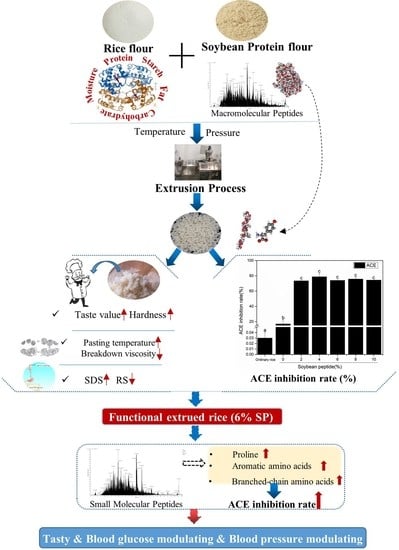
The Effect of Soybean Peptides on Improving Quality and the ACE Inhibitory Bioactivity of Extruded Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemicals
2.3. Experimental Methods
2.3.1. Extruded Process
2.3.2. Taste Value and Palatability
2.3.3. Pasting Characteristics
2.3.4. Starch Digestibility In Vitro
2.3.5. ACE-Inhibitory Activity Analysis
2.3.6. Identification and Analysis of Endogenous Polypeptides by LC-MS/MS
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effect of Soybean Protein Contents on the Taste and Palatability of Extruded Cooking Rice
3.2. Effect of Soybean Protein Contents on the Pasting Properties of Extruded Cooking Rice
3.3. Effect of Soybean Protein Contents on the Starch Digestibility Properties of Extruded Cooking Rice
3.4. Response Surface Methodology (RSM) Analysis
3.5. ACE Inhibitory Action In Vitro of Different Soybean Protein Contents
3.6. Peptides Molecular Change of Different Soybean Protein Content during Extrusion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, S.; Benchamas, G.; Huang, G. Whole processing and use of rice polishings. Innov. Food Sci. Emerg. 2020, 63, 102373. [Google Scholar] [CrossRef]
- Min, B.; Mcclung, A.M.; Chen, M. Phytochemicals and Antioxidant Capacities in Rice Brans of Different Color. J. Food Sci. 2011, 76, C117–C126. [Google Scholar] [CrossRef] [PubMed]
- Descalsota-Empleo, G.I.; Amparado, A.; Inabangan-Asilo, M.A.; Tesoro, F.; Stangoulis, J.; Reinke, R.; Mallikarjuna Swamy, B.P. Genetic mapping of QTL for agronomic traits and grain mineral elements in rice. Crop J. 2019, 7, 560–572. [Google Scholar] [CrossRef]
- Tiozon, R.J.N.; Fernie, A.R.; Sreenivasulu, N. Meeting human dietary vitamin requirements in the staple rice via strategies of biofortification and post-harvest fortification. Trends Food Sci. Technol. 2021, 109, 65–82. [Google Scholar] [CrossRef]
- Dalbhagat, C.G.; Mahato, D.K.; Mishra, H.N. Effect of extrusion processing on physicochemical, functional and nutritional characteristics of rice and rice-based products: A review. Trends Food Sci. Technol. 2019, 85, 226–240. [Google Scholar] [CrossRef]
- Sharif, M.K.; Rizvi, S.S.H.; Paraman, I. Characterization of supercritical fluid extrusion processed rice-soy crisps fortified with micronutrients and soy protein. LWT-Food Sci. Technol. 2014, 56, 414–420. [Google Scholar] [CrossRef]
- Ravindran, G.; Carr, A.; Hardacre, A. A comparative study of the effects of three galactomannans on the functionality of extruded pea-rice blends. Food Chem. 2011, 124, 1620–1626. [Google Scholar] [CrossRef]
- Brennan, C.; Brennan, M.; Derbyshire, E.; Tiwari, B.K. Effects of extrusion on the polyphenols, vitamins and antioxidant activity of foods. Trends Food Sci. Technol. 2011, 22, 570–575. [Google Scholar] [CrossRef]
- Chen, J.; Suetsuna, K.; Yamauchi, F. Isolation and characterization of immunostimulative peptides from soybean. J. Nutr. Biochem. 1995, 6, 310–313. [Google Scholar] [CrossRef]
