high strength sheet steels for automotive applications: An overview. Jestech 2012, 15, 1–12. [Google Scholar]
- Huang, J.N.; Tang, Z.Y.; Ding, H.; Misra, R.D.K. The significant impact of phase fraction and austenite stability on the mechanical properties of a low-alloyed TRIP-aided steel: An insight into experimental analysis and predictions. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2019, 759, 40–46. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.-J.; De Cooman, B.C. Austenite stability of ultrafine-grained transformation-induced plasticity steel with Mn partitioning. Scr. Mater. 2011, 65, 225–228. [Google Scholar] [CrossRef]
- Wang, J.J.; van der Zwaag, S. Stabilization mechanisms of retained austenite in transformation-induced plasticity steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2001, 32, 1527–1539. [Google Scholar] [CrossRef]
- Sakuma, Y.; Matsumura, O.; Takechi, H. Mechanical-properties and retained austenite in intercritically heat-treated bainite-transformed steel and their variation with Si and Mn additions. Metall. Trans. A Phys. Metall. Mater. Sci. 1991, 22, 489–498. [Google Scholar] [CrossRef]
- Reisner, G.; Werner, E.A.; Kerschbaummayr, P.; Papst, I.; Fischer, F.D. The modeling of retained austenite in low-alloyed TRIP steels. JOM J. Miner. Met. Mater. Soc. 1997, 49, 62–65. [Google Scholar] [CrossRef]
- Bai, D.Q.; Di Chiro, A.; Yue, S. Stability of retained austenite in a Nb microalloyed Mn-Si TRIP steel. In Proceedings International Conference on Microalloying Steels; RodriguezIbabe, J.M., Gutierrez, I., Lopez, B., Eds.; Trans Tech Publications Ltd: Stafa, Zurich, Switzerland, 1998; pp. 253–260. [Google Scholar]
- De Meyer, M.; Vanderschueren, D.; De Cooman, B.C. The influence of the substitution of Si by Al on the properties of cold rolled C-Mn-Si TRIP steels. ISIJ Int. 1999, 39, 813–822. [Google Scholar] [CrossRef]
- Timokhina, I.B.; Hodgson, P.D.; Pereloma, E.V. Effect of microstructure on the stability of retained austenite in transformation-induced-plasticity steels. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2004, 35, 2331–2341. [Google Scholar] [CrossRef] [Green Version]
- Matlock, D.K.; Brautigam, V.E.; Speer, J.G. Application of the quenching and partitioning (Q&P) process to a medium-carbon, high-Si microalloyed bar steel. In Thermec 2003, Pts 1–5; Chandra, T., Torralba, J.M., Sakai, T., Eds.; Trans Tech Publications Ltd.: Zurich, Switzerland, 2003; pp. 1089–1094. [Google Scholar]
- Speer, J.; Matlock, D.K.; De Cooman, B.C.; Schroth, J.G. Carbon partitioning into austenite after martensite transformation. Acta Mater. 2003, 51, 2611–2622. [Google Scholar] [CrossRef]
- Speer, J.G.; Edmonds, D.V.; Rizzo, F.C.; Matlock, D.K. Partitioning of carbon from supersaturated plates of ferrite, with application to steel processing and fundamentals of the bainite transformation. Curr. Opin. Solid State Mater. Sci. 2004, 8, 219–237. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Singh, S.B.; Das, S.; Haldar, A.; Bhattacharjee, D. Development and characterisation of C-Mn-Al-Si-Nb TRIP aided steel. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2011, 528, 2394–2400. [Google Scholar] [CrossRef]
- De Cooman, B.C. Structure-properties relationship in TRIP steels containing carbide-free bainite. Curr. Opin. Solid State Mater. Sci. 2004, 8, 285–303. [Google Scholar] [CrossRef]
- Zhao, Z.Z.; Yin, H.X.; Zhao, A.M.; Gong, Z.Q.; He, J.G.; Tong, T.T.; Hu, H.J. The influence of the austempering temperature on the transformation behavior and properties of ultra-high-strength TRIP-aided bainitic-ferritic sheet steel. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2014, 613, 8–16. [Google Scholar] [CrossRef]
- Bigg, T.D.; Edmonds, D.V.; Eardley, E.S. Real-time structural analysis of quenching and partitioning (Q&P) in an experimental martensitic steel. J. Alloys Compd. 2013, 577, S695–S698. [Google Scholar] [CrossRef]
- Geandier, G.; Aeby-Gautier, E.; Settefrati, A.; Dehmas, M.; Appolaire, B. Study of diffusive transformations by high energy X-ray diffraction. C. R. Phys. 2012, 13, 257–267. [Google Scholar] [CrossRef]
- Allain, S.Y.P.; Geandier, G.; Hell, J.C.; Soler, M.; Danoix, F.; Goune, M. In-situ investigation of quenching and partitioning by high energy X-Ray diffraction experiments. Scr. Mater. 2017, 131, 15–18. [Google Scholar] [CrossRef]
- Allain, S.Y.P.; Geandier, G.; Hell, J.C.; Soler, M.; Danoix, F.; Goune, M. Effects of Q&P processing conditions on austenite carbon enrichment studied by in situ high-energy X-ray diffraction experiments. Metals 2017, 7, 232. [Google Scholar] [CrossRef] [Green Version]
