Biopolymeric Fibrous Aerogels: The Sustainable Alternative for Water Remediation
Abstract
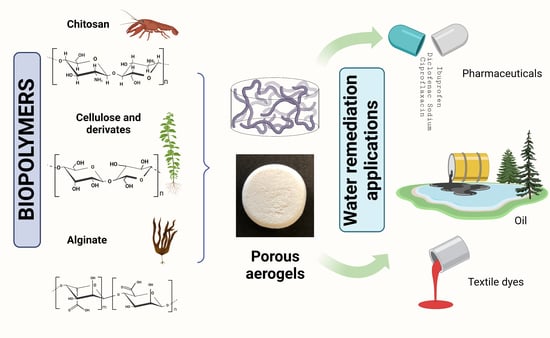
:1. Introduction
2. Biopolymers Employed
2.1. Cellulose
2.2. Chitosan
2.3. Alginate
2.4. Others
3. Preparation Methods
3.1. Supercritical Carbon Dioxide Drying
3.2. Freeze-Drying
3.3. Ambient Drying
4. Aerogel Application in Water Remediation
4.1. Oil Recovery
| Raw Material | Hydrophobic Modification | Porosity (%) | WCA (°) | Pollutants Treated | Absorption Capacity (g/g) | Recovery Methods | Reusability (Cycles Proved) | Regeneration % Last Cycle (Substance) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Bacterial cellulose hydrogel | TMCS | 99.6 | 137.1–146.5 | Inorganic oils (Gasoline, diesel, paraffin liquid) Organic oils (plant) Organic solvents (n-hexane, Acetone, Toluene, Chlorobenzene, Dichloromethane, Chloroform) | 80–175 | Mechanical compression | 10 | Stable (Diesel) | [82] |
| Bamboo | MTMS | 99.4 | 140 | Inorganic oils (Gasoline, diesel, pump, mineral, motor) Organic oils (corn) Organic solvents (Acetone, Ethanol, Toluene, Hexane, Chloroform, Dimethyl sulfoxide) | 45–99 | Mechanical compression | 35 | 84 (gasoline) | [83] |
| Bamboo chopsticks | - | - | 145 | Inorganic oils (pump, crude, diesel, gasoline) Organic oils (colza) Organic solvents (Hexane, Octane, Decane, Hexadecane, Chloroform, Toluene) | 30–130 | Distillation, combustion, mechanical compression | 6 | Stable (n-Hexane distillation) 62.1 (Hexadecane combustion) 61.4 (gasoline compression) | [80] |
| Bamboo fungus | MTMS | 93.3 | 152.1 | Oils (Corn, Silicone, Gasoline, vaccuum pump) Organic solvents (Acetone, Toluene, Cyclohexane, Methylene chloride, Chloroform, Carbon tetrachloride) | 20–42 | Solvent extraction | 10 | Stable (Chloroform, Methylene chloride, silicone oil, Cyclohexane) | [91] |
| Bamboo pulp | - | - | 135.9 | Inorganic oils (pump, liquid paraffin) Organic oils (Sesame) Organic solvents (Chloroform, Heptane, Dimethylacetamide, Paraxylene, sym-Dichloroethane, Petroleum ether, Hexane, Ethanol) | 50–150 | Extraction, distillation | 5 | Stable (pump oil, heptane) | [79] |
| Cellulose from waste board (fluting, box, milkcarton) | MTMS/HDTMS | 99.48–99.79 | 107.2–159 | Inorganic oils (Marine diesel, kerosene, gasoline, motor) Organic oils (castor, linseed) Organic solvents (Chloroform, Hexane, Dimethyl sulfoxide, Toluene, Acetone, Ethanol) | 65–180 | Mechanical compression | 30 | 70–80 (marine diesel oil) | [78] |
| Cellulose from waste newspaper | - | - | 132 | Inorganic oils (Gasoline, pump) Organic oils (olive, cooking) Organic solvents (Ethyl acetate, Methylbenzene, Benzene, Acetone, Chloroform, Methanol, Ethanol). | 30–52 | Distillation (gasoline), Combustion (ethanol), Mechanical compression (pump oil) | 5 | Stable (gasoline, ethanol) 68.5 (pump oil) | [84] |
| Chitosan | - | 97.67–97.98 | - | Inorganic oils (pump, crude, diesel, gasoline) Organic oils (oleic acid) Organic solvents (Ethanol, Acetone, Ethyl acetate, Toluene, Tetraethoxysilane, Ethyleneglycol, Carbon tetrachlorice) | 13.11–32.39 | Solvent extraction | 10 | 65.97 (diesel) | [85] |
| Chitosan | MTMS | 96.8 | 152.3 | Inorganic oils (vaccuum pump) Organic oils (corn) Organic solvents (Hexane, petroleum ether, Cyclohexane, Toluene, Ethyl acetate, Dichloromethane, Chloroform) | 31–63 | Mechanical compression | 10 | Stable (Chloroform, Dichloromethane, vacuum oil, Toluene, Hexane) | [89] |
| Cotton/Cotton-Cellulose | MTMS | 99.4–99.7 | 130.3–142.8 | Inorganic oils (machine) | 40–100 | Distillation | 5 | 70 (ethanol) | [9] |
| Cotton-SDS | - | - | - | Inorganic oils (vacuum pump) Organic solvents (Cyclohexane, Ethyl acetate) | 145–207 | - | - | - | [87] |
| Lignin-PVA | MTMS | 87.54 | 143 | Inorganic oils (kerosene) Organic oils (soybean) Organic solvents (methylbenzene, petroleum ether, n-heptane, trichloromethane) | 300–1200 | Solvent extraction | 10 | Stable (toluene) | [90] |
| Lignin-SA-GO | MTMS | - | 144–161 | Inorganic oils (pump) Organic solvents (Isooctane, Chloroform, Dichloromethane, n-hexane, Xylene) | 5.296–13.214 | Mechanical compression | 10 | 39.08 (pump oil) 76.1 (Chloroform) | [24] |
| Pineapple leaves | MTMS | 96.98–98.85 | 138.6–146.1 | Inorganic oils (motor) | 26.6–37.9 | - | - | - | [88] |
| Sisal leaves | Cu | 99.25–99.76 | 150.3 | Inorganic oils (pump, gasoline) Organic oils (Soybean) Organic solvents (Trichloromethane, Dichloromethane, Dimethyl sulfoxide, Methylbenzene, Cyclohexane, n-hexane) | 68–165 | Distillation | 10 | 40–85 (trichloromethane) | [93] |