- Tsuruki, T.; Kishi, K.; Takahashi, M.; Tanaka, M.; Matsukawa, T.; Yoshikawa, M. Soymetide, an immunostimulating peptide derived from soybean β-conglycinin, is an fMLP agonist. FEBS Lett. 2003, 540, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Yimit, D.; Hoxur, P.; Amat, N.; Uchikawa, K.; Yamaguchi, N. Effects of soybean peptide on immune function, brain function, and neurochemistry in healthy volunteers. Nutrition 2012, 28, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Tovar, A.R.; Murguia, F.; Cruz, C.; Hernandez-Pando, R.; Aguilar-Salinas, C.A.; Pedraza-Chaverri, J.; Correa-Rotter, R.; Torres, N. A soy protein diet alters hepatic lipid metabolism gene expression and reduces serum lipids and renal fibrogenic cytokines in rats with chronic nephrotic syndrome. J. Nutr. 2002, 132, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Mora, L.; Toldrá, F. Health relevance of antihypertensive peptides in foods. Curr. Opin. Food Sci. 2018, 19, 8–14. [Google Scholar] [CrossRef]
- Bejjani, S.; Wu, J. Transport of IRW, an Ovotransferrin-Derived Antihypertensive Peptide, in Human Intestinal Epithelial Caco-2 Cells. J. Agric. Food Chem. 2013, 61, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Mikiashvili, N.; Bonku, R.; Smith, I.N. Allergenicity, antioxidant activity and ACE-inhibitory activity of protease hydrolyzed peanut flour. Food Chem. 2021, 360, 129992. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, M.S.; Asiri, Y.I.; Uddin, J.; El-Seedi, H.R.; Musharraf, S.G. Repurposing of pharmaceutical drugs by high-throughput approach for antihypertensive activity as inhibitors of angiotensin-converting enzyme (ACE) using HPLC-ESI-MS/MS method. Arab. J. Chem. 2021, 14, 103279. [Google Scholar] [CrossRef]
- Sharifi, A.M.; Akbarloo, N.; Darabi, R.; Larijani, B. Study of correlation between elevation of blood pressure and tissue ACE activity during development of hypertension in 1K1C rats. Vasc. Pharmacol. 2004, 41, 15–20. [Google Scholar] [CrossRef]
- Chen, X.; Luo, J.; Fu, L.; Cai, D.; Lu, X.; Liang, Z.; Zhu, J.; Li, L. Structural, physicochemical, and digestibility properties of starch-soybean peptide complex subjected to heat moisture treatment. Food Chem. 2019, 297, 124957. [Google Scholar] [CrossRef]
- Lapointe, N.; Rouleau, J. Activation of vascular tissue angiotensin-converting enzyme (ACE) in heart failure: Effects of ACE inhibitors. J. Am. Coll. Cardiol. 2002, 39, 776–779. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Ye, R.; Wu, Y.; Xia, W. Comparison of analytical methods to assay inhibitors of angiotensin I-converting enzyme. Food Chem. 2013, 141, 3329–3334. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, J.; Zhang, X.; Li, Y.; Zhao, J.; Li, T.; Zhou, B.; Yang, H.; Qiao, L. Enrichment of soybean dietary fiber and protein fortified rice grain by dry flour extrusion cooking: The physicochemical, pasting, taste, palatability, cooking and starch digestibility properties. RSC Adv. 2018, 8, 22669–26682. [Google Scholar] [CrossRef] [PubMed]
