Pulsed Electric Field-Assisted Ethanolic Extraction of Date Palm Fruits: Bioactive Compounds, Antioxidant Activity and Physicochemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
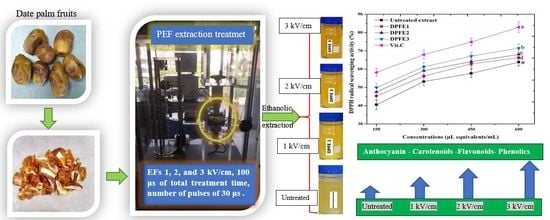
2.2. PEF Treatment
2.3. Ethanolic Extraction
2.4. Determination of Total Flavonoids Content
2.5. Determination of Total Carotenoids Content
2.6. Determination of Total Anthocyanins Content
2.7. Antioxidant Activity Measurement
2.7.1. Reducing Power Assay
2.7.2. DPPH Radical Scavenging Activity
2.8. Measurement of Physicochemical Characteristics of Date Palm Fruit Extracts
2.9. Determination of 5-(Hydroxymethyl) Furfural (5-HMF)
2.10. Determination of Volatile Flavors Compounds
2.11. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic (TPC) and Flavonoids Contents (TFC)
3.2. Impact on Total Anthocyanin Contents (TAC)
3.3. Total Carotenoids Contents (TCC)
3.4. Antioxidant Activities
3.4.1. DPPH Assay
3.4.2. Reducing Power Assay
3.5. Color Parameters
3.6. TSS, pH, TA, EC, and 5-HMF
3.7. Volatile Flavor Compounds
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nasser, R.A.; Salem, M.Z.; Hiziroglu, S.; Al-Mefarrej, H.A.; Mohareb, A.S.; Alam, M.; Aref, I.M. Chemical analysis of different parts of date palm (Phoenix dactylifera L.) using ultimate, proximate and thermo-gravimetric techniques for energy production. Energies 2016, 9, 374. [Google Scholar] [CrossRef]
- Al-Abdoulhadi, I.; Al-Ali, S.; Khurshid, K.; Al-Shryda, F.; Al-Jabr, A.; Abdallah, A.B. Assessing fruit characteristics to standardize quality norms in date cultivars of Saudi Arabia. Indian J. Sci. Technol. 2011, 4, 1262–1266. [Google Scholar]
- Al-Farsi, M.A.; Lee, C.Y. Optimization of phenolics and dietary fibre extraction from date seeds. Food Chem. 2008, 108, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Fageer, A.S.; Eltayeb, M.M.; Mohamed Ahmed, I.A. Chemical composition, antioxidant capacity, and mineral extractability of Sudanese date palm (Phoenix dactylifera L.) fruits. Food Sci. Nutr. 2014, 2, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Parvin, S.; Easmin, D.; Sheikh, A.; Biswas, M.; Sharma, S.C.D.; Jahan, M.G.S.; Islam, M.A.; Shovon, M.; Roy, N. Nutritional analysis of date fruits (Phoenix dactylifera L.) in perspective of Bangladesh. Am. J. Life Sci. 2015, 3, 274–278. [Google Scholar] [CrossRef]
- Assirey, E.A.R. Nutritional composition of fruit of 10 date palm (Phoenix dactylifera L.) cultivars grown in Saudi Arabia. J. Taibah Univ. Sci. 2015, 9, 75–79. [Google Scholar] [CrossRef]
- Saeeduddin, M.; Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Awad, F.N.; Hu, B.; Lei, S.; Zeng, X. Quality assessment of pear juice under ultrasound and commercial pasteurization processing conditions. LWT-Food Sci. Technol. 2015, 64, 452–458. [Google Scholar] [CrossRef]
- Liu, Z.-W.; Zeng, X.-A.; Sun, D.-W.; Han, Z.; Aadil, R.M. Synergistic effect of thermal and pulsed electric field (PEF) treatment on the permeability of soya PC and DPPC vesicles. J. Food Eng. 2015, 153, 124–131. [Google Scholar] [CrossRef]
