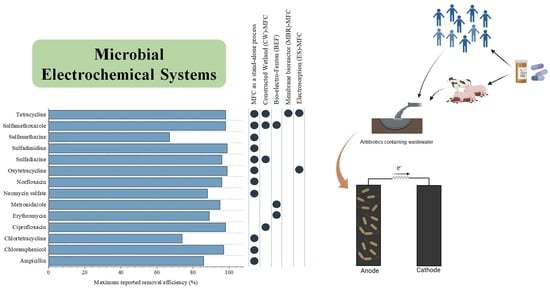
A Review of Stand-Alone and Hybrid Microbial Electrochemical Systems for Antibiotics Removal from Wastewater
Abstract
:1. Introduction
2. Stand-Alone Microbial Electrochemical Systems for Antibiotics Removal
2.1. Process Schemes and Antibiotics Removal Efficiencies
2.2. Process Parameters
3. Hybrid Microbial Electrochemical Systems for Antibiotics Removal
3.1. Constructed Wetland–Microbial Fuel Cell (CW-MFC)
3.2. Microbial Electrochemical Systems Coupled with Other Physicochemical Processes
4. Summary and Outlook
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [Green Version]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Zhang, X.; Shan, X.; Liu, Y. Contribution of antibiotics to the fate of antibiotic resistance genes in anaerobic treatment processes of swine wastewater: A review. Bioresour. Technol. 2020, 299, 122654. [Google Scholar] [CrossRef]
- Zhao, R.; Feng, J.; Liu, J.; Fu, W.; Li, X.; Li, B. Deciphering of microbial community and antibiotic resistance genes in activated sludge reactors under high selective pressure of different antibiotics. Water Res. 2019, 151, 388–402. [Google Scholar] [CrossRef]
- Qiao, M.; Ying, G.-G.; Singer, A.; Zhu, Y.-G. Review of antibiotic resistance in China and its environment. Environ. Int. 2018, 110, 160–172. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Yan, Z.; Zhang, Y.; Xu, W.; Kong, D.; Shan, Z.; Wang, N. Behavior of antibiotic resistance genes under extremely high-level antibiotic selection pressures in pharmaceutical wastewater treatment plants. Sci. Total Environ. 2018, 612, 119–128. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, S.; Chen, J.; Tyagi, R.; Li, J. Physical, chemical, and biological impact (hazard) of hospital wastewater on environment: Presence of pharmaceuticals, pathogens, and antibiotic-resistance genes. In Environmental and Health Impact of Hospital Wastewater, Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 79–102. [Google Scholar] [CrossRef]
- Abadi, A.T.B.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World Health Organization Report: Current Crisis of Antibiotic Resistance. BioNanoScience 2019, 9, 778–788. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Leng, L.; Wei, L.; Xiong, Q.; Xu, S.; Li, W.; Lv, S.; Lu, Q.; Wan, L.; Wen, Z.; Zhou, W. Use of microalgae based technology for the removal of antibiotics from wastewater: A review. Chemosphere 2020, 238, 124680. [Google Scholar] [CrossRef]
- Liu, X.-H.; Guo, X.; Liu, Y.; Lu, S.; Xi, B.; Zhang, J.; Wang, Z.; Bi, B. A review on removing antibiotics and antibiotic resistance genes from wastewater by constructed wetlands: Performance and microbial response. Environ. Pollut. 2019, 254, 112996. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Liang, L.; Duan, X.; Li, R.; Lu, X.; Yan, B.; Li, N.; Wang, S. Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review. J. Hazard. Mater. 2021, 408, 124461. [Google Scholar] [CrossRef]
- Oberoi, A.S.; Jia, Y.; Zhang, H.; Khanal, S.K.; Lu, H. Insights into the Fate and Removal of Antibiotics in Engineered Biological Treatment Systems: A Critical Review. Environ. Sci. Technol. 2019, 53, 7234–7264. [Google Scholar] [CrossRef]
- Shi, H.; NI, J.; Zheng, T.; Wang, X.; Wu, C.; Wang, Q. Remediation of wastewater contaminated by antibiotics. A review. Environ. Chem. Lett. 2020, 18, 345–360. [Google Scholar] [CrossRef]
- Wen, X.-J.; Niu, C.-G.; Zhang, L.; Liang, C.; Zeng, G.-M. A novel Ag2O/CeO2 heterojunction photocatalysts for photocatalytic degradation of enrofloxacin: Possible degradation pathways, mineralization activity and an in depth mechanism insight. Appl. Catal. B Environ. 2018, 221, 701–714. [Google Scholar] [CrossRef]
- Sun, F.; Liu, H.; Liang, B.; Song, R.; Yan, Q.; Wang, A. Reductive degradation of chloramphenicol using bioelectrochemical system (BES): A comparative study of abiotic cathode and biocathode. Bioresour. Technol. 2013, 143, 699–702. [Google Scholar] [CrossRef]