- Champagne, E.T.; Bett, K.L.; Vinyard, B.T.; Mcclung, A.M.; Barton, F.E.; Moldenhauer, K.; Linscombe, S.; McKenzie, K. Correlation Between Cooked Rice Texture and Rapid Visco Analyser Measurements. Cereal Chem. 1999, 76, 764–771. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. S2), S33. [Google Scholar] [PubMed]
- Jimsheena, V.K.; Gowda, L.R. Colorimetric, High-Throughput Assay for Screening Angiotensin I-Converting Enzyme Inhibitors. Anal. Chem. 2009, 81, 9388–9394. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.N.; Pappin, D.J.; Creasy, D.M.; Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 1999, 20, 3551–3567. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M.; Matic, I.; Hilger, M.; Nagaraj, N.; Selbach, M.; Olsen, J.V. A practical guide to the MaxQuant computational platform for SILAC-based quantitative proteomics. Nat. Protoc. 2009, 4, 698–705. [Google Scholar] [CrossRef]
- Chen, H.; Chen, D.; He, L.; Wang, T.; Lu, H.; Yang, F.; Deng, F.; Chen, Y.; Tao, Y.; Li, M.; et al. Correlation of taste values with chemical compositions and Rapid Visco Analyser profiles of 36 indica rice (Oryza sativa L.) varieties. Food Chem. 2021, 349, 129176. [Google Scholar] [CrossRef]
- Lu, S.; Cik, T.; Lii, C.; Lai, P.; Chen, H. Effect of amylose content on structure, texture and α-amylase reactivity of cooked rice. Food Sci. Technol. 2013, 54, 224–228. [Google Scholar] [CrossRef]
- Okadome, H.N.F.R. Application of instrument-based multiple texture measurement of cooked milled-rice grains to rice quality evaluation. JARQ Jpn. Agric. Res. Q. 2005, 39, 261–268. [Google Scholar] [CrossRef]
- Tao, K.; Yu, W.; Prakash, S.; Gilbert, R.G. High-amylose rice: Starch molecular structural features controlling cooked rice texture and preference. Carbohydr. Polym. 2019, 219, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Park, I.M.; Ibáñez, A.M.; Zhong, F.; Shoemaker, C.F. Gelatinization and Pasting Properties of Waxy and Non-waxy Rice Starches. Starch-Stärke 2007, 59, 388–396. [Google Scholar] [CrossRef]
- Tao, K.; Li, C.; Yu, W.; Gilbert, R.G.; Li, E. How amylose molecular fine structure of rice starch affects functional properties. Carbohydr. Polym. 2019, 204, 24–31. [Google Scholar] [CrossRef]
- Bhat, F.M.; Riar, C.S. Effect of composition, granular morphology and crystalline structure on the pasting, textural, thermal and sensory characteristics of traditional rice cultivars. Food Chem. 2019, 280, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Horibata, T.F.U.H.; Nakamoto, M.; Fuwa, H.; Inouchi, N. Structural and physicochemical characteristics of endosperm starches of rice cultivars recently bred in Japan. J. Appl. Glycosci. JAG 2004, 51, 303–313. [Google Scholar] [CrossRef]
- Tao, K.; Yu, W.; Prakash, S.; Gilbert, R.G. Investigating cooked rice textural properties by instrumental measurements. Food Sci. Hum. Wellness 2020, 9, 130–135. [Google Scholar] [CrossRef]
