Hybrid Filtration Process for Gas Desulfurization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
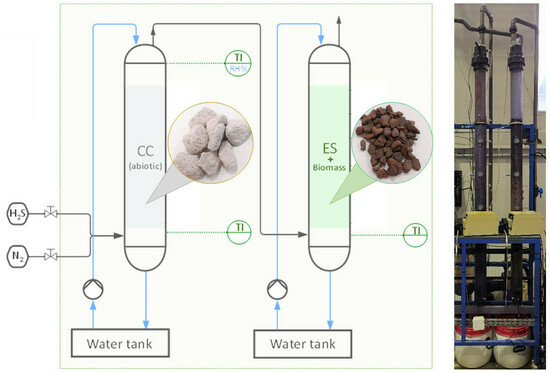
2.2. Experimental Setup
2.3. Operating Conditions
2.4. Gas Analysis
2.5. Water Analysis
2.6. Microbial Community Analysis
3. Results and Discussion
3.1. Performance of the Process
3.2. Resilience of the Process
3.3. Microbial Community Analysis
3.4. Pro and Cons of the Hybrid Filtration Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Portner, H.-O.; Roberts, D.; Trisos, C.; Simpson, N. Summary for Policymakers: Climate Change 2022: Impacts, Adaptation, and Vulnerability. In Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2022. [Google Scholar] [CrossRef]
- UN-Habitat. The Sustainable Development Goals Report 2022|UN-Habitat. Available online: https://unhabitat.org/the-sustainable-development-goals-report-2022 (accessed on 22 March 2023).
- Golmakani, A.; Ali Nabavi, S.; Wadi, B.; Manovic, V. Advances, Challenges, and Perspectives of Biogas Cleaning, Upgrading, and Utilisation. Fuel 2022, 317, 123085. [Google Scholar] [CrossRef]
- Abanades, S.; Abbaspour, H.; Ahmadi, A.; Das, B.; Ehyaei, M.A.; Esmaeilion, F.; El Haj Assad, M.; Hajilounezhad, T.; Jamali, D.H.; Hmida, A.; et al. A Critical Review of Biogas Production and Usage with Legislations Framework across the Globe. Int. J. Environ. Sci. Technol. 2022, 19, 3377–3400. [Google Scholar] [CrossRef] [PubMed]
- Almenglo, F.; González-Cortés, J.J.; Ramírez, M.; Cantero, D. Recent Advances in Biological Technologies for Anoxic Biogas Desulfurization. Chemosphere 2023, 321, 138084. [Google Scholar] [CrossRef] [PubMed]
- Mulu, E.; M’Arimi, M.M.; Ramkat, R.C. A Review of Recent Developments in Application of Low Cost Natural Materials in Purification and Upgrade of Biogas. Renew. Sustain. Energy Rev. 2021, 145, 111081. [Google Scholar] [CrossRef]
- Piechota, G.; Igliński, B. Biomethane in Poland—Current Status, Potential, Perspective and Development. Energies 2021, 14, 1517. [Google Scholar] [CrossRef]
- Chan, Y.H.; Lock, S.S.M.; Wong, M.K.; Yiin, C.L.; Loy, A.C.M.; Cheah, K.W.; Chai, S.Y.W.; Li, C.; How, B.S.; Chin, B.L.F.; et al. A State-of-the-Art Review on Capture and Separation of Hazardous Hydrogen Sulfide (H2S): Recent Advances, Challenges and Outlook. Environ. Pollut. 2022, 314, 120219. [Google Scholar] [CrossRef] [PubMed]
- Mutegoa, E.; Sahini, M.G. Approaches to Mitigation of Hydrogen Sulfide during Anaerobic Digestion Process—A Review. Heliyon 2023, 9, e19768. [Google Scholar] [CrossRef]
- Alguacil, F.J. Recent Advances in H2S Removal from Gas Streams. Appl. Sci. 2023, 13, 3217. [Google Scholar] [CrossRef]
- Pudi, A.; Rezaei, M.; Signorini, V.; Andersson, M.P.; Baschetti, M.G.; Mansouri, S.S. Hydrogen Sulfide Capture and Removal Technologies: A Comprehensive Review of Recent Developments and Emerging Trends. Sep. Purif. Technol. 2022, 298, 121448. [Google Scholar] [CrossRef]
