Rheological and Mechanical Behavior of Silk Fibroin Reinforced Waterborne Polyurethane
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of SF Powder
2.3. Synthesis of WPU
2.4. Preparation of the WPU17-SF Dispersions and Films
2.5. Measurement of Rheological Behavior
2.6. Measurement of Particle Size and Zeta Potential
2.7. FTIR Spectroscopy
2.8. SEM and TEM Imaging
2.9. Measurement of Mechanical Properties
2.10. Statistical Analyses
3. Results and Discussion
3.1. Steady-Shear Flow Behavior of WPU Dispersions
3.2. Dynamic Oscillation Behavior of WPU Dispersions
3.3. Dynamic Oscillation Behavior of WPU17-SF Dispersions
3.4. Particle Size and Zeta Potential of the WPU17-SF Dispersions
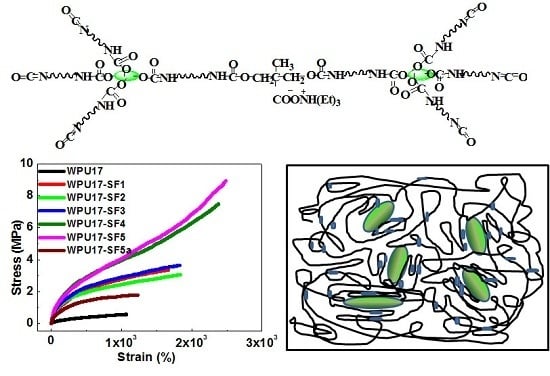
3.5. Structure and Properties of the WPU17-SF Films
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghosh, B.; Urban, M.W. Self-repairing oxetane-substituted chitosan polyurethane networks. Science 2009, 323, 1458–1460. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Miura, Y.; Macosko, C.W. Graphene/polyurethane nanocomposites for improved gas barrier and electrical conductivity. Chem. Mater. 2010, 22, 3441–3450. [Google Scholar] [CrossRef]
- Florian, P.; Jena, K.K.; Allauddin, S.; Narayan, R.; Raju, K.V.S.N. Preparation and characterization of waterborne hyperbranched polyurethane-urea and their hybrid coatings. Ind. Eng. Chem. Res. 2010, 49, 4517–4527. [Google Scholar] [CrossRef]
- Jung, Y.C.; Kim, H.H.; Kim, Y.A.; Kim, J.H.; Cho, J.W.; Endo, M.; Dresselhaus, M.S. Optically active multi-walled carbon nanotubes for transparent, conductive memory-shape polyurethane film. Macromolecules 2010, 43, 6106–6112. [Google Scholar] [CrossRef]
- Jabbari, E.; Khakpour, M. Morphology of and release behavior from porous polyurethane microspheres. Biomaterials 2000, 21, 2073–2079. [Google Scholar] [CrossRef]
- Bazyleva, A.B.; Hasan, M.A.; Fulem, M.; Becerra, M.; Shaw, J.M. Bitumen and heavy oil rheological properties: Reconciliation with viscosity measurements. J. Chem. Eng. Data 2009, 55, 1389–1397. [Google Scholar] [CrossRef]
- Hasan, M.A.; Fulem, M.; Bazyleva, A.; Shaw, J.M. Rheological properties of nanofiltered athabasca bitumen and maya crude oil. Energy Fuels 2009, 23, 5012–5021. [Google Scholar] [CrossRef]
- Hasan, M.A.; Shaw, J.M. Rheology of reconstituted crude oils: Artifacts and asphaltenes. Energy Fuels 2010, 24, 6417–6427. [Google Scholar] [CrossRef]
- Kim, A.; Hasan, M.; Nahm, S.; Cho, S. Evaluation of compressive mechanical properties of Al-foams using electrical conductivity. Compos. Struct. 2005, 71, 191–198. [Google Scholar] [CrossRef]
- Kim, A.K.; Hasan, M.A.; Choen, S.S.; Lee, H.J. The constitutive behavior of metallic foams using nanoindentation technique and Fe modeling. Key Eng. Mater. 2005. [Google Scholar] [CrossRef]