- Chung, H.; Liu, Q.; Lee, L.; Wei, D. Relationship between the structure, physicochemical properties and in vitro digestibility of rice starches with different amylose contents. Food Hydrocoll. 2011, 25, 968–975. [Google Scholar] [CrossRef]
- Chen, X.; He, X.; Fu, X.; Zhang, B.; Huang, Q. Complexation of rice starch/flour and maize oil through heat moisture treatment: Structural, in vitro digestion and physicochemical properties. Int. J. Biol. Macromol. 2017, 98, 557–564. [Google Scholar] [CrossRef]
- Hasjim, J.; Lavau, G.C.; Gidley, M.J.; Gilbert, R.G. In Vivo and In Vitro Starch Digestion: Are Current In Vitro Techniques Adequate? Biomacromolecules 2010, 11, 3600–3608. [Google Scholar] [CrossRef]
- Piecyk, M.; Drużyńska, B.; Worobiej, E.; Wołosiak, R.; Ostrowska-Ligęza, E. Effect of hydrothermal treatment of runner bean (Phaseolus coccineus) seeds and starch isolation on starch digestibility. Food Res. Int. 2013, 50, 428–437. [Google Scholar] [CrossRef]
- Zou, W.; Schulz, B.L.; Tan, X.; Sissons, M.; Warren, F.J.; Gidley, M.J.; Gilbert, R.G. The role of thermostable proteinaceous α-amylase inhibitors in slowing starch digestion in pasta. Food Hydrocoll. 2019, 90, 241–247. [Google Scholar] [CrossRef]
- Tuaño, A.P.P.; Barcellano, E.C.G.; Rodriguez, M.S. Resistant starch levels and in vitro starch digestibility of selected cooked Philippine brown and milled rices varying in apparent amylose content and glycemic index. Food Chem. Mol. Sci. 2021, 2, 100010. [Google Scholar] [CrossRef] [PubMed]
- Aalim, H.; Wang, D.; Luo, Z. Black rice (Oryza sativa L.) processing: Evaluation of physicochemical properties, in vitro starch digestibility, and phenolic functions linked to type 2 diabetes. Food Res. Int. 2021, 141, 109898. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chang, R.; Lu, H.; Ma, R.; Qiu, L.; Tian, Y. Effect of amino acids composing rice protein on rice starch digestibility. Food Sci. Technol. 2021, 146, 111417. [Google Scholar] [CrossRef]
- Chi, C.; Li, X.; Zhang, Y.; Chen, L.; Li, L. Understanding the mechanism of starch digestion mitigation by rice protein and its enzymatic hydrolysates. Food Hydrocolloid. 2018, 84, 473–480. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Frias, J.; Peñas, E.; Zieliński, H.; Giménez-Bastida, J.A.; Wiczkowski, W.; Zielińska, D.; Martínez-Villaluenga, C. Simultaneous release of peptides and phenolics with antioxidant, ACE-inhibitory and anti-inflammatory activities from pinto bean (Phaseolus vulgaris L. var. pinto) proteins by subtilisins. J. Funct. Foods 2015, 18, 319–332. [Google Scholar] [CrossRef]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef]
- Firouzabadi, N.; Tajik, N.; Shafiei, M.; Ebrahimi, S.A.; Bakhshandeh, H. Interaction of A-240T and A2350G related genotypes of angiotensin-converting enzyme (ACE) is associated with decreased serum ACE activity and blood pressure in a healthy Iranian population. Eur. J. Pharmacol. 2011, 668, 241–247. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides 2012, 33, 178–185. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef]