- Soni Castro, P.; Martinez Zuniga, G.; Holmes, W.; Buchireddy, P.R.; Gang, D.D.; Revellame, E.; Zappi, M.; Hernandez, R. Review of the Adsorbents/Catalysts for the Removal of Sulfur Compounds from Natural Gas. Gas Sci. Eng. 2023, 115, 205004. [Google Scholar] [CrossRef]
- Das, J.; Ravishankar, H.; Lens, P.N.L. Biological Biogas Purification: Recent Developments, Challenges and Future Prospects. J. Environ. Manag. 2022, 304, 114198. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.; Colón, J.; Ramírez, M. Life Cycle Assessment of Biofiltration. In From Biofiltration to Promising Options in Gaseous Fluxes Biotreatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–108. ISBN 978-0-12-819064-7. [Google Scholar]
- Soreanu, G.; Béland, M.; Falletta, P.; Edmonson, K.; Seto, P. Investigation on the Use of Nitrified Wastewater for the Steady-State Operation of a Biotrickling Filter for the Removal of Hydrogen Sulphide in Biogas. J. Environ. Eng. Sci. 2008, 7, 543–552. [Google Scholar] [CrossRef]
- Cattaneo, C.R.; Muñoz, R.; Korshin, G.V.; Naddeo, V.; Belgiorno, V.; Zarra, T. Biological Desulfurization of Biogas: A Comprehensive Review on Sulfide Microbial Metabolism and Treatment Biotechnologies. Sci. Total Environ. 2023, 893, 164689. [Google Scholar] [CrossRef] [PubMed]
- González-Cortés, J.J.; Quijano, G.; Ramírez, M.; Cantero, D. Methane Concentration and Bacterial Communities’ Dynamics during the Anoxic Desulfurization of Landfill Biogas under Diverse Nitrate Sources and Hydraulic Residence Times. J. Environ. Chem. Eng. 2023, 11, 109285. [Google Scholar] [CrossRef]
- Rocher-Rivas, R.; González-Sánchez, A.; Ulloa-Mercado, G.; Muñoz, R.; Quijano, G. Biogas Desulfurization and Calorific Value Enhancement in Compact H2S/CO2 Absorption Units Coupled to a Photobioreactor. J. Environ. Chem. Eng. 2022, 10, 108336. [Google Scholar] [CrossRef]
- Cano, P.I.; Colón, J.; Ramírez, M.; Lafuente, J.; Gabriel, D.; Cantero, D. Life Cycle Assessment of Different Physical-Chemical and Biological Technologies for Biogas Desulfurization in Sewage Treatment Plants. J. Clean. Prod. 2018, 181, 663–674. [Google Scholar] [CrossRef]
- González-Cortés, J.J.; Almenglo, F.; Ramírez, M.; Cantero, D. Simultaneous Removal of Ammonium from Landfill Leachate and Hydrogen Sulfide from Biogas Using a Novel Two-Stage Oxic-Anoxic System. Sci. Total Environ. 2021, 750, 141664. [Google Scholar] [CrossRef] [PubMed]
- Poser, M.; Silva, L.R.D.E.; Peu, P.; Dumont, É.; Couvert, A. A Two-Stage Biogas Desulfurization Process Using Cellular Concrete Filtration and an Anoxic Biotrickling Filter. Energies 2022, 15, 3762. [Google Scholar] [CrossRef]
- Poser, M.; Duarte E Silva, L.R.; Peu, P.; Couvert, A.; Dumont, É. Cellular Concrete Waste: An Efficient New Way for H2S Removal. Sep. Purif. Technol. 2023, 309, 123014. [Google Scholar] [CrossRef]