- Kim, A.K.; Hasan, M.A.; Lee, H.J.; Cho, S.S. Characterization of submicron mechanical properties of Al-alloy foam using nanoindentation technique. Mater. Sci. Forum 2005. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Tang, C.-M.; Tseng, H.-J. Biocompatibility of poly(ether)urethane-gold nanocomposites. J. Biomed. Mater. Res. Part A 2006, 79A, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.H.; Lodge, T.P. Nanocasting nanoporous inorganic and organic materials from polymeric bicontinuous microemulsion templates. Polym. J. 2012, 44, 131–146. [Google Scholar] [CrossRef]
- Jones, B.H.; Lodge, T.P. Nanoporous materials derived from polymeric bicontinuous microemulsions. Chem. Mater. 2010, 22, 1279–1281. [Google Scholar] [CrossRef]
- Liu, Y.D.; Choi, H.J. Recent progress in smart polymer composite particles in electric and magnetic fields. Polym. Int. 2013, 62, 147–151. [Google Scholar] [CrossRef]
- Dijkstra, D.J.; Langstein, G. Alternative feedstocks: A continuing trend in the polymer industry? Polym. Int. 2012, 61, 6–8. [Google Scholar] [CrossRef]
- Pei, A.; Malho, J.-M.; Ruokolainen, J.; Zhou, Q.; Berglund, L.A. Strong nanocomposite reinforcement effects in polyurethane elastomer with low volume fraction of cellulose nanocrystals. Macromolecules 2011, 44, 4422–4427. [Google Scholar] [CrossRef]
- Phua, S.L.; Yang, L.; Toh, C.L.; Huang, S.; Tsakadze, Z.; Lau, S.K.; Mai, Y.W.; Lu, X. Reinforcement of polyether polyurethane with dopamine-modified clay: The role of interfacial hydrogen bonding. ACS Appl. Mater. Interfaces 2012, 4, 4571–4578. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, E. Bioconjugation of hydrogels for tissue engineering. Curr. Opin. Biotechnol. 2011, 22, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Nurunnabi, M.; Morshed, M.; Paul, A.; Polini, A.; Kuila, T.; Al Hariri, M.; Lee, Y.-K.; Jaffa, A.A. Recent advances in application of biosensors in tissue engineering. BioMed Res. Int. 2014, 2014, 307519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q.; Shen, W.-D.; Xiang, R.-L.; Zhuge, L.-J.; Gao, W.-J.; Wang, W.-B. Formation of silk fibroin nanoparticles in water-miscible organic solvent and their characterization. J. Nanopart Res. 2007, 9, 885–900. [Google Scholar] [CrossRef]
- Drummy, L.F.; Farmer, B.L.; Naik, R.R. Correlation of the [small β]-sheet crystal size in silk fibers with the protein amino acid sequence. Soft Matter 2007, 3, 877–882. [Google Scholar] [CrossRef]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, B.; Guo, F.; Du, J.; Gu, P.; Lin, X.; Yang, W.; Zhang, H.; Lu, M.; Huang, Y.; et al. Injectable silk fibroin/polyurethane composite hydrogel for nucleus pulposus replacement. J. Mater. Sci. Mater. Med. 2012, 23, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yan, Y.; Xu, W. Physical characteristics and properties of waterborne polyurethane materials reinforced with silk fibroin powder. J. Polym. Sci. Polym. Phys. 2010, 48, 940–950. [Google Scholar] [CrossRef]
- Tao, Y.; Xu, W.; Yan, Y.; Wu, H. Structure and properties of composites compression-molded from silk fibroin powder and waterborne polyurethane. Polym. Adv. Technol. 2012, 23, 639–644. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Otaigbe, J.U.; Nanda, A.K.; Wicks, D.A. Rheological behavior of aqueous polyurethane dispersions: Effects of solid content, degree of neutralization, chain extension, and temperature. Macromolecules 2005, 38, 4014–4023. [Google Scholar] [CrossRef]
