An Insight into Enamel Resin Infiltrants with Experimental Compositions
Abstract
:1. Introduction
2. Materials and Methods
3. Results
| Author (Year) | Country | Resin Infiltrant | Objective of the Study | Alternative Formulations | Test | Conclusions |
|---|---|---|---|---|---|---|
| Andrade Neto et al. (2016) [34] | Brazil |
| To evaluate the influence on DC and anti-caries efficacy | 10%wt HAp after HT | XRD, FT-IR, DC, TEM, Knoop hardness | 10%wt HAp after 2 or 5 h of HT were equally effective in increasing DC and enhancing anti-caries capacity |
| Araujo et al. (2015) [32] | Brazil |
| To evaluate the influence on bond strength | Different monomer (UDMA and BisEMA) and solvent (ethanol and HEMA) | Microtensile bond strength test | The resin matrix did not affect bond strength. Ethanol negatively influenced the bond strength, possibly interefering with the polymerization reaction. |
| Araujo GSA et al. (2013) [12] | Brazil |
| To evaluate the influence of monomers and solvents on mechanical properties and penetrativity | UDMA, BisEMA, HEMA, ethanol | DC, Knoop hardness, softening ratio, E- modulus, confocal laser scanning microscope | Hydrophobic monomers and solvents, in particular ethanol, resulted in decreased mechanical properties and did not improve penetrativity. |
| Askar et al. (2018) [31] | Germany |
| Penetration/filling ability in non-, micro- and cavitated inter-proximal natural caries lesions (ICDAS 2, 3, and 5). | Organic filler particles | Dual-fluorescence staining and confocal microscopy | MFIR showed similar penetration depth as IR with improved filling ability. |
| Bagheri et al. (2020) [48] | Iran |
| To evaluate the masking effects of artificial WSLs | BAG and n-FHAP | Spectrophotometry | n-FHAP-based compositions showed good masking effects after 14 days. |
| Cuppini et al. (2021) [37] | Brazil |
| To evaluate the influence on mechanical properties and citotoxicity | Ionic liquid-loaded microcapsules (MC-IL) | SEM, ultimate tensile strength, contact angle, and surfaces free energy | MC-IL increased surface properties without influencing mechanical properties and cytotoxicity. |
| Dai et al. (2022) [43] | China |
| To evaluate the remineralization ability and mechanical properties | Amorphous calium-phosphate nanoparticles (NACP) | Citotoxicity, DC, microhardness, color change, Ca and P ion release, and pH cycling. | Incorporating of 30 wt% NACP resulted in longer Ca and P release, higher DC, higher hardness, and biocompatibility |
| Fischer et al. (2021a) [44] | Poland |
| To evaluate the depth penetration into root cementum | PMMAn-MTZ | SEM and light microscopy | Experimental resin showed good penetration into decalcified root and in the root dentin. |
| Fischer et al. (2021b) [45] | Poland |
| To evaluate the influence on citotoxicity | PMMMn-MTZ | Mouse subcuta- neous connective tissue fibroblasts, fluorescence microscope, and viability assessment | No differences in cytotoxicity among the tested groups. |
| Flor-Ribeiro et al. (2019) [36] | Brazil |
| To evaluate the influence on mechanical properties and bacteriostaticity | Ionic salt (DPI) and chitosan | DC, E-modulus, flexural strength, water sorption/solubility, and antibacterial ability. | The concentration of 0.5 mol% DPI and 0.12 wt% chitosan could improve mechanical properties and enhance antibacterial activity in the experimental infiltrant resin. |
| Gaglianone et al. (2020) [38] | Brazil |
| To evaluate the influence on physical properties and penetration depth. | Pre-heating (25 and 55 °C) and different formulation with HEMA or ethanol as solvents. | DC, E-modulus, flexural strength, contact angle, confocal microscopy | Pre-heating and the adding of ethanol as solvent resulted in altered mechanical properties and did not enhance penetration depth. |