- Chung, T.H.; Dhar, B.R. A multi-perspective review on microbial electrochemical technologies for food waste valorization. Bioresour. Technol. 2021, 342, 125950. [Google Scholar] [CrossRef]
- Dhar, B.R.; Ryu, H.; Ren, H.; Domingo, J.W.S.; Chae, J.; Lee, H.-S. High Biofilm Conductivity Maintained Despite Anode Potential Changes in a Geobacter -Enriched Biofilm. ChemSusChem 2016, 9, 3485–3491. [Google Scholar] [CrossRef]
- Lee, H.-S.; Dhar, B.R.; Ranjan, D.B.; Rittmann, B.E.; Ryu, H.; Domingo, J.W.S.; Ren, H.; Chae, J. The Roles of Biofilm Conductivity and Donor Substrate Kinetics in a Mixed-Culture Biofilm Anode. Environ. Sci. Technol. 2016, 50, 12799–12807. [Google Scholar] [CrossRef]
- Zakaria, B.S.; Dhar, B.R. Progress towards catalyzing electro-methanogenesis in anaerobic digestion process: Fundamentals, process optimization, design and scale-up considerations. Bioresour. Technol. 2019, 289, 121738. [Google Scholar] [CrossRef]
- Chung, T.H.; Meshref, M.N.; Hai, F.I.; Al-Mamun, A.; Dhar, B.R. Microbial electrochemical systems for hydrogen peroxide synthesis: Critical review of process optimization, prospective environmental applications, and challenges. Bioresour. Technol. 2020, 313, 123727. [Google Scholar] [CrossRef]
- Barua, S.; Zakaria, B.S.; Chung, T.; Hai, F.; Haile, T.; Al-Mamun, A.; Dhar, B.R. Microbial electrolysis followed by chemical precipitation for effective nutrients recovery from digested sludge centrate in WWTPs. Chem. Eng. J. 2019, 361, 256–265. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Ni, B.-J.; Zhang, X. Microbial fuel cell for nutrient recovery and electricity generation from municipal wastewater under different ammonium concentrations. Bioresour. Technol. 2019, 292, 121992. [Google Scholar] [CrossRef]
- Al-Mamun, A.; Ahmad, W.; Baawain, M.S.; Khadem, M.; Dhar, B.R. A review of microbial desalination cell technology: Configurations, optimization and applications. J. Clean. Prod. 2018, 183, 458–480. [Google Scholar] [CrossRef]
- Rahman, S.; Jafary, T.; Al-Mamun, A.; Baawain, M.S.; Choudhury, M.R.; Alhaimali, H.; Siddiqi, S.A.; Dhar, B.R.; Sana, A.; Lam, S.S.; et al. Towards upscaling microbial desalination cell technology: A comprehensive review on current challenges and future prospects. J. Clean. Prod. 2021, 288, 125597. [Google Scholar] [CrossRef]
- Chung, T.H.; Dhar, B.R. Biosensors and Bioelectronics Paper-based platforms for microbial electrochemical cell-based biosensors: A review. Biosens. Bioelectron. 2021, 192, 113485. [Google Scholar] [CrossRef]
- Chung, T.H.; Meshref, M.N.; Dhar, B.R. A review and roadmap for developing microbial electrochemical cell-based biosensors for recalcitrant environmental contaminants, emphasis on aromatic compounds. Chem. Eng. J. 2021, 424, 130245. [Google Scholar] [CrossRef]
- Yan, W.; Guo, Y.; Xiao, Y.; Wang, S.; Ding, R.; Jiang, J.; Gang, H.; Wang, H.; Yang, J.; Zhao, F. The changes of bacterial communities and antibiotic resistance genes in microbial fuel cells during long-term oxytetracycline processing. Water Res. 2018, 142, 105–114. [Google Scholar] [CrossRef]
- Miran, W.; Jang, J.; Nawaz, M.; Shahzad, A.; Lee, D.S. Biodegradation of the sulfonamide antibiotic sulfamethoxazole by sulfamethoxazole acclimatized cultures in microbial fuel cells. Sci. Total Environ. 2018, 627, 1058–1065. [Google Scholar] [CrossRef]
- Wu, D.; Sun, F.; Chua, F.J.D.; Zhou, Y. Enhanced power generation in microbial fuel cell by an agonist of electroactive biofilm–Sulfamethoxazole. Chem. Eng. J. 2020, 384, 123238. [Google Scholar] [CrossRef]
- Liang, B.; Cheng, H.-Y.; Kong, D.-Y.; Gao, S.-H.; Sun, F.; Cui, D.; Kong, F.-Y.; Zhou, A.-J.; Liu, W.-Z.; Ren, N.-Q.; et al. Accelerated Reduction of Chlorinated Nitroaromatic Antibiotic Chloramphenicol by Biocathode. Environ. Sci. Technol. 2013, 47, 5353–5361. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Liu, Y.; Deng, L.; Chen, Z. Evaluation of a continuous flow microbial fuel cell for treating synthetic swine wastewater containing antibiotics. Sci. Total Environ. 2021, 756, 144133. [Google Scholar] [CrossRef]