| Sugarcane bagasse | SCCP | 92.8–99.2 | 140.1 | Inorganic oils (crude) | 17.41–23.08 | Solvent extraction | 10 | 70 (crude oil) | [92] |
| Winter melon | - | >97.5 | 135 | Inorganic oils (pump, crude, diesel, gasoline) Organic oils (corn, sesame, sunflower) Organic solvents (Toluene, Cyclohexane, Hexane, Ethylene glycol, Butyl stereate, Dimethylformamide, Chloroform, Acetone, 2-propanol, Ethanol, Methanol) | 15–50 | Distillation | 5 | Stable (ethanol, acetone, gasoline) 48 (crude oil) | [81] |
4.2. Dye Uptake
| Raw Material | Second Compound | Porosity (%) | WCA (°) | Dyes | Absorption Capacity (mg/g) | Reusability (Cycles Proved) | Ref. |
|---|---|---|---|---|---|---|---|
| Cellulose | Chitosan | 98.8 | - | Methylene blue | 780 | 6 | [105] |
| Biomass (pear fruit) | Graphene | - | - | Crystal violet (CV), methylene blue (MB), rhodamine B (RhB) | 57–74 | 5 | [106] |
| Alginate/gelatin | Graphene oxide | 93.5 | - | Methylene blue (MB) and Congo red (CR), | 196.8–322.6 | 5 | [107] |
| Cellulose | Polyaniline | - | - | Acid Red G, methylene blue | 600.7–1369.6 | 3 | [108] |
| Cellulose | Zinc Oxide-x | 90.64–93.84 | 109.68 | Methyl orange | 9000 | 7 | [109] |
| Cellulose | Polyethylene glycol diglycidyl ether (PEGDE) | 93.02 | - | Methyl blue | 917.43 | 5 | [110] |
| Pectin | Graphene oxide | - | - | Methyl orange, rhodamine B | 419–719 | 5 | [53] |
| Cellulose (extracted from waste reed) and chitosan. | - | - | - | Congo red | 380.23–260.41 | 6 | [111] |
| Cellulose (coconut) | Polyvinyl alcohol/xanthan gum | 96.30–98.32 | - | Methylene blue | 625 | 3 | [112] |
| (Ligno)cellulose/cellulose (Wheat Straw) | TEMPO | 99.61 | - | Methylene blue | 5–20 | 1 | [113] |
| Chitosan | Zeolite | - | - | Indigo carmine, methylene blue | 108–221 | 3 | [98] |
| Cellulose | Polidopamine | 143–150 | Methylene blue, rhodamine B | 11.5–16.5 | 1 | [104] |
4.3. Pharmaceuticals Recovery
5. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R. Global Threats to Human Water Security and River Biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B. The Challenge of Micropollutants in Aquatic Systems. Science (1979) 2006, 313, 1072–1077. [Google Scholar] [CrossRef]
- Yu, C.; Han, X. Adsorbent Material Used in Water Treatment-a Review. In Proceedings of the 2015 2nd International Workshop on Materials Engineering and Computer Sciences, Jinan, China, 10–11 October 2015; Atlantis Press: Paris, France, 2015; pp. 286–289. [Google Scholar]
- Maleki, H. Recent Advances in Aerogels for Environmental Remediation Applications: A Review. Chem. Eng. J. 2016, 300, 98–118. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Fu, Z.; Corker, J.; Papathanasiou, T.; Wang, Y.; Zhou, Y.; Madyan, O.A.; Liao, F.; Fan, M. Critical Review on the Thermal Conductivity Modelling of Silica Aerogel Composites. J. Build. Eng. 2022, 57, 104814. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Feng, J.; Le, N.T.; Le, A.T.T.; Hoang, N.; Tan, V.B.C.; Duong, H.M. Cellulose Aerogel from Paper Waste for Crude Oil Spill Cleaning. Ind. Eng. Chem. Res. 2013, 52, 18386–18391. [Google Scholar] [CrossRef]
- Feng, J.; Nguyen, S.T.; Fan, Z.; Duong, H.M. Advanced Fabrication and Oil Absorption Properties of Super-Hydrophobic Recycled Cellulose Aerogels. Chem. Eng. J. 2015, 270, 168–175. [Google Scholar] [CrossRef]
- Cheng, H.; Gu, B.; Pennefather, M.P.; Nguyen, T.X.; Phan-Thien, N.; Duong, H.M. Cotton Aerogels and Cotton-Cellulose Aerogels from Environmental Waste for Oil Spillage Cleanup. Mater. Des. 2017, 130, 452–458. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An Overview on Silica Aerogels Synthesis and Different Mechanical Reinforcing Strategies. J. Non. Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Sun, X.; Bao, M.; Li, Y. Construction of a Hydrophobic Magnetic Aerogel Based on Chitosan for Oil/Water Separation Applications. Int. J. Biol. Macromol. 2020, 165, 1869–1880. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, L.; Zhang, L.; Yan, J.; Lu, H.; Fan, W.; Liu, T. Immobilization of NiS Nanoparticles on N-Doped Carbon Fiber Aerogels as Advanced Electrode Materials for Supercapacitors. Nano Res. 2016, 9, 2747–2759. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Muhetaer, M.; Wang, K. Silver Nanoparticles Cross-Linked Polyimide Aerogels with Improved High Temperature Microstructure Stabilities and High Mechanical Performances. Microporous Mesoporous Mater. 2020, 297, 110035. [Google Scholar] [CrossRef]