| Hashemian et al. (2021) [49] | Iran |
| To improve the physical properties and enhance remineralization | PUA, nano-FHA and FD-BG fillers | CA, penetration coefficient, field emission SEM, EDS | The combination of PUA and TEGDMA may improve mechanical properties and inclusion of FD-BG may include reminerilizing effects. |
| Inagaki et al. (2016a) [30] | Brazil |
| To evaluate the influence of mechanical properties and bacteriostaticity. | CHX and different hydrophobic monomers. | DC, Knoop hardness, microbiological assay. | TEGDMA/UDMA/CHX had improved antimicrobiological properties among groups without influencing mechanical properties. |
| Inagaki et al. (2016b) [33] | Brazil |
| To evaluate the influence on chemio-physical properties | CHX and different hydrophobic monomers. | Sorption and solubility, softening ratio, Flexural strength, and E-modulus. | The monomers’ characteristics more relevantly influence the physio-chemical characteristics of the resin than the inclusion of CHX. The combination of TEGDMA/UDMA resulted in higher properties. |
| Kielbassa et al. (2020) [28] | Austria |
| To evaluate penetrativity of internal and external infiltration | AgNP | Confocal laser scanning microscope | AgNP did not influence the penetrativity of Icon. Internal and external infiltration was considered a viable operative chance to treat proximal lesions extending to dentin. |
| Lausch et al. (2017) [8] | Germany |
| Penetration/filling ability in occlusal natural caries lesions (ICDAS 2, 3 and 5). | Organic filler particles | Dual-fluorescence staining and confocal microscopy | MFIR could combine the penetration depth of RI + the sealing ability of a fissure sealant. |
| Mathias et al. (2019) [39] | Brazil |
| To evaluate the influence on mechanical properties | DPI and different solvents (HEMA or ethanol) | Degree of conversion, water sorption/solubility, cohesive strength, contact angle. | 0.5 mol% DPI may compensate some worsening in mechanical properties in HEMA and ethanol-diluted resin infiltrants. Ethanol worsened the properties tested. |
| Nedeljkovic et al. (2022) [52] | Holland |
| Remineralization after artificial cariogenic challenges. | Hybrid-glass monomer/oligomers | Knoop microhardness, surface roughness SEM | Hybrid glass monomers performed better than control thanks to its acid/cariogenic higher stability. |
| Nóbrega et al. (2020) [41] | Brazil |
| To evaluate the influence on mechanical properties | Different composition and different enamel carious layers after 21 days | Microhardness and fluorescence microscopy | The combination TEGDMA/ BisEMA was the less affected by hydrolytic degradation. |
| Nowak-Wachol et al. (2022) [46] | Poland |
| To evaluate the penetrativity into decalcified root cementum | YF3 | SEM/EDX | 4% YF3 hampered penetrativity, while no differences were observed with 2% YF3 |
| Obeid et al. (2022) [40] | Brazil |
| To increase mechanical properties and enhance anticaries effect | Bioactive hybrid nanofibers containing silica (SiO2) or calcium-doped silica (SiO2–CaP) | DC, Knoop hardness, EDX | Bioactive nanofibers resulted in increased SH, with Si-O2CaP enabling higher remineralizing potential. |
| Paris and Meyer-Lueckel (2010) [11] | Germany |
| To evaluate the penetration ability | Infiltrant materials with different penetration coefficient | Microradiography after 400 days in demineralizing environment | TEGDMA showed higher penetration ability. Supposedly, ethanol contrasted with the polymerization reaction. |
| Paris et al. (2012) [50] | Germany |