- Wen, H.; Zhu, H.; Yan, B.; Xu, Y.; Shutes, B. Treatment of typical antibiotics in constructed wetlands integrated with microbial fuel cells: Roles of plant and circuit operation mode. Chemosphere 2020, 250, 126252. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Song, H.-L.; Lu, Y.; Yang, X.-L. Antibiotic resistance genes, bacterial communities, and functions in constructed wetland-microbial fuel cells: Responses to the co-stresses of antibiotics and zinc. Environ. Pollut. 2020, 265, 115084. [Google Scholar] [CrossRef]
- Song, H.-L.; Li, H.; Zhang, S.; Yang, Y.-L.; Zhang, L.-M.; Xu, H.; Yang, X.-L. Fate of sulfadiazine and its corresponding resistance genes in up-flow microbial fuel cell coupled constructed wetlands: Effects of circuit operation mode and hydraulic retention time. Chem. Eng. J. 2018, 350, 920–929. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Feng, Y.; Li, B.; Yu, M.; Xu, X.; Cai, L. Bio-Electron-Fenton (BEF) process driven by sediment microbial fuel cells (SMFCs) for antibiotics desorption and degradation. Biosens. Bioelectron. 2019, 136, 8–15. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Ge, R.; Yang, S.; Zhai, Y.; Hua, T.; Ondon, B.S.; Zhou, Q.; Li, F. Microbial electro-Fenton: A promising system for antibiotics resistance genes degradation and energy generation. Sci. Total Environ. 2020, 699, 134160. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, Z.; Huang, L.; Pan, Y.; Quan, X.; Puma, G.L. Intensified degradation and mineralization of antibiotic metronidazole in photo-assisted microbial fuel cells with Mo-W catalytic cathodes under anaerobic or aerobic conditions in the presence of Fe(III). Chem. Eng. J. 2019, 376, 119566. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, L.; Yang, F. Destruction of tetracycline hydrochloride antibiotics by FeOOH/TiO2 granular activated carbon as expanded cathode in low-cost MBR/MFC coupled system. J. Membr. Sci. 2017, 525, 202–209. [Google Scholar] [CrossRef]
- Yang, W.; Han, H.; Zhou, M.; Yang, J. Simultaneous electricity generation and tetracycline removal in continuous flow electrosorption driven by microbial fuel cells. RSC Adv. 2015, 5, 49513–49520. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Li, D. Cometabolic degradation of chloramphenicol via a meta-cleavage pathway in a microbial fuel cell and its microbial community. Bioresour. Technol. 2017, 229, 104–110. [Google Scholar] [CrossRef]
- Wang, L.; Liang, D.; Shi, Y. Profiling of co-metabolic degradation of tetracycline by the bio-cathode in microbial fuel cells. RSC Adv. 2021, 12, 509–516. [Google Scholar] [CrossRef]
- Chung, T.H.; Dhar, B.R. A Mini-Review on Applications of 3D Printing for Microbial Electrochemical Technologies. Front. Energy Res. 2021, 9. [Google Scholar] [CrossRef]
- Dhar, B.R.; Lee, H.-S. Membranes for bioelectrochemical systems: Challenges and research advances. Environ. Technol. 2013, 34, 1751–1764. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Huang, W.; Liu, Y.; Tang, M.; Zhang, L.; Sun, J. Biodegradation of oxytetracycline and electricity generation in microbial fuel cell with in situ dual graphene modified bioelectrode. Bioresour. Technol. 2018, 270, 482–488. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.H.; Guo, W.; Lee, D.; Nghiem, D.L.; Zhang, J.; Liang, S.; Varjani, S.; Wang, J. Performance of microbial fuel cell for treating swine wastewater containing sulfonamide antibiotics. Bioresour. Technol. 2020, 311, 123588. [Google Scholar] [CrossRef]
- Topcu, Ş.; Taşkan, E. Effect of the tetracycline antibiotics on performance and microbial community of microbial fuel cell. Bioprocess Biosyst. Eng. 2021, 44, 595–605. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, N.; Guo, W.; Wang, Y.; Huang, X.; Wu, P.; Dang, Z.; Zhang, X.; Xian, J. Simultaneous electricity production and antibiotics removal by microbial fuel cells. J. Environ. Manag. 2018, 217, 565–572. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, B.; Ge, R.; Song, T.-S.; Yu, J.; Xie, J. Degradation characterization and pathway analysis of chlortetracycline and oxytetracycline in a microbial fuel cell. RSC Adv. 2018, 8, 28613–28624. [Google Scholar] [CrossRef] [Green Version]