- Medina-Meza, I.G.; Barbosa-Cánovas, G.V. Assisted extraction of bioactive compounds from plum and grape peels by ultrasonics and pulsed electric fields. J. Food Eng. 2015, 166, 268–275. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Zeng, X.-A.; Rahaman, A.; Siddeeg, A.; Aadil, R.M.; Ahmed, Z.; Li, J.; Niu, D. Combined impact of pulsed electric field and ultrasound on bioactive compounds and FT-IR analysis of almond extract. J. Food Sci. Technol. 2019, 56, 2355–2364. [Google Scholar] [CrossRef]
- Rahaman, A.; Siddeeg, A.; Manzoor, M.F.; Zeng, X.-A.; Ali, S.; Baloch, Z.; Li, J.; Wen, Q.-H. Impact of pulsed electric field treatment on drying kinetics, mass transfer, colour parameters and microstructure of plum. J. Food Sci. Technol. 2019, 56, 2670–2678. [Google Scholar] [CrossRef] [PubMed]
- Siddeeg, A.; Zeng, X.-A.; Rahaman, A.; Manzoor, M.F.; Ahmed, Z.; Ammar, A.-F. Quality characteristics of the processed dates vinegar under influence of ultrasound and pulsed electric field treatments. J. Food Sci. Technol. 2019, 1–10. [Google Scholar] [CrossRef]
- Mtaoua, H.; Sánchez-Vega, R.; Ferchichi, A.; Martín-Belloso, O. Impact of High-Intensity Pulsed Electric Fields or Thermal Treatment on the Quality Attributes of Date Juice through Storage. J. Food Process. Preserv. 2017, 41, e13052. [Google Scholar] [CrossRef]
- Delgado, A.E.; Zheng, L.; Sun, D.-W. Influence of ultrasound on freezing rate of immersion-frozen apples. Food Bioprocess Technol. 2009, 2, 263–270. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yu, Q.; Zeng, X.A.; Han, Z.; Sun, D.W.; Muhammad-Aadil, R. Effects of pulsed electric field on selected properties of L-tryptophan. Int. J. Food Sci. Technol. 2015, 50, 1130–1136. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Han, Z.; Sahar, A.; Khalil, A.A.; Rahman, U.U.; Khan, M.; Mehmood, T. Combined effects of pulsed electric field and ultrasound on bioactive compounds and microbial quality of grapefruit juice. J. Food Process. Preserv. 2018, 42, e13507. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.; Rahman, M.; Sharif, K.; Mohamed, A.; Sahena, F.; Jahurul, M.; Ghafoor, K.; Norulaini, N.; Omar, A. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R.; Lu, R.; Wang, M.; Qian, P.; Yang, W. Effect of PEF on microbial inactivation and physical–chemical properties of green tea extracts. LWT-Food Sci. Technol. 2008, 41, 425–431. [Google Scholar] [CrossRef]
- López, N.; Puértolas, E.; Condón, S.; Álvarez, I.; Raso, J. Effects of pulsed electric fields on the extraction of phenolic compounds during the fermentation of must of Tempranillo grapes. Innov. Food Sci. Emerg. Technol. 2008, 9, 477–482. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zeng, X.A.; Ngadi, M. Enhanced extraction of phenolic compounds from onion by pulsed electric field (PEF). J. Food Process. Preserv. 2018, 42, e13755. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Leong, S.Y.; Burritt, D.J.; Oey, I. Evaluation of the anthocyanin release and health-promoting properties of Pinot Noir grape juices after pulsed electric fields. Food Chem. 2016, 196, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Segovia, F.J.; Luengo, E.; Corral-Pérez, J.J.; Raso, J.; Almajano, M.P. Improvements in the aqueous extraction of polyphenols from borage (Borago officinalis L.) leaves by pulsed electric fields: Pulsed electric fields (PEF) applications. Ind. Crop. Prod. 2015, 65, 390–396. [Google Scholar] [CrossRef]