| To evaluate the penetration ability in primary teeth | Infiltrant materials with different penetration coefficient and solvents in primary teeth | Confocal laser scanner microscopy | The penetration coefficient did not influence the penetration ability of the experimental resins in primary teeth. The solvent was a detrimental factor. |
| Prodan et al. (2022) [47] | Romania |
| To evaluate the influence on mechanical properties | Different monomer mixtures with BaF2 | DC, Water sorption, SEM, Fl release, flexural strength, Young’s modulus, residual monomers | Best mixture was found in Bis-GMA/HEMA/ TEGDMA |
| Sfalcin et al. (2017) [35] | Brazil |
| To evaluate the influence on mechanical properties | Bioactive fillers | FT-IR, universal testing machine, DC, Knoop hardness, softening ratio, tensile cohesive strength, E-modulus, water sorption/solubility. | Bioactive fillers increased chemo-mechanical properties of experimental resins compared to Icon, except for softening ratio. |
| Skucha-Nowak et al. (2016) [18] | Poland |
| To improve penetrativity and bacteriostaticity | MTZ + YbF3 | Back-scattered SEM | No improvement in penetration depth (probably due to the grain size of YbF3). No information on bacteriostaticity after inclusion of MTZ. |
| Villegas et al. (2020) [29] | Argentina |
| To add antibacterial activity | ZnO@NP | Microdilution method, infiltration, SEM/EDS | Experimental group showed antibacterial activity and better infiltration than the control. |
| Wang et al. (2021) [42] | Brazil |
| To evaluate the influence on mechanical properties and penetration depth | Self-etching capacity with PAM | Confocal laser scanning microscope, viscosity, pH. | Icon and CA demonstrated the lower penetrativity, while this was improved in the formulation TEGDMA/PAM |
| Yu et al. (2020) [51] | China |
| To add antibacterial activity | DMADDM | Citotoxicity, AFM, colour, biofilm formation, | Experimental resins showed good antibacterial activity after 1 month and did not influence the surface roughness and colour stability. |
4. Discussion
4.1. Mechanical Properties
4.2. Penetration Depth
4.3. Antibacterial Activity
4.4. Remineralization Activity
4.5. Color Stability
4.6. Root Caries
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Schwendicke, F.; Dörfer, C.E.; Schlattmann, P.; Foster Page, L.; Thomson, W.M.; Paris, S. Socioeconomic inequality and caries: A systematic review and meta-analysis. J. Dent. Res. 2015, 94, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Wegehaupt, F.J.; Attin, T. The role of fluoride and casein phosphopeptide/amorphous calcium phosphate in the prevention of erosive/abrasive wear in an in vitro model using hydrochloric acid. Caries Res. 2010, 44, 358–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kielbassa, A.M.; Muller, J.; Gernhardt, C.R. Closing the gap between oral hygiene and minimally invasive dentistry: A review on the resin infiltration technique of incipient (Proximal) enamel lesions. Quintessence Int. 2009, 40, 663–681. [Google Scholar] [PubMed]
- Bagattoni, S.; Gozzi, I.; Lardani, L.; Piana, G.; Mazzoni, A.; Breschi, L.; Mazzitelli, C. Case report of a novel interim approach to prevent early posteruptive enamel breakdown of molar-incisor hypomineralization-affected molars. J. Am. Dent. Assoc. 2021, 152, 560–566. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, D.G.; Park, C.J.; Cho, L.R. Minimally invasive treatment for esthetic enhancement of white spot lesion in adjacent tooth. J. Adv. Prosthodont. 2013, 5, 359–363. [Google Scholar] [CrossRef] [Green Version]
- Borges, A.B.; Caneppele, T.M.; Masterson, D.; Maia, L.C. Is resin infiltration an effective esthetic treatment for enamel development defects and white spot lesions? A systematic review. J. Dent. 2017, 56, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Meyer Lueckel, H.; Paris, S.; Kielbassa, A.M. Surface layer erosion of natural caries lesions with phosphoric and hydrochloric acid gels in preparation for resin infiltration. Caries Res. 2007, 41, 223–230. [Google Scholar] [CrossRef]