- Catal, T.; Yavaser, S.; Enisoglu-Atalay, V.; Bermek, H.; Ozilhan, S. Monitoring of neomycin sulfate antibiotic in microbial fuel cells. Bioresour. Technol. 2018, 268, 116–120. [Google Scholar] [CrossRef]
- Ondon, B.S.; Li, S.; Zhou, Q.; Li, F. Simultaneous removal and high tolerance of norfloxacin with electricity generation in microbial fuel cell and its antibiotic resistance genes quantification. Bioresour. Technol. 2020, 304, 122984. [Google Scholar] [CrossRef]
- Bagchi, S.; Behera, M. Evaluating the Effect of the Antibiotic Ampicillin on Performance of a Low-Cost Microbial Fuel Cell. J. Hazard. Toxic Radioact. Waste 2020, 24, 04020011. [Google Scholar] [CrossRef]
- Wu, W.; Lesnik, K.L.; Xu, S.; Wang, L.; Liu, H. Impact of tobramycin on the performance of microbial fuel cell. Microb. Cell Factories 2014, 13, 91. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Jiang, X.; Zhou, S.; Wu, M.; Pan, M.; Chen, H. Microbial community compositional analysis for membrane bioreactor treating antibiotics containing wastewater. Chem. Eng. J. 2017, 325, 300–309. [Google Scholar] [CrossRef]
- Xue, W.; Li, F.; Zhou, Q. Degradation mechanisms of sulfamethoxazole and its induction of bacterial community changes and antibiotic resistance genes in a microbial fuel cell. Bioresour. Technol. 2019, 289, 121632. [Google Scholar] [CrossRef]
- Wang, J.; He, M.-F.; Zhang, D.; Ren, Z.; Song, T.-S.; Xie, J. Simultaneous degradation of tetracycline by a microbial fuel cell and its toxicity evaluation by zebrafish. RSC Adv. 2017, 7, 44226–44233. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; You, L.; Zhang, J.; Yang, T.; Zhang, W.; Zhang, Z.; Liu, P.; Wu, S.; Zhao, F.; Ma, J. Biodegradation of sulfadiazine in microbial fuel cells: Reaction mechanism, biotoxicity removal and the correlation with reactor microbes. J. Hazard. Mater. 2018, 360, 402–411. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Jiang, L.; Wagner, T.; Sutton, N.B.; Ji, R.; Langenhoff, A.A. Improving removal of antibiotics in constructed wetland treatment systems based on key design and operational parameters: A review. J. Hazard. Mater. 2021, 407, 124386. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Fink, G.; Schlüsener, M.P.; Sidrach-Cardona, R.; Martín-Villacorta, J.; Ternes, T.; Becares, E. Removal of antibiotics from urban wastewater by constructed wetland optimization. Chemosphere 2011, 83, 713–719. [Google Scholar] [CrossRef]
- Kataki, S.; Chatterjee, S.; Vairale, M.G.; Dwivedi, S.K.; Gupta, D.K. Constructed wetland, an eco-technology for wastewater treatment: A review on types of wastewater treated and components of the technology (macrophyte, biolfilm and substrate). J. Environ. Manag. 2021, 283, 111986. [Google Scholar] [CrossRef]
- Rühmland, S.; Wick, A.; Ternes, T.; Barjenbruch, M. Fate of pharmaceuticals in a subsurface flow constructed wetland and two ponds. Ecol. Eng. 2015, 80, 125–139. [Google Scholar] [CrossRef]
- Gupta, S.; Srivastava, P.; Patil, S.A.; Yadav, A.K. A comprehensive review on emerging constructed wetland coupled microbial fuel cell technology: Potential applications and challenges. Bioresour. Technol. 2021, 320, 124376. [Google Scholar] [CrossRef]
- Guadarrama-Pérez, O.; Gutiérrez-Macías, T.; García-Sánchez, L.; Guadarrama-Pérez, V.H.; Estrada-Arriaga, E.B. Recent advances in constructed wetland-microbial fuel cells for simultaneous bioelectricity production and wastewater treatment: A review. Int. J. Energy Res. 2019, 43, 5106–5127. [Google Scholar] [CrossRef]
- Xu, H.; Song, H.-L.; Singh, R.P.; Yang, Y.-L.; Xu, J.-Y.; Yang, X.-L. Simultaneous reduction of antibiotics leakage and methane emission from constructed wetland by integrating microbial fuel cell. Bioresour. Technol. 2021, 320, 124285. [Google Scholar] [CrossRef]