- Xu, E.; Campanella, O.H.; Ye, X.; Jin, Z.; Liu, D.; Bemiller, J.N. Advances in conversion of natural biopolymers: A reactive extrusion (REX)–enzyme-combined strategy for starch/protein-based food processing. Trends Food Sci. Technol. 2020, 99, 167–180. [Google Scholar] [CrossRef]
- Martin, M.; Deussen, A. Effects of natural peptides from food proteins on angiotensin converting enzyme activity and hypertension. Crit. Rev. Food Sci. 2019, 59, 1264–1283. [Google Scholar] [CrossRef] [PubMed]
- Quirós, A.; Dávalos, A.; Lasunción, M.A.; Ramos, M.; Recio, I. Bioavailability of the antihypertensive peptide LHLPLP: Transepithelial flux of HLPLP. Int. Dairy J. 2008, 18, 279–286. [Google Scholar] [CrossRef]
- Chen, P.; Liu, L.; Zhang, X.; Massounga Bora, A.F.; Li, X.; Zhao, M.; Hao, X.; Wang, Y. Antioxidant activity of Cheddar cheese during its ripening time and after simulated gastrointestinal digestion as affected by probiotic bacteria. Int. J. Food Prop. 2019, 22, 218–229. [Google Scholar] [CrossRef]
- Stuknytė, M.; Cattaneo, S.; Masotti, F.; De Noni, I. Occurrence and fate of ACE-inhibitor peptides in cheeses and in their digestates following in vitro static gastrointestinal digestion. Food Chem. 2015, 168, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Young, J.F.; Løkke, M.M.; Lametsch, R.; Aluko, R.E.; Therkildsen, M. Revalorisation of bovine collagen as a potential precursor of angiotensin I-converting enzyme (ACE) inhibitory peptides based on in silico and in vitro protein digestions. J. Funct. Foods 2016, 24, 196–206. [Google Scholar] [CrossRef]
- Jung, W.; Mendis, E.; Je, J.; Park, P.; Son, B.W.; Kim, H.C.; Choi, Y.K.; Kim, S.-K. Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2006, 94, 26–32. [Google Scholar] [CrossRef]
- Aluko, R.E. Structure and function of plant protein-derived antihypertensive peptides. Curr. Opin. Food Sci. 2015, 4, 44–50. [Google Scholar] [CrossRef]



| SP (%) | Peak Visc. | Trough Visc. | Breakdown Visc. | Final Visc. | Setback Visc. | Pasting Temp. |
|---|---|---|---|---|---|---|
| 0 | 115.52 ± 9.9 a | 56.72 ± 9.77 a | 59.95 ± 5.62 a | 103.08 ± 2.56 a | −13.32 ± 7.53 d | 67.82 ± 7.60 a |
| 2 | 94.29 ± 9.28 b | 58.87 ± 9.97 a | 38.44 ± 2.77 c | 106.53 ± 2.29 a | 15.40 ± 4.93 b | 67.94 ± 7.63 a |
| 4 | 96.94 ± 4.98 b | 49.65 ± 9.08 a | 47.79 ± 3.43 b | 93.24 ± 2.81 b | −1.40 ± 8.45 c | 68.07 ± 6.94 a |
| 6 | 79.11 ± 4.84 c | 45.58 ± 9.74 ab | 27.07 ± 2.68 d | 84.93 ± 4.72 c | 6.66 ± 5.42 bc | 68.90 ± 7.08 ab |
| 8 | 56.12 ± 4.81 d | 30.74 ± 10.02 b | 19.13 ± 3.53 e | 69.90 ± 4.58 d | 15.86 ± 4.86 b | 72.45 ± 7.44 b |
| 10 | 40.06 ± 5.03 e | 29.55 ± 10.14 b | 7.93 ± 5.43 f | 72.79 ± 2.87 d | 35.63 ± 5.09 a | 74.25 ± 6.90 b |
| No. | Sequence Length | No. of Type | Relative Amount (%) | Pro | Leu | Ile | Val | Phe | Ala | Trp | Tyr |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1: SP | |||||||||||
| 1 | 8 | 5 | 3.226 | 9 | 2 | 2 | 6 | 1 | 0 | 2 | 0 |
| 2 | 9 | 18 | 11.613 | 18 | 14 | 11 | 6 | 4 | 4 | 2 | 6 |