- Lausch, J.; Askar, H.; Paris, S.; Meyer-Lueckel, H. Micro-filled resin infiltration of fissure caries lesions in vitro. J. Dent. 2017, 57, 73–76. [Google Scholar] [CrossRef]
- Ulrich, I.; Mueller, J.; Wolgin, M.; Frank, W.; Kielbassa, A.M. Tridimensional surface roughness analysis after resin infiltration of (deproteinized) natural subsurface carious lesions. Clin. Oral Investig. 2015, 19, 1473–1483. [Google Scholar] [CrossRef]
- Kielbassa, A.M.; Ulrich, I.; Werth, W.D.; Schüller, C.; Frank, W.; Schmidl, R. External and internal resin infiltration of natural proximal subsurface caries lesions: A valuable enhancement of the internal tunnel restoration. Quintessence Int. 2017, 48, 357–368. [Google Scholar]
- Paris, S.; Meyer-Lueckel, H. Infiltrants inhibit progression of natural caries lesions in vitro. J. Dent. 2010, 80, 1276–1280. [Google Scholar] [CrossRef] [PubMed]
- Araujo, G.S.A.; Sfalcin, R.A.; Araujo, T.G.F.; Alonso, R.C.B.; Puppin-Rontani, R.M. Evaluation of polymerization characteristics and penetration into enamel caries lesions of experimental infiltrants. J. Dent. 2013, 41, 1014–1019. [Google Scholar] [CrossRef] [Green Version]
- Aswani, R.; Chandrappa, V.; Uloopi, K.S.; Chandrasekhar, R.; RojaRamya, K.S. Resin infiltration of artificial enamel lesions: Evaluation of penetration depth, surface roughness and color stability. Int. J. Clin. Pediatr. Dent. 2019, 12, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Bitter, K.; Naumann, M.; Dorfer, C.E.; Meyer-Lueckel, H. Resin infiltration of proximal caries lesions differing in ICDAS codes. Eur. J. Oral Sci. 2011, 119, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Martignon, S.; Ekstrand, K.R.; Gomez, J.; Lara, J.S.; Cortes, A. Infiltrating/sealing proximal caries lesions: A 3-year randomized clinical trial. J. Dent. Res. 2012, 91, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Memarpour, M.; Abendizade, A.; Rafiee, A.; Hashemian, A. Penetration ability and microhardness of infiltrant resin and two pit and fissure sealants in primary teeth with early enamel lesions. Sci. Rep. 2022, 17, 4652. [Google Scholar] [CrossRef]
- Paris, S.; Lausch, J.; Selje, T.; Dorfer, C.E.; Meyer-Luckel, H. Comparison of sealant and infiltrant penetration into pit and fissure caries lesions in vitro. J. Dent. 2014, 42, 432–438. [Google Scholar] [CrossRef]
- Skucha-Nowak, M.; Machorowska Pieniazec, A.; Tanasiewicz, M. Assessing the penetrating abilities of experimental preparation with dental infiltrant features using optical microscope: Preliminary study. Adv. Clin. Exp. Med. 2016, 25, 961–969. [Google Scholar] [CrossRef]
- Perdigao, J. Resin infiltration of enamel white spot lesions: An ultramorphological analysis. J. Esthet. Restor. Dent. 2020, 32, 317–324. [Google Scholar] [CrossRef]
- Sönmez, H.; Saat, S. A clinical evaluation of deproteinization and different cavity designs on resin restoration performance in MIH-affected molars: Two-year results. J. Clin. Pediatr. Dent. 2017, 41, 336–342. [Google Scholar] [CrossRef]
- Dogra, S.; Goyal, V.; Gupta, A.; Joshi, S.; Kataria, V.; Saini, J.; Nagpal, M.; Narula, P. Spectrophotometric evaluation of color change in tooth enamel defects using resin infiltrate: An in vivo Study. J. Clin. Pediatr. Dent. 2020, 13, 150–154. [Google Scholar] [CrossRef]
- Youssef, A.; Farid, M.; Zayed, M.; Lynch, E.; Alam, M.K.; Kielbassa, A.M. Improving oral health: A short-term split-mouth randomized clinical trial revealing the superiority of resin infiltration over remineralization of white spot lesions. Quintessence Int. 2020, 51, 696–709. [Google Scholar] [PubMed]