- Madbouly, S.A.; Otaigbe, J.U.; Nanda, A.K.; Wicks, D.A. Rheological behavior of poss/polyurethane–urea nanocomposite films prepared by homogeneous solution polymerization in aqueous dispersions. Macromolecules 2007, 40, 4982–4991. [Google Scholar] [CrossRef]
- Xu, W.L. Grinding Block for processing organic nanometer powder. In Method of Precessing Organic Nanopowder; Guo, W.Q., Li, W.B., Eds.; Google Patents: Wuhan, China, 2004. [Google Scholar]
- Fulem, M.; Becerra, M.; Hasan, M.A.; Zhao, B.; Shaw, J.M. Phase behaviour of maya crude oil based on calorimetry and rheometry. Fluid Phase Equilibria 2008, 272, 32–41. [Google Scholar] [CrossRef]
- Ostwald, W. Ueber die geschwindigkeitsfunktion der viskosität disperser systeme. I. Kolloid-Zeitschrift 1925, 36, 99–117. [Google Scholar] [CrossRef]
- Gómez-Dı́az, D.; Navaza, J.M. Rheology of aqueous solutions of food additives: Effect of concentration, temperature and blending. J. Food Eng. 2003, 56, 387–392. [Google Scholar] [CrossRef]
- Winter, H. Can the gel point of a cross-linking polymer be detected by the G′–G′′ crossover? Polym. Eng. Sci. 1987, 27, 1698–1702. [Google Scholar] [CrossRef]
- Chambon, F.; Winter, H.H. Stopping of crosslinking reaction in a PDMS polymer at the gel point. Polym. Bull. 1985, 13, 499–503. [Google Scholar] [CrossRef]
- Chambon, F.; Winter, H.H. Linear viscoelasticity at the gel point of a crosslinking pdms with imbalanced stoichiometry. J. Rheol. 1987, 31, 683–697. [Google Scholar] [CrossRef]
- Scanlan, J.C.; Winter, H.H. Composition dependence of the viscoelasticity of end-linked poly(dimethylsiloxane) at the gel point. Macromolecules 1991, 24, 47–54. [Google Scholar] [CrossRef]
- Kjøniksen, A.-L.; Nyström, B. Effects of polymer concentration and cross-linking density on rheology of chemically cross-linked poly(vinyl alcohol) near the gelation threshold. Macromolecules 1996, 29, 5215–5222. [Google Scholar] [CrossRef]
- Schultz, K.M.; Baldwin, A.D.; Kiick, K.L.; Furst, E.M. Gelation of covalently cross-linked PEG-heparin hydrogels. Macromolecules 2009, 42, 5310–5316. [Google Scholar] [CrossRef] [PubMed]
- Te Nijenhuis, K.; Winter, H.H. Mechanical properties at the gel point of a crystallizing poly(vinyl chloride) solution. Macromolecules 1989, 22, 411–414. [Google Scholar] [CrossRef]
- Muthukumar, M. Screening effect on viscoelasticity near the gel point. Macromolecules 1989, 22, 4656–4658. [Google Scholar] [CrossRef]
- Tho, I.; Kjoniksen, A.L.; Nystrom, B.; Roots, J. Characterization of association and gelation of pectin in methanol-water mixtures. Biomacromolecules 2003, 4, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Fan, W.; Marcellan, A.; Hourdet, D.; Creton, C. Large strain and fracture properties of poly(dimethylamide)/silica hybrid hydrogels. Macromolecules 2010, 43, 2554–2563. [Google Scholar] [CrossRef]