- Budtova, T. Cellulose II Aerogels: A Review. Cellulose 2019, 26, 81–121. [Google Scholar] [CrossRef]
- Kong, Y.; Shen, X.; Cui, S.; Fan, M. Facile Synthesis of an Amine Hybrid Aerogel with High Adsorption Efficiency and Regenerability for Air Capture via a Solvothermal-Assisted Sol–Gel Process and Supercritical Drying. Green Chem. 2015, 17, 3436–3445. [Google Scholar] [CrossRef]
- Li, J.; Xia, C.; Cheng, R.; Lan, J.; Chen, F.; Li, X.; Li, S.; Chen, J.; Zeng, T.; Hou, H. Passivation of Multiple Heavy Metals in Lead–Zinc Tailings Facilitated by Straw Biochar-Loaded N-Doped Carbon Aerogel Nanoparticles: Mechanisms and Microbial Community Evolution. Sci. Total Environ. 2022, 803, 149866. [Google Scholar] [CrossRef]
- Li, Z.; Shao, L.; Ruan, Z.; Hu, W.; Lu, L.; Chen, Y. Converting Untreated Waste Office Paper and Chitosan into Aerogel Adsorbent for the Removal of Heavy Metal Ions. Carbohydr. Polym. 2018, 193, 221–227. [Google Scholar] [CrossRef]
- Shang, Y.; Gao, R.; Wang, Y.; Dong, X.; Li, X.; Tang, J.; Wang, X.; Liu, J.; Xie, Y.; Li, J. Scalable Fabrication of Efficient and Recycling Wood Residue-Derived Sponge for Crude Oil Adsorption. J. Hazard. Mater. 2023, 441, 129979. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a Sorbent for Contaminant Management in Soil and Water: A Review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Luo, X.; Wang, C.; Huang, G.; Tan, Y.; Tang, W.; Kong, J.; Li, Z. Bio-Inspired Chitosan Aerogel Decorated with MOF-on-COF Heterostructure Hybrid as Recyclable Scavenger of Herbicides in Water. Sep. Purif. Technol. 2022, 298, 121616. [Google Scholar] [CrossRef]
- Yang, J.; He, T.; Li, X.; Wang, R.; Wang, S.; Zhao, Y.; Wang, H. Rapid Dipping Preparation of Superhydrophobic TiO2 Cotton Fabric for Multifunctional Highly Efficient Oil-Water Separation and Photocatalytic Degradation. Colloids Surf. A Phys. Eng. Asp. 2022, 657, 130590. [Google Scholar] [CrossRef]
- Zou, X.; Yao, L.; Zhou, S.; Chen, G.; Wang, S.; Liu, X.; Jiang, Y. Sulfated Lignocellulose Nanofibril Based Composite Aerogel towards Adsorption–Photocatalytic Removal of Tetracycline. Carbohydr. Polym. 2022, 296, 119970. [Google Scholar] [CrossRef] [PubMed]
- Tomé, L.I.N.; Reis, M.S.; de Sousa, H.C.; Braga, M.E.M. Chitosan-Xanthan Gum PEC-Based Aerogels: A Chemically Stable PEC in ScCO2. Mater. Chem. Phys. 2022, 287, 126294. [Google Scholar] [CrossRef]
- Jiang, Y.-H.; Zhang, Y.-Q.; Gao, C.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R. Superhydrophobic Aerogel Membrane with Integrated Functions of Biopolymers for Efficient Oil/Water Separation. Sep. Purif. Technol. 2022, 282, 120138. [Google Scholar] [CrossRef]
- Mariana, M.; HPS, A.K.; Yahya, E.B.; Olaiya, N.G.; Alfatah, T.; Suriani, A.B.; Mohamed, A. Recent Trends and Future Prospects of Nanostructured Aerogels in Water Treatment Applications. J. Water Process Eng. 2022, 45, 102481. [Google Scholar] [CrossRef]
- Peydayesh, M.; Vogt, J.; Chen, X.; Zhou, J.; Donat, F.; Bagnani, M.; Müller, C.R.; Mezzenga, R. Amyloid-Based Carbon Aerogels for Water Purification. Chem. Eng. J. 2022, 449, 137703. [Google Scholar] [CrossRef]
- Ozden, S.; Monti, S.; Tozzini, V.; Dutta, N.S.; Gili, S.; Caggiano, N.; Link, A.J.; Pugno, N.M.; Higgins, J.; Priestley, R.D. Egg Protein Derived Ultralightweight Hybrid Monolithic Aerogel for Water Purification. Mater. Today 2022, 59, 46–55. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, Properties and Applications of Polysaccharide Nanocrystals in Advanced Functional Nanomaterials: A Review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef]
- Nita, L.E.; Ghilan, A.; Rusu, A.G.; Neamtu, I.; Chiriac, A.P. New Trends in Bio-Based Aerogels. Pharmaceutics 2020, 12, 449. [Google Scholar] [CrossRef]
- Trigo, J.P.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M.E. High Value-Added Compounds from Fruit and Vegetable by-Products–Characterization, Bioactivities, and Application in the Development of Novel Food Products. Crit. Rev. Food Sci. Nutr. 2022, 60, 1388–1416. [Google Scholar] [CrossRef]
- Long, L.-Y.; Weng, Y.-X.; Wang, Y.-Z. Cellulose Aerogels: Synthesis, Applications, and Prospects. Polymers 2018, 10, 623. [Google Scholar] [CrossRef]