- Ritwik, P.; Jones, C.M.; Fan, Y.; Sarkar, N.K. Hydrolytic and color stability of resin infiltration: A preliminary in vitro trial. Contemp. Dent. Pract. 2016, 17, 377–381. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.Z.; Zuo, Q.L.; Liu, C.; Jiang, H.; Du, M.Q. Accelerated aging effects on color, microhardness and microstructure of ICON resin infiltration. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7722–7731. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.K.; Min, J.H.; Kwon, H.K.; Kim, B.I. Modification of surface pretreatment of white spot lesions to improve the safety and efficacy of resin infiltration. Korean J. Orthod. 2014, 44, 195–202. [Google Scholar] [CrossRef] [Green Version]
- Zakizade, M.; Davoudi, A.; Akhavan, A.; Shirban, F. Effect of resin infiltration technique on improving surface hardness of enamel lesions: A systematic review and meta-analysis. J. Evid. Base Dent. Pract. 2020, 20, 101405. [Google Scholar] [CrossRef]
- Tawakoli, P.N.; Attin, T.; Mohn, D. Oral biofilm and caries-infiltrant interactions on enamel. J. Dent. 2016, 48, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Kielbassa, A.M.; Leimer, M.R.; Hartmann, J.; Harm, S.; Pasztorek, M.; Ulrich, I.B. Ex vivo investigation on internal tunnel approach/internal resin infiltration and external nanosilver-modified resin infiltration of proximal caries exceeding into dentin. PLoS ONE 2020, 15, e0228249. [Google Scholar] [CrossRef] [Green Version]
- Villegas, N.A.; Silvero Compagnucci, M.J.; Sainz Aja, M.; Rocca, D.M.; Becerra, M.C.; Fabian Molina, G.; Palma, S.D. Novel antibacterial resin-based filling material containing nanoparticles for the potential one-step treatment of caries. J. Healthc. 2019, 13, 6367919. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, L.T.; Alonso, R.C.; Araujo, G.A.; de Souza-Junior, E.J.; Anibal, P.C.; Hofling, J.F.; Pascon, F.M.; Puppin-Rontani, R.M. Effect of monomer blend and chlorhexidine-adding on physical, mechanical and biological properties of experimental infiltrants. Dent. Mater. 2016, 32, e307–e313. [Google Scholar] [CrossRef]
- Askar, H.; Schwendicke, F.; Lausch, J.; Meyer-Lueckel, H.; Paris, S. Modified resin infiltration of non-, micro- and cavitated proximal caries lesions in vitro. J. Dent. 2018, 74, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Araújo, T.G.; Sfalcin, R.A.; de Araújo, G.S.; Alonso, R.C.; Puppin-Rontani, R.M. Bond strength of experimental low-viscosity resin materials to early enamel caries lesions: Effect of diluent/solvent addition. J. Adhes. Dent. 2015, 17, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, L.T.; Dainezi, V.B.; Alonso, R.C.; Paula, A.B.; Garcia-Godoy, F.; Puppin-Rontani, R.M.; Pascon, F.M. Evaluation of sorption/solubility, softening, flexural strength and elastic modulus of experimental resin blends with chlorhexidine. J. Dent. 2016, 49, 40–45. [Google Scholar] [CrossRef]
- Andrade Neto, D.M.; Carvalho, E.V.; Rodrigues, E.A.; Feitosa, V.P.; Sauro, S.; Mele, G.; Carbone, L.; Mazzetto, S.E.; Rodrigues, L.K.; Fechine, P.B.A. Novel hydroxyapatite nanorods improve anti-caries efficacy of enamel infiltrants. Dent. Mater. 2016, 32, 784–793. [Google Scholar] [CrossRef]
- Sfalcin, R.A.; Correr, A.B.; Morbidelli, L.R.; Araújo, T.G.F.; Feitosa, V.P.; Correr-Sobrinho, L.; Watson, T.F.; Sauro, S. Influence of bioactive particles on the chemical-mechanical properties of experimental enamel resin infiltrants. Clin. Oral Investig. 2017, 21, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Flor-Ribeiro, M.D.; Graziano, T.S.; Aguiar, F.H.B.; Stipp, R.N.; Marchi, G.M. Effect of iodonium salt and chitosan on the physical and antibacterial properties of experimental infiltrants. Braz. Oral Res. 2019, 33, e075. [Google Scholar] [CrossRef] [PubMed]