- Liu, S.; Feng, X.; Li, X. Bioelectrochemical approach for control of methane emission from wetlands. Bioresour. Technol. 2017, 241, 812–820. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Yang, Y.-L.; Yang, X.-L.; Wu, Y.; Zhang, S.; Song, H.-L. Effects of graphite and Mn ore media on electro-active bacteria enrichment and fate of antibiotic and corresponding resistance gene in up flow microbial fuel cell constructed wetland. Water Res. 2019, 165, 114988. [Google Scholar] [CrossRef]
- Zhang, S.; Song, H.-L.; Yang, X.-L.; Yang, Y.-L.; Yang, K.-Y.; Wang, X.-Y. Fate of tetracycline and sulfamethoxazole and their corresponding resistance genes in microbial fuel cell coupled constructed wetlands. RSC Adv. 2016, 6, 95999–96005. [Google Scholar] [CrossRef]
- Nielsen, L.; Biggs, M.J.; Skinner, W.; Bandosz, T.J. The effects of activated carbon surface features on the reactive adsorption of carbamazepine and sulfamethoxazole. Carbon 2014, 80, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Dai, M.; Zhang, Y.; Wu, Y.; Sun, R.; Zong, W.; Kong, Q. Mechanism involved in the treatment of sulfamethoxazole in wastewater using a constructed wetland microbial fuel cell system. J. Environ. Chem. Eng. 2021, 9, 106193. [Google Scholar] [CrossRef]
- Wen, H.; Zhu, H.; Yan, B.; Bañuelos, G.; Shutes, B.; Wang, X.; Cao, S.; Cheng, R.; Tian, L. High removal efficiencies of antibiotics and low accumulation of antibiotic resistant genes obtained in microbial fuel cell-constructed wetlands intensified by sponge iron. Sci. Total Environ. 2021, 806, 150220. [Google Scholar] [CrossRef]
- Wen, H.; Zhu, H.; Xu, Y.; Yan, B.; Shutes, B.; Bañuelos, G.; Wang, X. Removal of sulfamethoxazole and tetracycline in constructed wetlands integrated with microbial fuel cells influenced by influent and operational conditions. Environ. Pollut. 2021, 272, 115988. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, X.; Xue, Y. Application of calcium peroxide in water and soil treatment: A review. J. Hazard. Mater. 2017, 337, 163–177. [Google Scholar] [CrossRef]
- Sun, X.; Zu, K.; Liang, H.; Sun, L.; Zhang, L.; Wang, C.; Sharma, V.K. Electrochemical synthesis of ferrate(VI) using sponge iron anode and oxidative transformations of antibiotic and pesticide. J. Hazard. Mater. 2018, 344, 1155–1164. [Google Scholar] [CrossRef]
- Ding, J.; Su, M.; Wu, C.; Lin, K. Transformation of triclosan to 2,8-dichlorodibenzo-p-dioxin by iron and manganese oxides under near dry conditions. Chemosphere 2015, 133, 41–46. [Google Scholar] [CrossRef]
- Xie, H.; Yang, Y.; Liu, J.; Kang, Y.; Zhang, J.; Hu, Z.; Liang, S. Enhanced triclosan and nutrient removal performance in vertical up-flow constructed wetlands with manganese oxides. Water Res. 2018, 143, 457–466. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of classic Fenton’s peroxidation as an advanced oxidation technique. J. Hazard. Mater. 2003, 98, 33–50. [Google Scholar] [CrossRef]
- Sopaj, F.; Oturan, N.; Pinson, J.; Podvorica, F.I.; Oturan, M.A. Effect of cathode material on electro-Fenton process efficiency for electrocatalytic mineralization of the antibiotic sulfamethazine. Chem. Eng. J. 2020, 384, 123249. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, G.-Q.; Yuan, G.-E.; Liu, H.-Y.; Liu, J.-D.; Yang, F.-L. Anti-fouling performance and mechanism of anthraquinone/polypyrrole composite modified membrane cathode in a novel MFC–aerobic MBR coupled system. RSC Adv. 2015, 5, 22533–22543. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Liu, J.; Yang, F.; Ren, N. PPy/AQS (9, 10-anthraquinone-2-sulfonic acid) and PPy/ARS (Alizarin Red’s) modified stainless steel mesh as cathode membrane in an integrated MBR/MFC system. Desalination 2014, 349, 94–101. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhao, J.; Li, L.; Wang, Y.; Dai, X.; Yu, F.; Ma, J. Interfacial interaction between micro/nanoplastics and typical PPCPs and nanoplastics removal via electrosorption from an aqueous solution. Water Res. 2020, 184, 116100. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Zhang, C.; Hu, Y.; Zhou, M. Electrosorption driven by microbial fuel cells without electric grid energy consumption for simultaneous phenol removal and wastewater treatment. Electrochem. Commun. 2013, 34, 121–124. [Google Scholar] [CrossRef]