- Siddeeg, A.; Zeng, X.-A.; Ammar, A.-F.; Han, Z. Sugar profile, volatile compounds, composition and antioxidant activity of Sukkari date palm fruit. J. Food Sci. Technol. 2019, 56, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Wiktor, A.; Nowacka, M.; Dadan, M.; Rybak, K.; Lojkowski, W.; Chudoba, T.; Witrowa-Rajchert, D. The effect of pulsed electric field on drying kinetics, color, and microstructure of carrot. Dry. Technol. 2016, 34, 1286–1296. [Google Scholar] [CrossRef]
- Azhari, S.; Xu, Y.; Jiang, Q.; Xia, W. Physicochemical properties and chemical composition of Seinat (Cucumis melo var. tibish) seed oil and its antioxidant activity. Grasas Y Aceites 2014, 65, 008. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Wang, M.S.; Liu, Z.W.; Han, Z.; Zhang, Z.H.; Hong, J.; Jabbar, S. A potential of ultrasound on minerals, micro-organisms, phenolic compounds and colouring pigments of grapefruit juice. Int. J. Food Sci. Technol. 2015, 50, 1144–1150. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar]
- Siddeeg, A.; Xu, Y.; Jiang, Q.; Xia, W. In vitro antioxidant activity of protein fractions extracted from seinat (Cucumis melo var. tibish) seeds. CYTA-J. Food 2015, 13, 472–481. [Google Scholar] [CrossRef]
- De Oliveira, T.L.C.; de Carvalho, S.M.; de Araújo Soares, R.; Andrade, M.A.; das Graças Cardoso, M.; Ramos, E.M.; Piccoli, R.H. Antioxidant effects of Satureja montana L. essential oil on TBARS and color of mortadella-type sausages formulated with different levels of sodium nitrite. LWT-Food Sci. Technol. 2012, 45, 204–212. [Google Scholar] [CrossRef]
- Saricoban, C.; Yilmaz, M.T. Modelling the effects of processing factors on the changes in colour parameters of cooked meatballs using response surface methodology. World Appl. Sci. J. 2010, 9, 14–22. [Google Scholar]
- Agcam, E.; Akyıldız, A.; Evrendilek, G.A. Comparison of phenolic compounds of orange juice processed by pulsed electric fields (PEF) and conventional thermal pasteurisation. Food Chem. 2014, 143, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Aguiló-Aguayo, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Avoiding non-enzymatic browning by high-intensity pulsed electric fields in strawberry, tomato and watermelon juices. J. Food Eng. 2009, 92, 37–43. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmad, N.; Ahmed, Z.; Siddique, R.; Zeng, X.A.; Rahaman, A.; Muhammad Aadil, R.; Wahab, A. Novel extraction techniques and pharmaceutical activities of luteolin and its derivatives. J. Food Biochem. 2019, e12974. [Google Scholar] [CrossRef]
- Bhat, R.; Kamaruddin, N.S.B.C.; Min-Tze, L.; Karim, A. Sonication improves kasturi lime (Citrus microcarpa) juice quality. Ultrason. Sonochem. 2011, 18, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Odriozola-Serrano, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Phenolic acids, flavonoids, vitamin C and antioxidant capacity of strawberry juices processed by high-intensity pulsed electric fields or heat treatments. Eur. Food Res. Technol. 2008, 228, 239. [Google Scholar] [CrossRef]