- Kabir, S.M.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.; Ali, A.; Islam, M.N. Cellulose-Based Hydrogel Materials: Chemistry, Properties and Their Prospective Applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaman, A.; Huang, F.; Jiang, M.; Wei, W.; Zhou, Z. Preparation, Properties, and Applications of Natural Cellulosic Aerogels: A Review. Energy Built Environ. 2020, 1, 60–76. [Google Scholar] [CrossRef]
- Ait Benhamou, A.; Kassab, Z.; Nadifiyine, M.; Salim, M.H.; Sehaqui, H.; Moubarik, A.; el Achaby, M. Extraction, Characterization and Chemical Functionalization of Phosphorylated Cellulose Derivatives from Giant Reed Plant. Cellulose 2021, 28, 4625–4642. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and Its Derivatives: Towards Biomedical Applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Romero-Montero, A.; Tecante, A.; García-Arrazola, R.; Montiel, C.; del Valle, L.J.; Puiggalí, J.; Gimeno, M. Growth of Epithelial Cells on Films of Enzymatically Synthesized Poly (Gallic Acid) Crosslinked to Carboxymethylcellulose. RSC Adv. 2017, 7, 17660–17669. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhou, L.; Chen, L.; Duan, G.; Mei, C.; Huang, C.; Han, J.; Jiang, S. Anisotropic Nanocellulose Aerogels with Ordered Structures Fabricated by Directional Freeze-Drying for Fast Liquid Transport. Cellulose 2019, 26, 6653–6667. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Vasilyeva, N.Y.; Mikova, N.M.; Zhizhaev, A.M. Synthesis and Sulfation with Sulfamic Acid of Aerogels Based on Birch-Wood and Cotton Celluloses. J. Sib. Fed. Univ. Chem. 2022, 15, 57–68. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Grishechko, L.; Szczurek, A.; Fierro, V.; Pizzi, A.; Kuznetsov, B.; Celzard, A. Highly Mesoporous Organic Aerogels Derived from Soy and Tannin. Green Chem. 2012, 14, 3099–3106. [Google Scholar] [CrossRef]
- Grishechko, L.I.; Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Kuznetsov, B.N.; Pizzi, A.; Celzard, A. New Tannin–Lignin Aerogels. Ind. Crops. Prod. 2013, 41, 347–355. [Google Scholar] [CrossRef]
- Takeshita, S.; Zhao, S.; Malfait, W.J.; Koebel, M.M. Chemistry of Chitosan Aerogels: Three-dimensional Pore Control for Tailored Applications. Angew. Chem. Int. Ed. 2021, 60, 9828–9851. [Google Scholar] [CrossRef]
- Fan, S.; Chen, J.; Fan, C.; Chen, G.; Liu, S.; Zhou, H.; Liu, R.; Zhang, Y.; Hu, H.; Huang, Z. Fabrication of a CO2-Responsive Chitosan Aerogel as an Effective Adsorbent for the Adsorption and Desorption of Heavy Metal Ions. J. Hazard. Mater. 2021, 416, 126225. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Gou, S.; He, Y.; Fang, S.; Zhou, L.; Gou, G.; Liu, L. N-Methylene Phosphonic Chitosan Aerogels for Efficient Capture of Cu2+ and Pb2+ from Aqueous Environment. Carbohydr. Polym. 2021, 269, 118355. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Sadeghpour, A.; Malfait, W.J.; Konishi, A.; Otake, K.; Yoda, S. Formation of Nanofibrous Structure in Biopolymer Aerogel during Supercritical CO2 Processing: The Case of Chitosan Aerogel. Biomacromolecules 2019, 20, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, P.; Smirnova, I.; Athamneh, T.; Papastergiou, M.; Chriti, D.; Mali, G.; Čendak, T.; Raptopoulos, G.; Gurikov, P. Polyurea-Crosslinked Biopolymer Aerogel Beads. RSC Adv. 2020, 10, 40843–40852. [Google Scholar] [CrossRef]
- Valentin, R.; Bonelli, B.; Garrone, E.; di Renzo, F.; Quignard, F. Accessibility of the Functional Groups of Chitosan Aerogel Probed by FT-IR-Monitored Deuteration. Biomacromolecules 2007, 8, 3646–3650. [Google Scholar] [CrossRef] [PubMed]
- Rehm, B.H.A. Alginates: Biology and Applications; Springer: Berlin/Heidelberg, Germany, 2009; Volume 13, ISBN 3540926798. [Google Scholar]
- Mustapa, A.N.; Martín, Á.; Cocero, M.J. Alginate Aerogels Dried by Supercritical CO2 as Herbal Delivery Carrier. Malays. J. Anal. Sci. 2018, 22, 522–531. [Google Scholar]
- Forgács, A.; Papp, V.; Paul, G.; Marchese, L.; Len, A.; Dudás, Z.; Fábián, I.; Gurikov, P.; Kalmár, J. Mechanism of Hydration and Hydration Induced Structural Changes of Calcium Alginate Aerogel. ACS Appl. Mater. Interfaces 2021, 13, 2997–3010. [Google Scholar] [CrossRef]
- Sam, D.K.; Sam, E.K.; Durairaj, A.; Lv, X.; Zhou, Z.; Liu, J. Synthesis of Biomass-Based Carbon Aerogels in Energy and Sustainability. Carbohydr. Res. 2020, 491, 107986. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Grishechko, L.I.; Fierro, V.; Kuznetsov, B.N.; Pizzi, A.; Celzard, A. Tannin-Based Xerogels with Distinctive Porous Structures. Biomass. Bioenergy 2013, 56, 437–445. [Google Scholar] [CrossRef]