| 3 | 10 | 33 | 21.290 | 55 | 23 | 19 | 16 | 8 | 12 | 2 | 7 |
| 4 | 11 | 25 | 16.129 | 43 | 15 | 16 | 11 | 15 | 14 | 3 | 2 |
| 5 | 12 | 17 | 10.968 | 34 | 13 | 14 | 11 | 14 | 4 | 1 | 1 |
| 6 | 13 | 14 | 9.032 | 25 | 10 | 14 | 11 | 11 | 4 | 1 | 3 |
| 7 | 14 | 8 | 5.161 | 20 | 4 | 9 | 3 | 6 | 0 | 0 | 1 |
| 8 | 15 | 8 | 5.161 | 18 | 8 | 6 | 7 | 8 | 2 | 1 | 2 |
| 9 | 16 | 5 | 3.226 | 19 | 4 | 1 | 0 | 3 | 0 | 2 | 0 |
| 10 | 17 | 4 | 2.581 | 6 | 7 | 4 | 1 | 3 | 1 | 0 | 1 |
| 11 | 18 | 3 | 1.935 | 16 | 0 | 1 | 1 | 4 | 1 | 2 | 1 |
| 12 | 20 | 4 | 2.581 | 23 | 0 | 1 | 1 | 6 | 1 | 2 | 0 |
| 13 | 21 | 5 | 3.226 | 23 | 2 | 3 | 3 | 7 | 2 | 2 | 1 |
| 14 | 22 | 4 | 2.581 | 13 | 5 | 3 | 2 | 7 | 1 | 0 | 1 |
| 15 | 23 | 1 | 0.645 | 6 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| 16 | 25 | 1 | 0.645 | 6 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Sample 2: extruded rice (6% SP) | |||||||||||
| 1 | 8 | 7 | 4.667 | 9 | 4 | 1 | 2 | 4 | 0 | 0 | 1 |
| 2 | 9 | 27 | 18.000 | 32 | 15 | 13 | 13 | 9 | 8 | 7 | 1 |
| 3 | 10 | 35 | 23.333 | 44 | 22 | 13 | 24 | 13 | 16 | 14 | 1 |
| 4 | 11 | 28 | 18.667 | 36 | 16 | 13 | 20 | 15 | 16 | 2 | 2 |
| 5 | 12 | 14 | 9.333 | 20 | 9 | 7 | 12 | 6 | 7 | 0 | 1 |
| 6 | 13 | 10 | 6.667 | 12 | 6 | 6 | 7 | 2 | 3 | 0 | 3 |
| 7 | 14 | 9 | 6.000 | 11 | 3 | 7 | 9 | 3 | 0 | 1 | 2 |
| 8 | 15 | 4 | 2.667 | 9 | 2 | 2 | 2 | 2 | 0 | 0 | 0 |
| 9 | 16 | 2 | 1.333 | 6 | 0 | 2 | 2 | 0 | 0 | 0 | 0 |
| 10 | 18 | 1 | 0.667 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 11 | 19 | 2 | 1.333 | 12 | 0 | 2 | 0 | 2 | 0 | 0 | 0 |
| 12 | 20 | 1 | 0.667 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 13 | 21 | 4 | 2.667 | 21 | 0 | 2 | 1 | 5 | 0 | 0 | 0 |
| 14 | 22 | 4 | 2.667 | 21 | 0 | 2 | 0 | 5 | 0 | 0 | 0 |
| 15 | 23 | 1 | 0.667 | 6 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| 16 | 25 | 1 | 0.667 | 6 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Sample 3: extruded rice (0% SP) | |||||||||||
| 1 | 8 | 4 | 21.053 | 4 | 2 | 0 | 1 | 2 | 0 | 1 | 0 |
| 2 | 9 | 5 | 26.316 | 8 | 0 | 3 | 1 | 0 | 0 | 0 | 1 |
| 3 | 10 | 7 | 36.842 | 8 | 6 | 3 | 1 | 0 | 5 | 4 | 0 |
| 4 | 11 | 2 | 10.526 | 4 | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| 5 | 12 | 1 | 5.263 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, S.; Zhao, J.; Zu, Y.; Zheng, J.; Wang, C.; Liu, X. The Effect of Soybean Peptides on Improving Quality and the ACE Inhibitory Bioactivity of Extruded Rice. Processes 2022, 10, 1921. https://doi.org/10.3390/pr10101921
Hou S, Zhao J, Zu Y, Zheng J, Wang C, Liu X. The Effect of Soybean Peptides on Improving Quality and the ACE Inhibitory Bioactivity of Extruded Rice. Processes. 2022; 10(10):1921. https://doi.org/10.3390/pr10101921
Chicago/Turabian StyleHou, Shuangdi, Jiafeng Zhao, Yuan Zu, Jiaxuan Zheng, Chunyu Wang, and Xia Liu. 2022. "The Effect of Soybean Peptides on Improving Quality and the ACE Inhibitory Bioactivity of Extruded Rice" Processes 10, no. 10: 1921. https://doi.org/10.3390/pr10101921