- Cuppini, M.; Garcia, I.M.; de Souza, V.S.; Zatta, K.C.; Visioli, F.; Leitune, V.C.B.; Guterres, S.S.; Scholten, J.D.; Collares, F.M. Ionic liquid-loaded microcapsules doped into dental resin infiltrants. Bioact. Mater. 2021, 12, 2667–2675. [Google Scholar] [CrossRef] [PubMed]
- Gaglianone, L.A.; Pfeifer, C.S.; Mathias, C.; Puppin-Rontani, R.M.; Marchi, G.M. Can composition and preheating improve infiltrant characteristics and penetrability in demineralized enamel? Braz. Oral Res. 2020, 34, e099. [Google Scholar] [CrossRef]
- Mathias, C.; Gomes, R.S.; Dressano, D.; Braga, R.R.; Aguiar, F.H.B.; Marchi, G.M. Effect of diphenyliodonium hexafluorophosphate salt on experimental infiltrants containing different diluents. Odontology 2019, 107, 202–208. [Google Scholar] [CrossRef]
- Obeid, A.T.; Garcia, L.H.A.; Nascimento, T.R.L.; Castellano, L.R.C.; Bombonatti, J.F.S.; Honório, H.M.; Mondelli, R.F.L.; Sauro, S.; Campos Velo, M.M.A. Effects of hybrid inorganic-organic nanofibers on the properties of enamel resin infiltrants-An in vitro study. J. Mech. Behav. Biomed. Mater. 2022, 126, 105067. [Google Scholar] [CrossRef]
- Nóbrega, M.T.C.; Dantas, E.L.A.; Alonso, R.C.B.; Almeida, L.F.D.; Puppin-Rontani, R.M.; Sousa, F.B. Hydrolytic degradation of different infiltrant compositions within different histological zones of enamel caries like-lesions. Dent. Mater. J. 2020, 39, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Freitas, M.C.C.A.; Prakki, A.; Mosquim, V.; González, A.H.M.; Rios, D.; Honorio, H.M. Experimental self-etching resin infiltrants on the treatment of simulated carious white spot lesions. J. Mech. Behav. Biomed. Mater. 2021, 113, 104146. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Xie, Y.; Zhang, N.; Li, S.; Yang, K.; Zhu, M.; Weir, M.D.; Xu, H.H.K.; Zhang, K.; Zhao, Z.; et al. Novel nanostructured resin infiltrant containing calcium phosphate nanoparticles to prevent enamel white spot lesions. J. Mech. Behav. Biomed. Mater. 2022, 126, 104990. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Mertas, A.; Czuba, Z.P.; Skucha-Nowak, M. Study of cytotoxic properties of an experimental preparation with features of a dental infiltrant. Materials 2021, 14, 2442. [Google Scholar] [CrossRef]
- Fischer, M.; Scucha-Nowak, M.; Chmiela, B.; Korytkowska-Wałach, A. Assessment of the potential ability to penetrate into the hard tissues of the root of an experimental preparation with the characteristics of a dental infiltratant, enriched with an antimicrobial component-preliminary study. Materials 2021, 28, 5654. [Google Scholar] [CrossRef]
- Nowak-Wachol, A.; Korytkowska-Wałach, A.; Chmiela, B.; Wachol, K.; Łopaciński, M.; Wyszyńska, M.; Al-Dulaimi, Y.; Skucha-Nowak, M. Yttrium trifluoride as a marker of infiltration rate of decalcified root cementum: An in vitro study. Polymers 2022, 14, 780. [Google Scholar] [CrossRef] [PubMed]
- Prodan, D.; Moldovan, M.; Chisnoiu, A.M.; Saroși, C.; Cuc, S.; Filip, M.; Gheorghe, G.F.; Chisnoiu, R.M.; Furtos, G.; Cojocaru, I.; et al. Development of new experimental dental enamel resin infiltrants-synthesis and characterization. Materials 2022, 21, 803. [Google Scholar] [CrossRef]
- Bagheri, H.; Namdar, F.; Rivandi, M.H.; Asadi, S. Color properties of artificial white spot lesions treated by experimental resin infiltrants containing bioactive glass and nano-fluorohydroxyapatite. Int. J. Esthet. Dent. 2020, 5, 334–343. [Google Scholar]
- Hashemian, A.; Shahabi, S.; Behroozibakhsh, M.; Najafi, F.; Al-Bakhakh, B.A.J.; Hajizamani, H. A modified TEGDMA-based resin infiltrant using polyurethane acrylate oligomer and remineralising nano-fillers with improved physical properties and remineralisation potential. J. Dent. 2021, 113, 103810. [Google Scholar] [CrossRef]