- Allain, S.Y.P.; Gaudez, S.; Geandier, G.; Hell, J.C.; Goune, M.; Danoix, F.; Soler, M.; Aoued, S.; Poulon-Quintin, A. Internal stresses and carbon enrichment in austenite of Quenching and Partitioning steels from high energy X-ray diffraction experiments. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2018, 710, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Blonde, R.; Jimenez-Melero, E.; Zhao, L.; Wright, J.P.; Bruck, E.; van der Zwaag, S.; van Dijk, N.H. High-energy X-ray diffraction study on the temperature-dependent mechanical stability of retained austenite in low-alloyed TRIP steels. Acta Mater. 2012, 60, 565–577. [Google Scholar] [CrossRef]
- Ebner, S.; Suppan, C.; Stark, A.; Schnitzer, R.; Hofer, C. Austenite decomposition and carbon partitioning during quenching and partitioning heat treatments studied via in-situ X-ray diffraction. Mater. Des. 2019, 178, 107862. [Google Scholar] [CrossRef]
- Rieger, T.; Herrmann, K.; Carmele, D.; Meyer, S.; Lippmann, T.; Stark, A.; Bleck, W.; Klemradt, U. ‘Quenching and Partitioning’—An in situ approach to characterize the process kinetics and the final microstructure of TRIP-assisted steel. Adv. Mater. Res. 2012, 409, 713–718. [Google Scholar] [CrossRef] [Green Version]
- Song, C.; Yu, H.; Lu, J.; Zhou, T.; Yang, S. Stress partitioning among ferrite, martensite and retained austenite of a TRIP-assisted multiphase steel: An in-situ high-energy X-ray diffraction study. Mater. Sci. Eng. A 2018, 726, 1–9. [Google Scholar] [CrossRef]
- Yan, K.; Liss, K.-D.; Timokhina, I.B.; Pereloma, E.V. In situ synchrotron X-ray diffraction studies of the effect of microstructure on tensile behavior and retained austenite stability of thermo-mechanically processed transformation induced plasticity steel. Mater. Sci. Eng. A 2016, 662, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Rauch, E.F.; Veron, M. Automated crystal orientation and phase mapping in TEM. Mater. Charact. 2014, 98, 1–9. [Google Scholar] [CrossRef]
- Brahmi, A.; Borrelly, R. Manganese enrichment of cementite and solubility of carbon in low-carbon steels investigated by thermoelectric power measurements. Scr. Metall. Mater. 1995, 32. [Google Scholar] [CrossRef]
- Moreno, M.; Teixeira, J.; Ghanbaja, J.; Bonnet, F.; Allain, S. Evolution of cementite composition along the processing of cold-rolled and annealed Dual-Phase steels. Materialia 2019, 6, 100179. [Google Scholar] [CrossRef]
- Pereloma, E.V.; Timokhina, I.B.; Miller, M.K.; Hodgson, P.D. Three-dimensional atom probe analysis of solute distribution in thermomechanically processed TRIP steels. Acta Mater. 2007, 55, 2587–2598. [Google Scholar] [CrossRef]
- Cayron, C. ARPGE: A computer program to automatically reconstruct the parent grains from electron backscatter diffraction data. J. Appl. Crystallogr. 2007, 40, 1183–1188. [Google Scholar] [CrossRef] [Green Version]
- Bhadeshia, H.K.D.H.; Edmonds, D.V. Bainite transformation in a silicon steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 1979, 10, 895–907. [Google Scholar] [CrossRef]
- Kozeschnik, E.; Bhadeshia, H.K.D.H. Influence of silicon on cementite precipitation in steels. Mater. Sci. Technol. 2008, 24, 343–347. [Google Scholar] [CrossRef]
- Sandvik, B.P.J. The bainite reaction in Fe-Si-C alloys: The primary stage. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 1982, 13, 777–787. [Google Scholar] [CrossRef]

















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tournoud, Z.; De Geuser, F.; Renou, G.; Huin, D.; Donnadieu, P.; Deschamps, A. In-Situ Characterization by High-Energy X-ray Diffraction of the Phase Transformations Leading to Transformation-Induced Plasticity-Aided Bainitic Steel. Quantum Beam Sci. 2019, 3, 25. https://doi.org/10.3390/qubs3040025
Tournoud Z, De Geuser F, Renou G, Huin D, Donnadieu P, Deschamps A. In-Situ Characterization by High-Energy X-ray Diffraction of the Phase Transformations Leading to Transformation-Induced Plasticity-Aided Bainitic Steel. Quantum Beam Science. 2019; 3(4):25. https://doi.org/10.3390/qubs3040025
Chicago/Turabian StyleTournoud, Zélie, Frédéric De Geuser, Gilles Renou, Didier Huin, Patricia Donnadieu, and Alexis Deschamps. 2019. "In-Situ Characterization by High-Energy X-ray Diffraction of the Phase Transformations Leading to Transformation-Induced Plasticity-Aided Bainitic Steel" Quantum Beam Science 3, no. 4: 25. https://doi.org/10.3390/qubs3040025