- Groult, S.; Buwalda, S.; Budtova, T. Tuning Bio-Aerogel Properties for Controlling Drug Delivery. Part 2: Cellulose-Pectin Composite Aerogels. Biomater. Adv. 2022, 135, 212732. [Google Scholar] [CrossRef]
- He, Z.; Qin, M.; Han, C.; Bai, X.; Wu, Y.; Yao, D.; Zheng, Y. Pectin/Graphene Oxide Aerogel with Bamboo-like Structure for Enhanced Dyes Adsorption. Colloids Surf. A Phys. Eng. Asp. 2022, 652, 129837. [Google Scholar] [CrossRef]
- García-González, C.A.; Carenza, E.; Zeng, M.; Smirnova, I.; Roig, A. Design of Biocompatible Magnetic Pectin Aerogel Monoliths and Microspheres. RSC Adv. 2012, 2, 9816–9823. [Google Scholar] [CrossRef]
- Yang, X.; Xie, Y.; Wang, Y.; Qi, W.; Huang, R.; Su, R.; He, Z. Self-Assembled Microporous Peptide-Polysaccharide Aerogels for Oil–Water Separation. Langmuir 2018, 34, 10732–10738. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, S.; Aggeli, A.; Boden, N.; Koopmans, R.J.; Brydson, R.; Rayner, C.M. Peptide Aerogels Comprising Self-Assembling Nanofibrils. Micro. Nano Lett. 2007, 2, 24–29. [Google Scholar] [CrossRef]
- He, Z.; Zhang, X.; Batchelor, W. Cellulose Nanofibre Aerogel Filter with Tuneable Pore Structure for Oil/Water Separation and Recovery. RSC Adv. 2016, 6, 21435–21438. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, Y.; Xu, C.; Gao, M.; Zhu, M.; Jiang, L. Hierarchical Interface Engineering for Advanced Nanocellulosic Hybrid Aerogels with High Compressibility and Multifunctionality. Adv. Funct. Mater. 2021, 31, 2009349. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Zheng, Y.; Xu, Y.; Zheng, Z.; Chen, X.; Liu, W. Versatile Aerogels for Sensors. Small 2019, 15, 1902826. [Google Scholar] [CrossRef]
- Feng, J.; Su, B.-L.; Xia, H.; Zhao, S.; Gao, C.; Wang, L.; Ogbeide, O.; Feng, J.; Hasan, T. Printed Aerogels: Chemistry, Processing, and Applications. Chem. Soc. Rev. 2021, 50, 3842–3888. [Google Scholar] [CrossRef]
- García-González, C.A.; Sosnik, A.; Kalmár, J.; de Marco, I.; Erkey, C.; Concheiro, A.; Alvarez-Lorenzo, C. Aerogels in Drug Delivery: From Design to Application. J. Control. Release 2021, 332, 40–63. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-Based Aerogels—Promising Biodegradable Carriers for Drug Delivery Systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Tu, H.; Zhu, M.; Duan, B.; Zhang, L. Recent Progress in High-strength and Robust Regenerated Cellulose Materials. Adv. Mater. 2021, 33, 2000682. [Google Scholar] [CrossRef] [PubMed]
- Pircher, N.; Carbajal, L.; Schimper, C.; Bacher, M.; Rennhofer, H.; Nedelec, J.-M.; Lichtenegger, H.C.; Rosenau, T.; Liebner, F. Impact of Selected Solvent Systems on the Pore and Solid Structure of Cellulose Aerogels. Cellulose 2016, 23, 1949–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garemark, J.; Yang, X.; Sheng, X.; Cheung, O.; Sun, L.; Berglund, L.A.; Li, Y. Top-down Approach Making Anisotropic Cellulose Aerogels as Universal Substrates for Multifunctionalization. ACS Nano 2020, 14, 7111–7120. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Budtova, T. Tailoring the Morphology and Properties of Starch Aerogels and Cryogels via Starch Source and Process Parameter. Carbohydr. Polym. 2021, 255, 117344. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Fang, Y.; Ni, X.; Wu, K.; Wang, Y.; Jiang, F.; Riffat, S.B. Fabrication and Characterization of a Novel Konjac Glucomannan-Based Air Filtration Aerogels Strengthened by Wheat Straw and Okara. Carbohydr. Polym. 2019, 224, 115129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Kuang, Y.; Xiao, M.; Su, Y.; Jiang, F. Microstructure and Filtration Performance of Konjac Glucomannan-Based Aerogels Strengthened by Wheat Straw. Int. J. Low-Carbon Technol. 2019, 14, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, S.E.; Staiger, M.P.; Deb-Choudhury, S.; Ranford, S. Protein-Based Aerogels: Processing and Morphology. Biobased Aerogels Polysacch. Protein-Based Mater. 2018, 58, 67–102. [Google Scholar]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, Drying Process and Medical Application of Polysaccharide-Based Aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef]
- Prakash, S.S.; Brinker, C.J.; Hurd, A.J.; Rao, S.M. Silica Aerogel Films Prepared at Ambient Pressure by Using Surface Derivatization to Induce Reversible Drying Shrinkage. Nature 1995, 374, 439–443. [Google Scholar] [CrossRef]