- Das, S.; Irin, F.; Ma, L.; Bhattacharia, S.K.; Hedden, R.C.; Green, M.J. Rheology and morphology of pristine graphen/polyacrylamide gels. ACS Appl. Mater. Interfaces 2013, 5, 8633–8640. [Google Scholar] [CrossRef] [PubMed]
- Braatz, J.A.; Yasuda, Y.; Olden, K.; Yamada, K.M.; Heifetz, A.H. Functional peptide-polyurethane conjugates with extended circulatory half-lives. Bioconjugate Chem. 1993, 4, 262–267. [Google Scholar] [CrossRef]
- Maji, P.K.; Guchhait, P.K.; Bhowmick, A.K. Effect of the microstructure of a hyperbranched polymer and nanoclay loading on the morphology and properties of novel polyurethane nanocomposites. ACS Appl. Mater. Interfaces 2009, 1, 289–300. [Google Scholar] [CrossRef]
- Cao, X.; Dong, H.; Li, C.M. New nanocomposite materials reinforced with flax cellulose nanocrystals in waterborne polyurethane. Biomacromolecules 2007, 8, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hehl, J.; Su, Y.; Mattheis, C.; Greiner, A.; Agarwal, S. Smart secondary polyurethane dispersions. Polym. Int. 2013, 62, 1750–1757. [Google Scholar] [CrossRef]
- Yao, X.; Qi, X.; He, Y.; Tan, D.; Chen, F.; Fu, Q. Simultaneous reinforcing and toughening of polyurethane via grafting on the surface of microfibrillated cellulose. ACS Appl. Mater. Interfaces 2014, 6, 2497–2507. [Google Scholar] [CrossRef] [PubMed]








| Samples | Weight of Raw Materials (g) | SF Content (wt %) | |||||
|---|---|---|---|---|---|---|---|
| SF | PPG | DMPA | TDI | TEA | H2O | ||
| WPU17 | 0 | 23.60 | 1.58 | 7.20 | 1.20 | 150 | 0 |
| WPU17-SF1 | 0.34 | 23.60 | 1.58 | 7.20 | 1.20 | 150 | 1 |
| WPU17-SF2 | 0.68 | 23.60 | 1.58 | 7.20 | 1.20 | 150 | 2 |
| WPU17-SF3 | 1.01 | 23.60 | 1.58 | 7.20 | 1.20 | 150 | 3 |
| WPU17-SF4 | 1.35 | 23.60 | 1.58 | 7.20 | 1.20 | 150 | 4 |
| WPU17-SF5 | 1.68 | 23.60 | 1.58 | 7.20 | 1.20 | 150 | 5 |
| Samples | n′ | df a | n′′ | df b | N* (mol/m3) | Dh (nm) | ξ (mV) |
|---|---|---|---|---|---|---|---|
| WPU17 | 0.27 | 2.35 | 0.26 | 2.36 | 0.205 | 56.2 | −32.0 ± 1.8 |
| WPU17-SF1 | 0.32 | 2.32 | 0.31 | 2.33 | 0.036 | 63.6 | −36.6 ± 4.4 |
| WPU17-SF2 | 0.27 | 2.35 | 0.27 | 2.35 | 0.081 | 93.8 | −38.4 ± 4.8 |
| WPU17-SF3 | 0.35 | 2.30 | 0.37 | 2.29 | 0.048 | 111.5 | −51.5 ± 6.4 |
| WPU17-SF4 | 0.39 | 2.27 | 0.35 | 2.30 | 0.081 | 133.9 | −53.7 ± 9.0 |
| WPU17-SF5 | 0.27 | 2.35 | 0.23 | 2.38 | 0.095 | 112.8 | −40.5 ± 5.7 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.; Hasan, A.; Deeb, G.; Hu, C.; Han, H. Rheological and Mechanical Behavior of Silk Fibroin Reinforced Waterborne Polyurethane. Polymers 2016, 8, 94. https://doi.org/10.3390/polym8030094
Tao Y, Hasan A, Deeb G, Hu C, Han H. Rheological and Mechanical Behavior of Silk Fibroin Reinforced Waterborne Polyurethane. Polymers. 2016; 8(3):94. https://doi.org/10.3390/polym8030094
Chicago/Turabian StyleTao, Yongzhen, Anwarul Hasan, George Deeb, Changkai Hu, and Huipeng Han. 2016. "Rheological and Mechanical Behavior of Silk Fibroin Reinforced Waterborne Polyurethane" Polymers 8, no. 3: 94. https://doi.org/10.3390/polym8030094