Wound Dressing Double-Crosslinked Quick Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Modified Nanocellulose
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Oxidation of Cellulose Nanocrystals
2.3. Functionalization of Carboxymethyl Cellulose Nanofibers
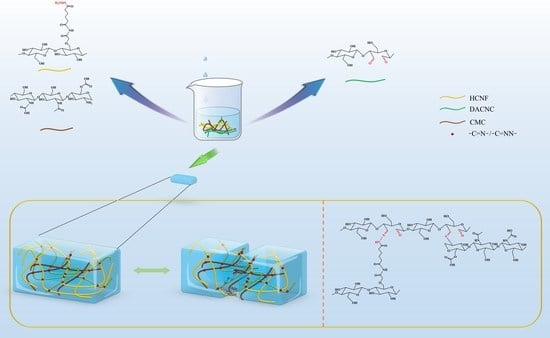
2.4. Preparation of DACNC/CMC Hydrogels and CMC/ DACNC/ HCNF Hydrogels
2.5. Verification of Modified Raw Materials and Hydrogels
2.6. Self-Healing Research
2.7. In Vitro Cytocompatibility Evaluation
3. Results and Discussion
3.1. Formation of CMC/DACNC/HCNF Hydrogels
3.2. Fluid Absorption Capacity
3.3. Mechanical Strength
3.4. Self-Healing Performance
3.5. Antibacterial Activity
3.6. In Vitro Cytotoxicity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dąbrowska, A.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.; Rossi, R. The relationship between skin function, barrier properties, and body-dependent factors. Skin Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monavarian, M.; Kader, S.; Moeinzadeh, S.; Jabbari, E. Regenerative Scar-Free Skin Wound Healing. Tissue Eng. Part B Rev. 2019, 25, 294–311. [Google Scholar] [CrossRef] [PubMed]
- Maksimova, N.; Lyundup, A.; Lyubimov, R.; Mel’Nichenko, G.; Nikolenko, V. Pathophysiological aspects of wound healing in normal and diabetic foot. Ann. Russ. Acad. Med. Sci. 2014, 69, 110–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portou, M.; Baker, D.; Abraham, D.; Tsui, J. The innate immune system, toll-like receptors and dermal wound healing: A review. Vasc. Pharmacol. 2015, 71, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Xu, F.-J. Rational design and latest advances of polysaccharide-based hydrogels for wound healing. Biomater. Sci. 2020, 8, 2084–2101. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Mohamad, N.; Low, W.-L.; Martin, C.; Amin, M.C.I.M. Microwaved bacterial cellulose-based hydrogel microparticles for the healing of partial thickness burn wounds. Drug Deliv. Transl. Res. 2017, 7, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.; Yang, K.; Fu, Y.V.; Xu, T.; Li, S.; Zhang, D.; Wang, L.; Lee, C. A Novel Double-Crosslinking-Double-Network Design for Injectable Hydrogels with Enhanced Tissue Adhesion and Antibacterial Capability for Wound Treatment. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Veld, R.C.O.; Walboomers, X.F.; Jansen, J.A.; Wagener, F.A. Design Considerations for Hydrogel Wound Dressings: Strategic and Molecular Advances. Tissue Eng. Part B Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef]
- Cao, J.; Wu, P.; Cheng, Q.; He, C.; Chen, Y.; Zhou, J. Ultrafast Fabrication of Self-Healing and Injectable Carboxymethyl Chitosan Hydrogel Dressing for Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 24095–24105. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X. Alginate hydrogel dressings for advanced wound management. Int. J. Biol. Macromol. 2020, 162, 1414–1428. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, X.; Zhang, X.; Cao, H.; Mao, L.; Yuan, M.; Liao, W. Novel fenugreek gum-cellulose composite hydrogel with wound healing synergism: Facile preparation, characterization and wound healing activity evaluation. Int. J. Biol. Macromol. 2020, 160, 1242–1251. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; Huang, Z.; Wang, X.; Chen, L.; Zhang, Y.; Zhang, L. On-Demand Dissolvable Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Cellulose Nanocrystal for Deep Partial Thickness Burn Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 41076–41088. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, B.; Zhang, M.; Wu, Z.; Wei, J.; Jiang, Y.; Sheng, N.; Liang, Q.; Zhang, D.; Chen, S. All-natural injectable hydrogel with self-healing and antibacterial properties for wound dressing. Cellulose 2020, 27, 2637–2650. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, G.; Peng, Y.; Chen, L.; Fu, S. Effects of cellulose nanofibrils on dialdehyde carboxymethyl cellulose based dual responsive self-healing hydrogel. Cellulose 2019, 26, 8813–8827. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Kazemi, M.S.; Mohammadi, Z.; Amini, M.; Yousefi, M.; Tarighi, P.; Eftekhari, S.; Tehrani, M.R. Thiolated chitosan-lauric acid as a new chitosan derivative: Synthesis, characterization and cytotoxicity. Int. J. Biol. Macromol. 2019, 136, 823–830. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef] [PubMed]
- Lamm, M.E.; Li, K.; Qian, J.; Wang, L.; Lavoine, N.; Newman, R.; Gardner, D.J.; Li, T.; Hu, L.; Ragauskas, A.J.; et al. Recent Advances in Functional Materials through Cellulose Nanofiber Templating. Adv. Mater. 2021, 33, e2005538. [Google Scholar] [CrossRef]
- Tang, J.; Javaid, M.U.; Pan, C.; Yu, G.; Berry, R.M.; Tam, K.C. Self-healing stimuli-responsive cellulose nanocrystal hydrogels. Carbohydr. Polym. 2020, 229, 115486. [Google Scholar] [CrossRef] [PubMed]
- Grishkewich, N.; Mohammed, N.; Tang, J.; Tam, K.C. Recent advances in the application of cellulose nanocrystals. Curr. Opin. Colloid Interface Sci. 2017, 29, 32–45. [Google Scholar] [CrossRef]
- Mali, P.; Sherje, A.P. Cellulose nanocrystals: Fundamentals and biomedical applications. Carbohydr. Polym. 2022, 275, 118668. [Google Scholar] [CrossRef]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef] [PubMed]
- El Miri, N.; Heggset, E.B.; Wallsten, S.; Svedberg, A.; Syverud, K.; Norgren, M. A comprehensive investigation on modified cellulose nanocrystals and their films properties. Int. J. Biol. Macromol. 2022, 219, 998–1008. [Google Scholar] [CrossRef]
- Hudson, S.P.; Langer, R.; Fink, G.R.; Kohane, D.S. Injectable in situ cross-linking hydrogels for local antifungal therapy. Biomaterials 2010, 31, 1444–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, D.; Singh, A.K.; Dravid, A.; Bellare, J.R. Multiscale Porosity in Compressible Cryogenically 3D Printed Gels for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 20437–20452. [Google Scholar] [CrossRef] [PubMed]
- El Fawal, G.F.; Abu-Serie, M.M.; Hassan, M.A.; Elnouby, M.S. Hydroxyethyl cellulose hydrogel for wound dressing: Fabrication, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018, 111, 649–659. [Google Scholar] [CrossRef]
- Sultana, T.; Hossain, M.; Rahaman, S.; Kim, Y.S.; Gwon, J.-G.; Lee, B.-T. Multi-functional nanocellulose-chitosan dressing loaded with antibacterial lawsone for rapid hemostasis and cutaneous wound healing. Carbohydr. Polym. 2021, 272, 118482. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Patwa, R.; Zandraa, O.; Saha, N.; Saha, P. Preparation and characterization of injectable self-antibacterial gelatin/carrageenan/bacterial cellulose hydrogel scaffolds for wound healing application. J. Drug Deliv. Sci. Technol. 2021, 63, 102415. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, A.; Chen, Y.; Wu, C. Wound Dressing Double-Crosslinked Quick Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Modified Nanocellulose. Polymers 2023, 15, 3389. https://doi.org/10.3390/polym15163389
Huang A, Chen Y, Wu C. Wound Dressing Double-Crosslinked Quick Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Modified Nanocellulose. Polymers. 2023; 15(16):3389. https://doi.org/10.3390/polym15163389
Chicago/Turabian StyleHuang, Anshan, Yehong Chen, and Chaojun Wu. 2023. "Wound Dressing Double-Crosslinked Quick Self-Healing Hydrogel Based on Carboxymethyl Chitosan and Modified Nanocellulose" Polymers 15, no. 16: 3389. https://doi.org/10.3390/polym15163389