- Paris, S.; Soviero, V.M.; Chatzidakis, A.J.; Meyer-Lueckel, H. Penetration of experimental infiltrants with different penetration coefficients and ethanol addition into natural caries lesions in primary molars. Caries Res. 2012, 46, 113–117. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Zhou, X.; Han, Q.; Zhou, W.; Liang, J.; Xu, H.H.K.; Ren, B.; Weir, M.D.; Li, M.; et al. Anti-caries effect of resin infiltrant modified by quaternary ammonium monomers. J. Dent. 2020, 97, 103355. [Google Scholar] [CrossRef] [PubMed]
- Nedeljkovic, I.; Abdelaziz, M.; Feilzer, A.J.; Szafert, S.; Gulia, N.; Dawaa, M.; Krejci, I.; Kleverlaan, C.J. Novel hybrid-glass-based material for infiltration of early caries lesions. Dent. Mater. 2022, 38, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Doméjean, S.; Ducamp, R.; Léger, S.; Holmgren, C. Resin infiltration of non-cavitated caries lesions: A systematic review. Med. Princ. Pract. 2015, 24, 216–221. [Google Scholar] [CrossRef]
- Mazzitelli, C.; Maravic, T.; Sabold, M.; Checchi, V.; Josic, U.; Breschi, L.; Mazzoni, A. Effect of shelf-life of a universal adhesive to dentin. Int. J. Adhes. Adhes. 2020, 102, 102673. [Google Scholar] [CrossRef]
- Mancuso, E.; Comba, A.; Mazzitelli, C.; Maravic, T.; Josic, U.; Del Bianco, F.; Tay, F.R.; Breschi, L.; Mazzoni, A. Bonding to dentin using an experimental zirconium oxynitrate etchant. J. Dent. 2021, 108, 103641. [Google Scholar] [CrossRef] [PubMed]
- Par, M.; Spanovic, N.; Mohn, D.; Attin, T.; Tauböck, T.T.; Tarle, Z. Curing potential of experimental resin composites filled with bioactive glass: A comparison between Bis-EMA and UDMA based resin systems. Dent. Mater. 2020, 36, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Rahiotis, C.; Zinelis, S.; Eliades, G.; Eliades, T. Setting characteristics of a resin infiltration system for incipient caries treatment. J. Dent. 2015, 43, 715–719. [Google Scholar] [CrossRef] [Green Version]
- Feitosa, V.P.; Sauro, S.; Ogliari, F.A.; Stansbury, J.W.; Carpenter, G.H.; Watson, T.F.; Sinhoreti, M.A.; Correr, A.B. The role of spacer carbon chain in acidic functional monomers on the physicochemical properties of self-etch dental adhesives. J. Dent. 2014, 42, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate- based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef]
- Gajewski, V.E.; Pfeifer, C.S.; Froes-Salgado, N.R.; Boaro, L.C.; Braga, R.R. Monomers used in resin composites: Degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar] [CrossRef]
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent advances and developments in composite dental restorative materials. J. Dent Res. 2011, 90, 402–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Malacarne, J.; Carvalho, R.M.; de Goes, M.F.; Svizero, N.; Pashley, D.H.; Tay, F.R.; Yiu, C.K.; Carrilho, M.R.O. Water sorption/solubility of dental adhesive resins. Dent. Mater. 2006, 22, 973–980. [Google Scholar] [CrossRef]
- Mazzitelli, C.; Monticelli, F.; Osorio, R.; Casucci, A.; Toledano, M.; Ferrari, M. Water sorption and solubility of different self-adhesive cements. Dent. Mater. 2009, 25, e37. [Google Scholar] [CrossRef]
- Park, J.; Eslick, J.; Ye, Q.; Misra, A.; Spencer, P. The influence of chemical structure on the properties in methacrylate-based dentin adhesives. Dent. Mater. 2011, 27, 1086–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslan, S.; Kaplan, M.H. The effect of resin infiltration on the progression of proximal caries lesions: A randomized clinical trial. Med. Princ. Pract. 2020, 29, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Cagetti, M.G.; Cattaneo, S.; Hu, Y.Q.; Campus, G. Amelogenesis imperfecta: A non- invasive approach to improve esthetics in young patients. Report of two cases. J. Clin. Pediatr. Dent. 2017, 41, 332–335. [Google Scholar] [CrossRef]