- Toivonen, M.S.; Kaskela, A.; Rojas, O.J.; Kauppinen, E.I.; Ikkala, O. Ambient-dried Cellulose Nanofibril Aerogel Membranes with High Tensile Strength and Their Use for Aerosol Collection and Templates for Transparent, Flexible Devices. Adv. Funct. Mater. 2015, 25, 6618–6626. [Google Scholar] [CrossRef] [Green Version]
- Aravind, P.R.; Shajesh, P.; Soraru, G.D.; Warrier, K.G.K. Ambient Pressure Drying: A Successful Approach for the Preparation of Silica and Silica Based Mixed Oxide Aerogels. J. Solgel. Sci. Technol. 2010, 54, 105–117. [Google Scholar] [CrossRef]
- Françon, H.; Wang, Z.; Marais, A.; Mystek, K.; Piper, A.; Granberg, H.; Malti, A.; Gatenholm, P.; Larsson, P.A.; Wågberg, L. Ambient-Dried, 3D-Printable and Electrically Conducting Cellulose Nanofiber Aerogels by Inclusion of Functional Polymers. Adv. Funct. Mater. 2020, 30, 1909383. [Google Scholar] [CrossRef]
- Ismail, N.H.; Salleh, W.N.W.; Ismail, A.F.; Hasbullah, H.; Yusof, N.; Aziz, F.; Jaafar, J. Hydrophilic Polymer-Based Membrane for Oily Wastewater Treatment: A Review. Sep. Purif. Technol. 2020, 233, 116007. [Google Scholar] [CrossRef]
- Mruthunjayappa, M.H.; Sharma, V.T.; Dharmalingam, K.; Sanna Kotrappanavar, N.; Mondal, D. Engineering a Biopolymer-Based Ultrafast Permeable Aerogel Membrane Decorated with Task-Specific Fe–Al Nanocomposites for Robust Water Purification. ACS Appl. Bio. Mater. 2020, 3, 5233–5243. [Google Scholar] [CrossRef] [PubMed]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging Pollutants in Wastewater: A Review of the Literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef]
- Laitinen, O.; Suopajärvi, T.; Österberg, M.; Liimatainen, H. Hydrophobic, Superabsorbing Aerogels from Choline Chloride-Based Deep Eutectic Solvent Pretreated and Silylated Cellulose Nanofibrils for Selective Oil Removal. ACS Appl. Mater. Interfaces 2017, 9, 25029–25037. [Google Scholar] [CrossRef] [Green Version]
- Yuan, W.; Zhang, X.; Zhao, J.; Li, Q.; Ao, C.; Xia, T.; Zhang, W.; Lu, C. Ultra-Lightweight and Highly Porous Carbon Aerogels from Bamboo Pulp Fibers as an Effective Sorbent for Water Treatment. Results Phys. 2017, 7, 2919–2924. [Google Scholar] [CrossRef]
- Yang, S.; Chen, L.; Mu, L.; Hao, B.; Ma, P.-C. Low Cost Carbon Fiber Aerogel Derived from Bamboo for the Adsorption of Oils and Organic Solvents with Excellent Performances. RSC Adv. 2015, 5, 38470–38478. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Samad, Y.A.; Polychronopoulou, K.; Alhassan, S.M.; Liao, K. Carbon Aerogel from Winter Melon for Highly Efficient and Recyclable Oils and Organic Solvents Absorption. ACS Sustain. Chem. Eng. 2014, 2, 1492–1497. [Google Scholar] [CrossRef]
- Sai, H.; Fu, R.; Xing, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface Modification of Bacterial Cellulose Aerogels’ Web-like Skeleton for Oil/Water Separation. ACS Appl. Mater. Interfaces 2015, 7, 7373–7381. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Cai, Z.; Yan, N.; Liu, M.; Yu, Y. Highly Compressible and Hydrophobic Anisotropic Aerogels for Selective Oil/Organic Solvent Absorption. ACS Sustain. Chem. Eng. 2018, 7, 332–340. [Google Scholar] [CrossRef]
- Han, S.; Sun, Q.; Zheng, H.; Li, J.; Jin, C. Green and Facile Fabrication of Carbon Aerogels from Cellulose-Based Waste Newspaper for Solving Organic Pollution. Carbohydr. Polym. 2016, 136, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Lin, R.; Lin, C.; He, B.; Zheng, T.; Lu, L.; Cao, Y. An Environment-Friendly and Multi-Functional Absorbent from Chitosan for Organic Pollutants and Heavy Metal Ion. Carbohydr. Polym. 2016, 148, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhu, Y.; Wang, J.; Liu, Z.; Wu, J.; Zhang, T.; Li, P.; Qiu, F. Agricultural Bamboo Leaf Waste as Carbon Precursor for the Preparation of Cu-Al/Biomass Fiber Adsorption and Its Application in the Removal of Ammonia Nitrogen Pollutants from Domestic Wastewater. J. Wood Chem. Technol. 2021, 41, 137–149. [Google Scholar] [CrossRef]
- Zhang, H.; Lyu, S.; Zhou, X.; Gu, H.; Ma, C.; Wang, C.; Ding, T.; Shao, Q.; Liu, H.; Guo, Z. Super Light 3D Hierarchical Nanocellulose Aerogel Foam with Superior Oil Adsorption. J. Colloid Interface Sci. 2019, 536, 245–251. [Google Scholar] [CrossRef]
- Do, N.H.N.; Luu, T.P.; Thai, Q.B.; Le, D.K.; Chau, N.D.Q.; Nguyen, S.T.; Le, P.K.; Phan-Thien, N.; Duong, H.M. Advanced Fabrication and Application of Pineapple Aerogels from Agricultural Waste. Mater. Technol. 2020, 35, 807–814. [Google Scholar] [CrossRef]
- Yi, L.; Yang, J.; Fang, X.; Xia, Y.; Zhao, L.; Wu, H.; Guo, S. Facile Fabrication of Wood-Inspired Aerogel from Chitosan for Efficient Removal of Oil from Water. J. Hazard. Mater. 2020, 385, 121507. [Google Scholar] [CrossRef]