- Chen, R.; Sheehan, T.; Ng, J.L.; Brucks, M.; Su, X. Capacitive deionization and electrosorption for heavy metal removal. Environ. Sci. Water Res. Technol. 2020, 6, 258–282. [Google Scholar] [CrossRef]
- Zhao, W.; Qu, J.; Zhou, Y.; Zhao, J.; Feng, Y.; Guo, C.; Lu, Y.; Zhao, Y.; Peijnenburg, W.J.; Zhang, Y.-N. Continuous flow electrosorption-microbial fuel cell system for efficient removal of oxytetracycline without external electrical supply. Bioresour. Technol. 2019, 290, 121751. [Google Scholar] [CrossRef]
- Dhar, B.R.; Sim, J.; Ryu, H.; Ren, H.; Domingo, J.W.S.; Chae, J.; Lee, H.-S. Microbial activity influences electrical conductivity of biofilm anode. Water Res. 2017, 127, 230–238. [Google Scholar] [CrossRef]
- Saidi, M.; Fourcade, F.; Bellakhal, N.; Amrane, A.; Geneste, F. Nickel foam as a new material for chlortetracycline electrochemical oxidation: Biodegradability improvement and biological treatment. J. Electroanal. Chem. 2020, 878, 114543. [Google Scholar] [CrossRef]
- Yu, M.; Sun, C.; Wang, L.; Zang, K.; Li, M.; Zhou, L.; Zheng, Y. Semi-coke activated persulfate promotes simultaneous degradation of sulfadiazine and tetracycline in a binary mixture. Chem. Eng. J. 2021, 416, 129122. [Google Scholar] [CrossRef]
- Pérez-Moya, M.; Mansilla, H.D.; Graells, M. A practical parametrical characterization of the Fenton and the photo-Fenton sulfamethazine treatment using semi-empirical modeling. J. Chem. Technol. Biotechnol. 2011, 86, 826–831. [Google Scholar] [CrossRef]
- Kümmerer, K. Pharmaceuticals in the Environment. Annu. Rev. Environ. Resour. 2010, 35, 57–75. [Google Scholar] [CrossRef] [Green Version]
- Pulicharla, R.; Brar, S.K.; Rouissi, T.; Auger, S.; Drogui, P.; Verma, M.; Surampalli, R.Y. Degradation of chlortetracycline in wastewater sludge by ultrasonication, Fenton oxidation, and ferro-sonication. Ultrason. Sonochem. 2017, 34, 332–342. [Google Scholar] [CrossRef]
- Qiao, X.; Liu, X.; Zhang, W.; Cai, Y.; Zhong, Z.; Li, Y.; Lü, J. Superior photo–Fenton activity towards chlortetracycline degradation over novel g–C3N4 nanosheets/schwertmannite nanocomposites with accelerated Fe(III)/Fe(II) cycling. Sep. Purif. Technol. 2021, 279, 119760. [Google Scholar] [CrossRef]
- Jain, A.; He, Z. “NEW” resource recovery from wastewater using bioelectrochemical systems: Moving forward with functions. Front. Environ. Sci. Eng. 2018, 12, 1. [Google Scholar] [CrossRef]
- Tan, W.; Chong, S.; Fang, H.-W.; Pan, K.-L.; Mohamad, M.; Lim, J.; Tiong, T.; Chan, Y.; Huang, C.-M.; Yang, T. Microbial Fuel Cell Technology—A Critical Review on Scale-Up Issues. Processes 2021, 9, 985. [Google Scholar] [CrossRef]
- Jafary, T.; Al-Mamun, A.; Alhimali, H.; Baawain, M.S.; Rahman, M.S.; Rahman, S.; Dhar, B.R.; Aghbashlo, M.; Tabatabaei, M. Enhanced power generation and desalination rate in a novel quadruple microbial desalination cell with a single desalination chamber. Renew. Sustain. Energy Rev. 2020, 127, 109855. [Google Scholar] [CrossRef]
- Dhar, B.R.; Ryu, H.; Domingo, J.W.S.; Lee, H.-S. Ohmic resistance affects microbial community and electrochemical kinetics in a multi-anode microbial electrochemical cell. J. Power Sources 2016, 331, 315–321. [Google Scholar] [CrossRef]
- Rossi, R.; Hur, A.Y.; Page, M.A.; Thomas, A.O.; Butkiewicz, J.J.; Jones, D.W.; Baek, G.; Saikaly, P.E.; Cropek, D.M.; Logan, B.E. Pilot scale microbial fuel cells using air cathodes for producing electricity while treating wastewater. Water Res. 2022, 215, 118208. [Google Scholar] [CrossRef]
- Sim, J.; Reid, R.; Hussain, A.; An, J.; Lee, H.-S. Hydrogen peroxide production in a pilot-scale microbial electrolysis cell. Biotechnol. Rep. 2018, 19, e00276. [Google Scholar] [CrossRef]