- Lebrun, G.; Couvert, A.; Dumont, É. H2S Removal Using Cellular Concrete Waste as Filtering Material: Reactions Identification and Performance Assessment. J. Environ. Chem. Eng. 2019, 7, 102967. [Google Scholar] [CrossRef]
- Madigou, C.; Lê Cao, K.-A.; Bureau, C.; Mazéas, L.; Déjean, S.; Chapleur, O. Ecological Consequences of Abrupt Temperature Changes in Anaerobic Digesters. Chem. Eng. J. 2019, 361, 266–277. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, P.-Y. Conservative Fragments in Bacterial 16S rRNA Genes and Primer Design for 16S Ribosomal DNA Amplicons in Metagenomic Studies. PLoS ONE 2009, 4, e7401. [Google Scholar] [CrossRef] [PubMed]
- Escudié, F.; Auer, L.; Bernard, M.; Mariadassou, M.; Cauquil, L.; Vidal, K.; Maman, S.; Hernandez-Raquet, G.; Combes, S.; Pascal, G. FROGS: Find, Rapidly, OTUs with Galaxy Solution. Bioinformatics 2018, 34, 1287–1294. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Ben Jaber, M.; Couvert, A.; Amrane, A.; Le Cloirec, P.; Dumont, E. Hydrogen Sulfide Removal from a Biogas Mimic by Biofiltration under Anoxic Conditions. J. Environ. Chem. Eng. 2017, 5, 5617–5623. [Google Scholar] [CrossRef]
- Ben Jaber, M.; Couvert, A.; Amrane, A.; Rouxel, F.; Le Cloirec, P.; Dumont, E. Biofiltration of High Concentration of H2S in Waste Air under Extreme Acidic Conditions. New Biotechnol. 2016, 33, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Huynh Nhut, H.; Le Thi Thanh, V.; Tran Le, L. Removal of H2S in Biogas Using Biotrickling Filter: Recent Development. Process Saf. Environ. Prot. 2020, 144, 297–309. [Google Scholar] [CrossRef]
- Rodriguez, G.; Dorado, A.D.; Fortuny, M.; Gabriel, D.; Gamisans, X. Biotrickling Filters for Biogas Sweetening: Oxygen Transfer Improvement for a Reliable Operation. Process Saf. Environ. Prot. 2014, 92, 261–268. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P. Potential Flue Gas Desulfurization Gypsum Utilization in Agriculture: A Comprehensive Review. Renew. Sustain. Energy Rev. 2018, 82, 1969–1978. [Google Scholar] [CrossRef]







| Parameter | Definition | Nomenclature |
|---|---|---|
| Loading Rate LR (gH2S m−3 h−1) | (gH2S m−3): Inlet concentration | |
| Removal Capacity RC (gH2S m−3 h−1) | (gH2S m−3): Outlet concentration | |
| Empty Bed Residence Time EBRT (s) | (m3 s−1): Gas flow rate | |
| Removal Efficiency RE (%) | (m3): Packing bed volume |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germain, C.; Poser, M.; Peu, P.; Couvert, A.; Dumont, E. Hybrid Filtration Process for Gas Desulfurization. Processes 2023, 11, 3438. https://doi.org/10.3390/pr11123438
Germain C, Poser M, Peu P, Couvert A, Dumont E. Hybrid Filtration Process for Gas Desulfurization. Processes. 2023; 11(12):3438. https://doi.org/10.3390/pr11123438
Chicago/Turabian StyleGermain, Christelle, Morgane Poser, Pascal Peu, Annabelle Couvert, and Eric Dumont. 2023. "Hybrid Filtration Process for Gas Desulfurization" Processes 11, no. 12: 3438. https://doi.org/10.3390/pr11123438