- Yi, Y.; Liu, P.; Zhang, N.; Gibril, M.E.; Kong, F.; Wang, S. A High Lignin-Content, Ultralight, and Hydrophobic Aerogel for Oil-Water Separation: Preparation and Characterization. J. Porous Mater. 2021, 28, 1881–1894. [Google Scholar] [CrossRef]
- Yi, L.; Xia, Y.; Tan, Z.; Fang, X.; Zhao, L.; Wu, H.; Guo, S. Design of Tubelike Aerogels with Macropores from Bamboo Fungus for Fast Oil/Water Separation. J. Clean. Prod. 2020, 264, 121558. [Google Scholar] [CrossRef]
- Kumar, G.; Dora, D.T.K.; Jadav, D.; Naudiyal, A.; Singh, A.; Roy, T. Utilization and Regeneration of Waste Sugarcane Bagasse as a Novel Robust Aerogel as an Effective Thermal, Acoustic Insulator, and Oil Adsorbent. J. Clean. Prod. 2021, 298, 126744. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, L.; Zhang, T.; Qiu, F.; Yue, X.; Yang, D. Sustainable, Flexible, and Superhydrophobic Functionalized Cellulose Aerogel for Selective and Versatile Oil/Water Separation. ACS Sustain. Chem. Eng. 2019, 7, 9984–9994. [Google Scholar] [CrossRef]
- Long, Q.; Zhang, Z.; Qi, G.; Wang, Z.; Chen, Y.; Liu, Z.-Q. Fabrication of Chitosan Nanofiltration Membranes by the Film Casting Strategy for Effective Removal of Dyes/Salts in Textile Wastewater. ACS Sustain. Chem. Eng. 2020, 8, 2512–2522. [Google Scholar] [CrossRef]
- Feng, C.; Ren, P.; Li, Z.; Tan, W.; Zhang, H.; Jin, Y.; Ren, F. Graphene/Waste-Newspaper Cellulose Composite Aerogels with Selective Adsorption of Organic Dyes: Preparation, Characterization, and Adsorption Mechanism. New J. Chem. 2020, 44, 2256–2267. [Google Scholar] [CrossRef]
- Kasiri, M.B. Application of Chitosan Derivatives as Promising Adsorbents for Treatment of Textile Wastewater. Impact Prospect. Green Chem. Text. Technol. 2019, 14, 417–469. [Google Scholar]
- de Luna, M.S.; Sirignano, M. Upcycling Soot Particles into Chitosan-Based Aerogels for Water Purification from Organic Pollutants. J. Hazard. Mater. Lett. 2021, 2, 100019. [Google Scholar] [CrossRef]
- Marotta, A.; Luzzi, E.; Salzano de Luna, M.; Aprea, P.; Ambrogi, V.; Filippone, G. Chitosan/Zeolite Composite Aerogels for a Fast and Effective Removal of Both Anionic and Cationic Dyes from Water. Polymers 2021, 13, 1691. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Wen, F.; Chen, J.; Chen, W.; Huang, Y.; Wang, B. Mussel-Inspired Synthesis of Magnetic Carboxymethyl Chitosan Aerogel for Removal Cationic and Anionic Dyes from Aqueous Solution. Polymers 2021, 213, 123316. [Google Scholar] [CrossRef]
- Arabkhani, P.; Asfaram, A. Development of a Novel Three-Dimensional Magnetic Polymer Aerogel as an Efficient Adsorbent for Malachite Green Removal. J. Hazard. Mater. 2020, 384, 121394. [Google Scholar] [CrossRef]
- Tang, J.; Song, Y.; Zhao, F.; Spinney, S.; da Silva Bernardes, J.; Tam, K.C. Compressible Cellulose Nanofibril (CNF) Based Aerogels Produced via a Bio-Inspired Strategy for Heavy Metal Ion and Dye Removal. Carbohydr. Polym. 2019, 208, 404–412. [Google Scholar] [CrossRef]
- Grishkewich, N.; Li, Y.; Liu, K.; Tam, K.C. Synthesis and Characterization of Modified Cellulose Nanofibril Organosilica Aerogels for the Removal of Anionic Dye. J. Polym. Res. 2022, 29, 261. [Google Scholar] [CrossRef]
- Zou, Y.; Chen, X.; Yang, P.; Liang, G.; Yang, Y.; Gu, Z.; Li, Y. Regulating the Absorption Spectrum of Polydopamine. Sci. Adv. 2020, 6, eabb4696. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhao, J.; Zhu, J.; Guo, X.; Chen, P.; Duan, G.; Liu, X.; Li, Y. A Mussel-Inspired Polydopamine-Filled Cellulose Aerogel for Solar-Enabled Water Remediation. ACS Appl. Mater. Interfaces 2021, 13, 7617–7624. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sheikhi, A.; van de Ven, T.G.M. Reusable Green Aerogels from Cross-Linked Hairy Nanocrystalline Cellulose and Modified Chitosan for Dye Removal. Langmuir 2016, 32, 11771–11779. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, J.; Kumar, V.; Garg, A.K.; Dubey, P.; Tripathi, K.M.; Sonkar, S.K. Bio-Mass Derived Functionalized Graphene Aerogel: A Sustainable Approach for the Removal of Multiple Organic Dyes and Their Mixtures. New J. Chem. 2021, 45, 9073–9083. [Google Scholar] [CrossRef]
- Jiao, C.; Li, T.; Wang, J.; Wang, H.; Zhang, X.; Han, X.; Du, Z.; Shang, Y.; Chen, Y. Efficient Removal of Dyes from Aqueous Solution by a Porous Sodium Alginate/Gelatin/Graphene Oxide Triple-Network Composite Aerogel. J. Polym. Environ. 2020, 28, 1492–1502. [Google Scholar] [CrossRef]