- Giannetti, L.; Murri Dello Diago, A.; Silingardi, G.; Spinas, E. Superficial infiltration to treat white hypomineralized defects of enamel: Clinical trial with 12- month follow-up. J. Biol. Regul. Homeost. Agents 2018, 32, 1335–1338. [Google Scholar]
- Tulunoglu, O.; Tulunoglu, I.F.; Antonson, S.A.; Campillo-Funollet, M.; Antonson, D.; Munoz-Viveros, C. Effectiveness of an infiltrant on sealing of composite restoration margins with/without artificial caries. J. Contemp. Dent. Pract. 2014, 15, 717–725. [Google Scholar] [CrossRef]
- Meyer-Lueckel, H.; Paris, S. Progression of artificial enamel caries lesions after infiltration with experimental light curing resins. Caries Res. 2008, 42, 117–124. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef] [PubMed]
- Kielbassa, A.M.; Ulrich, I.; Treven, l.; Mueller, J. An updated review on the resin infiltration technique of incipient proximal enamel lesions. Med. Evol. 2010, 7, 4–7. [Google Scholar]
- Mazzitelli, C.; Ionescu, A.; Josic, U.; Brambilla, E.; Breschi, L.; Mazzoni, A. Microbial contamination of resin composites inside their dispensers: An increased risk of cross-infection? J. Dent. 2022, 116, 103893. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.B.; Fernandes, A.R.; Coelho, A.S.; Marto, C.M.; Ferreira, M.M.; Caramelo, F.; do Vale, F.; Carrilho, E. Therapies for white spot lesions-a systematic review. J. Evid. Dent. Pract. 2017, 17, 23–38. [Google Scholar] [CrossRef]
- Fanfoni, L.; Marsich, E.; Turco, G.; Breschi, L.; Cadenaro, M. Development of di-methacrylate quaternary ammonium monomers with antibacterial activity. Acta Biomater. 2021, 15, 13847. [Google Scholar] [CrossRef]
- Josic, U.; Mazzitelli, C.; Maravic, T.; Radovic, I.; Jacimovic, J.; Mancuso, E.; Florenzano, F.; Breschi, L.; Mazzoni, A. The influence of selective enamel etch and self-etch mode of universal adhesives’ application on clinical behavior of composite restorations placed on non-carious cervical lesions: A systematic review and meta-analysis. Dent. Mater. 2022, 38, 472–488. [Google Scholar] [CrossRef]
- Josic, U.; Maravic, T.; Mazzitelli, C.; Del Bianco, F.; Mazzoni, A.; Breschi, L. The effect of chlorhexidine primer application on the clinical performance of composite restorations: A literature review. J. Esthet. Restor. Dent. 2021, 33, 69–77. [Google Scholar] [CrossRef]
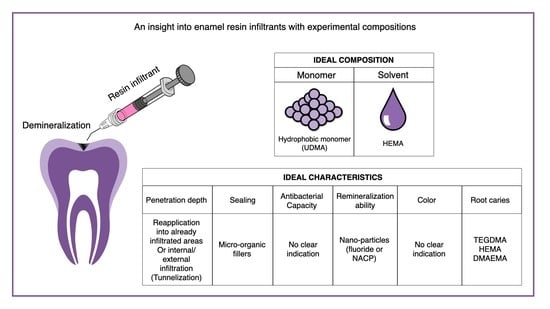
| Composition | Characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| Monomer | Solvent | Penetration Depth | Sealing | Antibacterial Capacity | Remineralization Ability | Color | Root Caries | |
| Ideal Resin Infiltrant | Hydrophobic monomer (UDMA) | HEMA | Re-application into already infiltrated areas or internal/external infiltration (tunnelization) | Micro-organic fillers | No clear indication | Nano-particles (fluoride or NACP) | No clear indication | TEGDMA/HEMA/ DMAEMA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzitelli, C.; Josic, U.; Maravic, T.; Mancuso, E.; Goracci, C.; Cadenaro, M.; Mazzoni, A.; Breschi, L. An Insight into Enamel Resin Infiltrants with Experimental Compositions. Polymers 2022, 14, 5553. https://doi.org/10.3390/polym14245553
Mazzitelli C, Josic U, Maravic T, Mancuso E, Goracci C, Cadenaro M, Mazzoni A, Breschi L. An Insight into Enamel Resin Infiltrants with Experimental Compositions. Polymers. 2022; 14(24):5553. https://doi.org/10.3390/polym14245553
Chicago/Turabian StyleMazzitelli, Claudia, Uros Josic, Tatjana Maravic, Edoardo Mancuso, Cecilia Goracci, Milena Cadenaro, Annalisa Mazzoni, and Lorenzo Breschi. 2022. "An Insight into Enamel Resin Infiltrants with Experimental Compositions" Polymers 14, no. 24: 5553. https://doi.org/10.3390/polym14245553