- Tiwari, B.; O’donnell, C.; Patras, A.; Brunton, N.; Cullen, P. Stability of anthocyanins and ascorbic acid in sonicated strawberry juice during storage. Eur. Food Res. Technol. 2009, 228, 717–724. [Google Scholar] [CrossRef]
- Puértolas, E.; Cregenzán, O.; Luengo, E.; Álvarez, I.; Raso, J. Pulsed-electric-field-assisted extraction of anthocyanins from purple-fleshed potato. Food Chem. 2013, 136, 1330–1336. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Hu, B.; Hashim, M.M.; Wu, T.; Wu, Z.; Khan, M.A.; Zeng, X. Synergistic impact of sonication and high hydrostatic pressure on microbial and enzymatic inactivation of apple juice. LWT-Food Sci. Technol. 2014, 59, 70–76. [Google Scholar] [CrossRef]
- Plaza, L.; Sánchez-Moreno, C.; De Ancos, B.; Elez-Martínez, P.; Martín-Belloso, O.; Cano, M.P. Carotenoid and flavanone content during refrigerated storage of orange juice processed by high-pressure, pulsed electric fields and low pasteurization. LWT-Food Sci. Technol. 2011, 44, 834–839. [Google Scholar] [CrossRef]
- Jabbar, S.; Abid, M.; Hu, B.; Wu, T.; Hashim, M.M.; Lei, S.; Zhu, X.; Zeng, X. Quality of carrot juice as influenced by blanching and sonication treatments. LWT-Food Sci. Technol. 2014, 55, 16–21. [Google Scholar] [CrossRef]
- Hasan, N.S.; Amom, Z.H.; Nor, A.; Norhafizah, M.; Norhaizan, M.E.; Azrina, A. Nutritional composition and in vitro evaluation of the antioxidant properties of various dates extracts (Phoenix dactylifera L.) from Libya. Asian J. Clin. Nutr. 2010, 2, 208–214. [Google Scholar] [CrossRef]
- Benmeddour, Z.; Mehinagic, E.; Le Meurlay, D.; Louaileche, H. Phenolic composition and antioxidant capacities of ten Algerian date (Phoenix dactylifera L.) cultivars: A comparative study. J. Funct. Foods 2013, 5, 346–354. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.-A.; Sun, D.-W.; Wang, M.-S.; Liu, Z.-W.; Zhang, Z.-H. Combined effects of sonication and pulsed electric field on selected quality parameters of grapefruit juice. LWT-Food Sci. Technol. 2015, 62, 890–893. [Google Scholar] [CrossRef]
- Meir, S.; Kanner, J.; Akiri, B.; Philosoph-Hadas, S. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J. Agric. Food Chem. 1995, 43, 1813–1819. [Google Scholar] [CrossRef]
- Anwar, F.; Przybylski, R. Effect of solvents extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.). Acta Sci. Pol. Technol. Aliment. 2012, 11, 293–302. [Google Scholar]
- Rivas, A.; Rodrigo, D.; Martinez, A.; Barbosa-Cánovas, G.; Rodrigo, M. Effect of PEF and heat pasteurization on the physical–chemical characteristics of blended orange and carrot juice. LWT-Food Sci. Technol. 2006, 39, 1163–1170. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R.; Wang, M.; Lu, R. Effects of pulsed electric fields on bioactive components, colour and flavour of green tea infusions. Int. J. Food Sci. Technol. 2009, 44, 312–321. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Physicochemical and nutritional characteristics of blueberry juice after high pressure processing. Food Res. Int. 2013, 50, 545–549. [Google Scholar] [CrossRef]