- Lyu, W.; Li, J.; Zheng, L.; Liu, H.; Chen, J.; Zhang, W.; Liao, Y. Fabrication of 3D Compressible Polyaniline/Cellulose Nanofiber Aerogel for Highly Efficient Removal of Organic Pollutants and Its Environmental-Friendly Regeneration by Peroxydisulfate Process. Chem. Eng. J. 2021, 414, 128931. [Google Scholar] [CrossRef]
- Hasanpour, M.; Motahari, S.; Jing, D.; Hatami, M. Investigation of Operation Parameters on the Removal Efficiency of Methyl Orange Pollutant by Cellulose/Zinc Oxide Hybrid Aerogel. Chemosphere 2021, 284, 131320. [Google Scholar] [CrossRef]
- Zhang, T.; Xiao, S.; Fan, K.; He, H.; Qin, Z. Preparation and Adsorption Properties of Green Cellulose-Based Composite Aerogel with Selective Adsorption of Methylene Blue. Polymer 2022, 258, 125320. [Google Scholar] [CrossRef]
- Liu, Y.; Ke, Y.; Shang, Q.; Yang, X.; Wang, D.; Liao, G. Fabrication of Multifunctional Biomass-Based Aerogel with 3D Hierarchical Porous Structure from Waste Reed for the Synergetic Adsorption of Dyes and Heavy Metal Ions. Chem. Eng. J. 2023, 451, 138934. [Google Scholar] [CrossRef]
- Nguyen, P.X.T.; Ho, K.H.; Do, N.H.N.; Nguyen, C.T.X.; Nguyen, H.M.; Tran, K.A.; Le, K.A.; Le, P.K. A Comparative Study on Modification of Aerogel-Based Biosorbents from Coconut Fibers for Treatment of Dye- and Oil-Contaminated Water. Mater. Today Sustain. 2022, 19, 100175. [Google Scholar] [CrossRef]
- Morcillo-Martín, R.; Espinosa, E.; Rabasco-Vílchez, L.; Sanchez, L.M.; de Haro, J.; Rodríguez, A. Cellulose Nanofiber-Based Aerogels from Wheat Straw: Influence of Surface Load and Lignin Content on Their Properties and Dye Removal Capacity. Biomolecules 2022, 12, 232. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhou, M.; Liang, L.; Jiang, L.; Wang, W. Ultrahigh-Surface-Area Activated Carbon Aerogels Derived from Glucose for High-Performance Organic Pollutants Adsorption. J. Colloid Interface Sci. 2019, 546, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, Y.; Luo, J.; Li, T.; Wang, D.; Luo, S.; Crittenden, J.C. 3D Hierarchical Porous-Structured Biochar Aerogel for Rapid and Efficient Phenicol Antibiotics Removal from Water. Chem. Eng. J. 2019, 368, 639–648. [Google Scholar] [CrossRef]
- Feng, Z.; Odelius, K.; Rajarao, G.K.; Hakkarainen, M. Microwave Carbonized Cellulose for Trace Pharmaceutical Adsorption. Chem. Eng. J. 2018, 346, 557–566. [Google Scholar] [CrossRef]
- Peydayesh, M.; Suter, M.K.; Bolisetty, S.; Boulos, S.; Handschin, S.; Nyström, L.; Mezzenga, R. Amyloid Fibrils Aerogel for Sustainable Removal of Organic Contaminants from Water. Adv. Mater. 2020, 32, 1907932. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, H.; Zeng, F.; Li, X.; Sun, J.; Hu, X.; Pan, Q.; Li, C.; Lin, H.; min Su, Z. Polyaniline as Interface Layers Promoting the In-Situ Growth of Zeolite Imidazole Skeleton on Regenerated Cellulose Aerogel for Efficient Removal of Tetracycline. J. Colloid Interface Sci. 2020, 579, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ren, W.; Li, Y.; Gao, J.; Yang, X.; Yao, J. Zeolitic Imidazolate Framework-67@ Cellulose Aerogel for Rapid and Efficient Degradation of Organic Pollutants. J. Solid State Chem. 2020, 291, 121621. [Google Scholar] [CrossRef]
- Lv, Y.; Liang, Z.; Li, Y.; Chen, Y.; Liu, K.; Yang, G.; Liu, Y.; Lin, C.; Ye, X.; Shi, Y. Efficient Adsorption of Diclofenac Sodium in Water by a Novel Functionalized Cellulose Aerogel. Environ. Res. 2021, 194, 110652. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Montero, A.; Valencia-Bermúdez, J.L.; Rosas-Meléndez, S.A.; Núñez-Tapia, I.; Piña-Barba, M.C.; Leyva-Gómez, G.; Del Prado-Audelo, M.L. Biopolymeric Fibrous Aerogels: The Sustainable Alternative for Water Remediation. Polymers 2023, 15, 262. https://doi.org/10.3390/polym15020262
Romero-Montero A, Valencia-Bermúdez JL, Rosas-Meléndez SA, Núñez-Tapia I, Piña-Barba MC, Leyva-Gómez G, Del Prado-Audelo ML. Biopolymeric Fibrous Aerogels: The Sustainable Alternative for Water Remediation. Polymers. 2023; 15(2):262. https://doi.org/10.3390/polym15020262
Chicago/Turabian StyleRomero-Montero, Alejandra, José Luis Valencia-Bermúdez, Samuel A. Rosas-Meléndez, Israel Núñez-Tapia, María Cristina Piña-Barba, Gerardo Leyva-Gómez, and María Luisa Del Prado-Audelo. 2023. "Biopolymeric Fibrous Aerogels: The Sustainable Alternative for Water Remediation" Polymers 15, no. 2: 262. https://doi.org/10.3390/polym15020262