- Peñacoba-Antona, L.; Senán-Salinas, J.; Aguirre-Sierra, A.; Letón, P.; Salas, J.J.; García-Calvo, E.; Esteve-Núñez, A. Assessing METland® Design and Performance Through LCA: Techno-Environmental Study With Multifunctional Unit Perspective. Front. Microbiol. 2021, 12, 652173. [Google Scholar] [CrossRef]
- Prado, A.; Ramírez-Vargas, C.A.; Arias, C.A.; Esteve-Núñez, A. Novel bioelectrochemical strategies for domesticating the electron flow in constructed wetlands. Sci. Total Environ. 2020, 735, 139522. [Google Scholar] [CrossRef]
- Pun, Á.; Boltes, K.; Letón, P.; Esteve-Nuñez, A. Detoxification of wastewater containing pharmaceuticals using horizontal flow bioelectrochemical filter. Bioresour. Technol. Rep. 2019, 7, 100296. [Google Scholar] [CrossRef]






| Antibiotic (Initial Concentration) | Wastewater (WW) | System Design | Maximum Removal Efficiency | Remarks/Key Findings | Ref. |
|---|---|---|---|---|---|
| Sulfamethoxazole (100 µg/L), Sulfamethazine (100 µg/L), and Sulfadiazine (100 µg/L) | Synthetic swine wastewater (glucose) | Dual chamber MFC | 59.4% (Sulfamethoxazole) | The removal efficiencies of sulfadiazine (16.8–19.5%) and sulfamethazine (14.0–16.3%) were relatively lower than sulfamethoxazole. Moreover, the sequential anode–cathode operation was more efficient than the single (anode) continuous mode. | [31] |
| Oxytetracycline (12.5–16.5 mg/L) | Synthetic WW (glucose) | Dual chamber MFC | 95% | Dual graphene-modified bioelectrode was developed for more efficient oxytetracycline removal. | [44] |
| Sulfamethoxazole (100 µg/L), Sulfamethazine (200 µg/L), and Sulfadiazine (300 µg/L) | Synthetic swine wastewater | Dual chamber MFC | 99% (Sulfamethoxazole) | The removal efficiencies of sulfadiazine (13.39–66.91%) and sulfamethazine (32.84–67.21%) were relatively low. | [45] |
| Tobramycin (0.1–1.9 g/L) | Synthetic WW (acetate) | Single chamber MFC | - | Anode biofilm was resilient against antibiotics in μg/L level but was sensitive to g/L level (inhibition ratio increased with increasing concentrations). | [52] |
| Neomycin sulfate (20–100 mg/L) | Synthetic WW (glucose) | Single chamber MFC | 88% (20 mg/L) | Antibiotic degradation efficiency decreased with increasing initial concentration. | [49] |
| Sulfamethoxazole (0.04–0.79 mM) | Synthetic WW (lactate) | Dual chamber MFC | 83.3% | Acclimatized biofilm showed better removal efficiency than unacclimatized one (70.1% vs. 83.3%). | [28] |
| Sulfamethoxazole (10–30 mg/L) | Synthetic WW (acetate) | Single chamber MFC | 96.1% | >85.1% removal efficiency achieved within 60 h. | [54] |
| Norfloxacin (4–128 mg/L) | Synthetic WW (acetate) | Single chamber MFC | 65.5% (4 mg/L) | Removal efficiencies decreased with increasing initial concentration (e.g., 48.4% removal with 128 mg/L). | [50] |
| Chlortetracycline and Oxytetracycline (10–60 mg/L) | Synthetic WW (glucose) | Dual chamber MFC | 78% | Higher degradability was observed for oxytetracycline (78% vs. 74.2%). | [48] |
| Sulfamethoxazole (20 mg/L) | Synthetic WW (acetate) | Dual chamber MFC | 98% | Compared to an open circuit control, removal efficiency almost doubled (47% vs. 98%). Moreover, sulfamethoxazole increased power density by 18.09%. | [29] |
| Ampicillin (10–25 mg/L) | Synthetic WW (sucrose) | Dual chamber MFC | 86% (10 mg/L) | Here, 25 mg/L negatively affected MFC performance and decreased removal efficiency of ampicillin. | [51] |
| Tetracycline, Chlortetracycline, and Oxytetracycline (0.25–50 mg/L) | Synthetic WW (acetate) | Dual chamber MFC | - | Increasing concentrations negatively affected MFC performance; chlortetracycline showed the highest toxicity among the three antibiotics tested. | [46] |
| Aureomycin (3–45 μg/L), Sulfadimidine (2–30 μg/L), Roxithromycin (1.2–18 μg/L), and Norfloxacin (1.2–18 μg/L) | Synthetic animal wastewater (glucose) | Single chamber MFC | 99.9–100% | After adding antibiotics, MFC voltage dropped from 0.51 V to 0.41 V. Inhibition intensity was ordered as sulfadimidine > aureomycin > roxithromycin > norfloxacin | [47] |
| Oxytetracycline (0.5–10 mg/L) | Synthetic WW (acetate) | Dual chamber MFC | 99% (10 mg/L) | Long-term (10 months) acclimation improved antibiotics removal efficiency. | [27] |