- Lopez, N.; Puertolas, E.; Condon, S.; Raso, J.; Alvarez, I. Enhancement of the solid-liquid extraction of sucrose from sugar beet (Beta vulgaris) by pulsed electric fields. LWT-Food Sci. Technol. 2009, 42, 1674–1680. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Soliva-Fortuny, R.; Martín-Belloso, O. Optimizing critical high-intensity pulsed electric fields treatments for reducing pectolytic activity and viscosity changes in watermelon juice. Eur. Food Res. Technol. 2010, 231, 509–517. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Satora, P.; Jurasz, E. Physicochemical and antioxidant properties of selected polish grape and fruit wines. Acta Sci. Pol. Technol. Aliment. 2008, 7, 35–45. [Google Scholar]
- Rahaman, A.; Zeng, X.-A.; Kumari, A.; Rafiq, M.; Siddeeg, A.; Manzoor, M.F.; Bloch, Z.; Ahmad, Z. Influence of ultrasound-assisted osmotic dehydration on texture, bioactive compounds and metabolites analysis of plum. Ultrason. Sonochemistry 2019, 58, 104643. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef] [PubMed]


| Sample | TPC (mg GAE/100 g) | TFC (mg CE/100 g) |
|---|---|---|
| Untreated extract | 62.50 ± 0.11 d | 3.20 ± 0.09 d |
| DPFE1 | 64.20 ± 0.41 c | 4.58 ± 0.71 c |
| DPFE2 | 65.90 ± 0.91 b | 5.80 ± 0.63 b |
| DPFE3 | 67.35 ± 0.71 a | 6.75 ± 0.55 a |
| Sample | TCC (µg/mL) | TAC (mg/L) |
|---|---|---|
| Untreated extract | 2.85 ± 0.12 d | 0.75 ± 0.09 d |
| DPFE1 | 3.29 ± 0.09 c | 0.94 ± 0.11 c |
| DPFE2 | 4.93 ± 0.07 b | 1.23 ± 0.03 b |
| DPFE3 | 6.10 ± 0.10 a | 2.08 ± 0.09 a |
| Sample | Color Parameters | |||||
|---|---|---|---|---|---|---|
| L* | a* | b* | Hue (ho) | C* | ΔE | |
| Untreated extract | 33.40 ± 0.19 a | −0.98 ± 0.17 a | 4.25 ± 0.16 b | −85.66 ± 0.33 b | 4.36 ± 0.10 b | --- |
| DPFE1 | 33.22 ± 0.13 a | −0.61 ± 0.11 a | 4.51 ± 0.18 b | −82.63 ± 0.29 b | 4.55 ± 0.11 b | 0.28 |
| DPFE2 | 33.12 ± 0.10 a | −0.58 ± 0.12 a | 4.70 ± 0.10 b | −81.93 ± 0.2b | 4.74 ± 0.20 b | 0.49 |
| DPFE3 | 32.96 ± 0.18 b | −0.29 ± 0.14 b | 5.00 ± 0.11 a | −73.21 ± 0.25 a | 5.08 ± 0.23 a | 1.33 |
| Parameter | Untreated Extract | Treated Extract | ||
|---|---|---|---|---|
| DPFE1 | DPFE2 | DPFE3 | ||
| EC (ms/cm) | 2.78 ± 0.11 a | 2.79 ± 0.11 a | 2.79 ± 0.11 a | 2.80 ± 0.11 a |
| TSS (°Brix) | 20.0 ± 0.09 a | 20.70 ± 0.03 a | 20.73 ± 0.10 a | 20.98 ± 0.11 a |
| pH | 5.85 ± 0.12 a | 5.79 ± 0.09 a | 5.78 ± 0.17 a | 5.76 ± 0.13 a |
| TA (%) | 0.10 ± 0.07 a | 0.11 ± 0.08 a | 0.11 ± 0.07 a | 0.12 ± 0.10 a |
| HMF (mg/L) | 4.95 ± 0.13 c | 5.11 ± 0.10 b | 5.13 ± 0.10 b | 5.94 ± 0.09 a |
| Area (%) | Constituent | |||
|---|---|---|---|---|
| Untreated Extract | DPFE1 | DPFE2 | DPFE3 | |
| 1.60 ± 0.02 c | 1.65 ± 0.01 b | 1.66 ± 0.03 b | 1.77 ± 0.01 a | Urea, N-butyl-N-nitroso- |
| 4.40 ± 0.04 c | 4.40 ± 0.00 c | 4.45 ± 0.03 b | 4.56 ± 0.00 a | Morpholine, 4-methyl-, 4-oxide |
| 5.14 ± 0.02 d | 5.28 ± 0.03 c | 5.35 ± 0.01 b | 5.44 ± 0.02 a | 1,3,5-Triazine-2,4,6-triamine |
| 9.45 ± 0.02 c | 9.46 ± 0.02 c | 9.55 ± 0.02 b | 9.63 ± 0.02 a | 4 H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- |
| 1.65 ± 0.03 b | 1.66 ± 0.04 b | 1.66 ± 0.02 b | 1.77 ± 0.07 a | 2(3H)-Furanone, dihydro-4-hydroxy- |