| Tetracycline (2–30 mg/L) | Synthetic WW (acetate) | Dual chamber MFC | 90% (10 mg/L) | Biocathode operation provided effective degradation of tetracycline than aerobic treatment. | [41] |
| Chloramphenicol (100–200 mg/L) | Synthetic WW (acetate) | Dual chamber MFC | 96.53% | pH, temperature, and initial antibiotic concentration had a significant impact; pH of 7.12, the temperature of 31.48 °C, and the initial chloramphenicol concentration of 106.37 mg/L were optimum. | [40] |
| Chloramphenicol (32 mg/L) | Synthetic WW (glucose) | Dual chamber MEC (0.5 V) | 96% | Biocathode could provide higher removal efficiencies than abiotic cathode (96% vs. 73%). | [30] |
| Chloramphenicol (30 mg/L) | Synthetic WW (glucose) | Dual chamber MFC | - | Biocathode could provide 3.2 times higher antibiotics removal rate than abiotic cathode. | [15] |
| Tetracycline (10–50 mg/L) | Synthetic WW (glucose) | Dual chamber MFC | 79.1% | Compared to traditional anaerobic treatment, MFC was more effective in tetracycline removal. | [55] |
| Sulfadiazine (10 mg/L) | - | Dual chamber MFC | 100% | After acclimation of biofilms, >80% of sulfadiazine could be removed in 24 h. | [56] |
| Antibiotic (Initial Concentration) | Carbon Source | Key Findings | Ref. |
|---|---|---|---|
| Sulfadiazine (2 mg/L) and Ciprofloxacin (2 mg/L) | Glucose |
| [65] |
| Sulfadiazine (4 mg/L) | Glucose |
| [34] |
| Tetracycline and Sulfamethoxazole (400–1600 µg/L) | Glucose |
| [66] |
| Sulfadiazine (2 mg/L) and Ciprofloxacin (2 mg/L) | Glucose |
| [63] |
| Sulfadiazine (2 mg/L) and Ciprofloxacin (2 mg/L) | Glucose |
| [33] |
| Sulfamethoxazole (5–100 µg/L), Tetracycline (5–50 µg/L) | Glucose |
| [32] |
| Sulfamethoxazole (4 mg/L) | Glucose |
| [68] |
| Sulfamethoxazole (100 µg/L), Tetracycline (50 µg/L) | Glucose |
| [69] |
| Sulfamethoxazole (60 µg/L), Tetracycline (25 µg/L) | Glucose, Sucrose, Starch, Acetate |
| [70] |
| Physicochemical Processes | Antibiotic (Initial Concentration) | Wastewater (WW) | Maximum Removal Efficiency | System Description | Ref. |
|---|---|---|---|---|---|
| AOP | Sulfamethoxazole and Norfloxacin | Sludge | 97.4% (Sulfamethoxazole), 96.1% (Norfloxacin) | A sediment-type BEF process was developed using γ-FeOOH graphene polyacrylamide carbonized aerogel (γ-FeOOH GPCA) cathode. | [35] |
| AOP | Erythromycin (50 μg/L) | Synthetic WW (acetate) | 88.73% | A BEF system was developed using a carbon nanotube (CNT)/γ-FeOOH cathode. | [36] |
| AOP | Metronidazole (80 mg/L) | Synthetic WW (acetate) | 94.5% | A photo-assisted BEF process was developed with Mo-W catalytic cathodes to intensify cathodic reduction under anaerobic conditions and oxidation under aerobic conditions. | [37] |
| Membrane | Tetracycline hydrochloride | Synthetic WW (Glucose) | 90% | An integrated MBR/MFC was developed with membrane cathode expanded with TiO2/FeOOH doped GAC. | [38] |
| Electrosorption | Tetracycline (20–100 mg/L) | Synthetic WW (Glucose) | 93.16% | Electricity generated by MFCs was used to operate an electro-sorption (ES) system. | [39] |
| Electrosorption | Oxytetracycline (2–10 mg/L) | Synthetic WW (acetate) | 98.8% (2 mg/L) | An electro-sorption (ES) system followed by MFCs was proposed for a two-stage treatment scheme, where MFCs powered the ES system and treated the ES effluent. | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, B.S.; Dhar, B.R. A Review of Stand-Alone and Hybrid Microbial Electrochemical Systems for Antibiotics Removal from Wastewater. Processes 2022, 10, 714. https://doi.org/10.3390/pr10040714
Zakaria BS, Dhar BR. A Review of Stand-Alone and Hybrid Microbial Electrochemical Systems for Antibiotics Removal from Wastewater. Processes. 2022; 10(4):714. https://doi.org/10.3390/pr10040714
Chicago/Turabian StyleZakaria, Basem S., and Bipro Ranjan Dhar. 2022. "A Review of Stand-Alone and Hybrid Microbial Electrochemical Systems for Antibiotics Removal from Wastewater" Processes 10, no. 4: 714. https://doi.org/10.3390/pr10040714