| 3.90 ± 0.01 c | 3.93 ± 0.02 c | 4.05 ± 0.05 b | 4.18 ± 0.09 a | Dimethylamine, N-(neopentyloxy)- |
| 5.38 ± 0.04 c | 5.40 ± 0.03 c | 5.48 ± 0.03 b | 5.66 ± 0.02 a | Isosorbide Dinitrate |
| 27.26 ± 0.03 c | 27.30 ± 0.02 b | 27.31 ± 0.01 b | 28.07 ± 0.00 a | 5-Hydroxymethylfurfural |
| 5.41 ± 0.03 c | 5.40 ± 0.00 c | 5.49 ± 0.02 b | 6.09 ± 0.03 a | 1,2,3-Propanetriol, 1-acetate |
| 1.26 ± 0.00 d | 1.35 ± 0.02 c | 1.44 ± 0.04 b | 2.19 ± 0.05 a | 2-Methyl-1-isopropyl(dimethyl)silyloxypropane |
| 7.85 ± 0.01 c | 7.88 ± 0.01 bc | 7.91 ± 0.03 a | 8.09 ± 0.04 a | 4 H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy |
| 1.92 ± 0.08 c | 1.92 ± 0.02 c | 2.09 ± 0.01 b | 2.33 ± 0.08 a | 2-Methyl-3,4,5,6-tetrahydropyrazine |
| 3.16 ± 0.03 c | 3.20 ± 0.04 bc | 3.21 ± 0.02 b | 3.41 ± 0.02 a | 3-Methyl-3-buten-1-ol, TMS derivative |
| 3.57 ± 0.02 c | 3.63 ± 0.08 b | 3.85 ± 0.02 a | 3.89 ± 0.07 a | Glycoluril |
| 2.11 ± 0.04 c | 2.15 ± 0.09 b | 2.19 ± 0.05 b | 2.44 ± 0.06 a | Propanamide, N,N-dimethyl- |
| 4.07 ± 0.09 c | 4.07 ± 0.03 c | 4.15 ± 0.03 b | 4.36 ± 0.01 a | Butanoic acid, 2-methyl-, 2-methylpropyl ester |
| 2.20 ± 0.07 d | 2.27 ± 0.02 c | 2.39 ± 0.02 b | 2.75 ± 0.02 a | 1-Nitro-2-acetamido-1,2-dideoxy-d-glucitol |
| 1.01 ± 0.00 d | 1.09 ± 0.01 c | 1.18 ± 0.04 b | 1.55 ± 0.09 a | β-d-Glucopyranose, 4-O-β-D-galactopyranosyl- |
| 3.44 ± 0.01 c | 3.45 ± 0.00 c | 3.56 ± 0.09 b | 3.77 ± 0.07 a | 3-Deoxy-d-mannoic lactone |
| 2.68 ± 0.02 d | 2.68 ± 0.03 c | 2.80 ± 0.05 b | 3.09 ± 0.03 b | 3-Deoxy-d-mannoic lactone |
| 1.06 ± 0.00 c | 1.11 ± 0.01 c | 2.13 ± 0.04 b | 2.55 ± 0.09 a | n-Hexadecanoic acid |
| 1.50 ± 0.03 d | 1.61 ± 0.02 c | 1.88 ± 0.03 b | 2.13 ± 0.07 a | 9,12-Octadecadienoic acid (Z, Z)- |
| ND | ND | ND | 2.02 ± 0.02 a | 11,13-Dihydroxy-tetradec-5 ynoic acid, methyl ester |
| ND | ND | 0.59 ± 0.05 b | 0.88 ± 0.04 a | 2-Myristynoyl pantetheine |
| ND | 0.53 ± 0.04 b | 0.90 ± 0.01 a | 0.92 ± 0.03 a | Paromomycin |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddeeg, A.; Faisal Manzoor, M.; Haseeb Ahmad, M.; Ahmad, N.; Ahmed, Z.; Kashif Iqbal Khan, M.; Aslam Maan, A.; Mahr-Un-Nisa; Zeng, X.-A.; Ammar, A.-F. Pulsed Electric Field-Assisted Ethanolic Extraction of Date Palm Fruits: Bioactive Compounds, Antioxidant Activity and Physicochemical Properties. Processes 2019, 7, 585. https://doi.org/10.3390/pr7090585
Siddeeg A, Faisal Manzoor M, Haseeb Ahmad M, Ahmad N, Ahmed Z, Kashif Iqbal Khan M, Aslam Maan A, Mahr-Un-Nisa, Zeng X-A, Ammar A-F. Pulsed Electric Field-Assisted Ethanolic Extraction of Date Palm Fruits: Bioactive Compounds, Antioxidant Activity and Physicochemical Properties. Processes. 2019; 7(9):585. https://doi.org/10.3390/pr7090585
Chicago/Turabian StyleSiddeeg, Azhari, Muhammad Faisal Manzoor, Muhammad Haseeb Ahmad, Nazir Ahmad, Zahoor Ahmed, Muhammad Kashif Iqbal Khan, Abid Aslam Maan, Mahr-Un-Nisa, Xin-An Zeng, and Al-Farga Ammar. 2019. "Pulsed Electric Field-Assisted Ethanolic Extraction of Date Palm Fruits: Bioactive Compounds, Antioxidant Activity and Physicochemical Properties" Processes 7, no. 9: 585. https://doi.org/10.3390/pr7090585