Calcium Phosphates–Chitosan Composite Layers Obtained by Combining Radio-Frequency Magnetron Sputtering and Matrix-Assisted Pulsed Laser Evaporation Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Titanium Substrates
2.1.2. Calcium Phosphate Tribasic Powder
2.1.3. Chitosan
2.2. Deposition Techniques
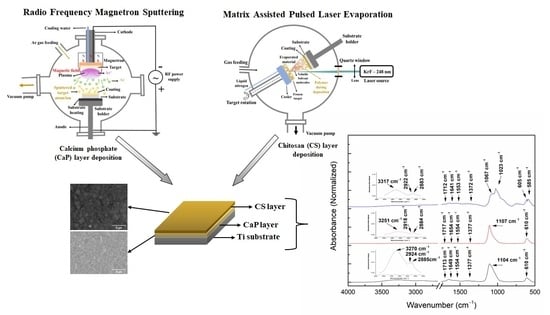
2.2.1. Calcium Phosphate Deposition by Radio-Frequency Magnetron Sputtering
2.2.2. Chitosan Deposition by Matrix-Assisted Pulsed Laser Evaporation
2.3. Characterization Techniques
3. Results and Discussion
3.1. X-ray Photoelectron Spectroscopy
3.1.1. XPS Analysis of CaP Coatings as a Function of Substrate Temperature
3.1.2. XPS Analysis of CaP_CS Coatings
3.2. Fourier Transform Infrared Spectroscopy
3.2.1. FTIR Analysis of CS Powder
3.2.2. Peak Fitting Analysis of FTIR Spectrum of CaP Powder
3.2.3. Peak Fitting Analysis of FTIR Spectra of CaP Coatings as Function of Substrate Temperature
3.2.4. Peak Fitting Analysis of FTIR Spectra of CaP_CS Samples
| Wavenumber (cm−1) | Wavenumber (cm−1) | FTIR Band Assignment | Ref. | ||||
|---|---|---|---|---|---|---|---|
| CaP_1 | CaP_2 | CaP_3 | CaP_1_CS | CaP_2_CS | CaP_3_CS | ||
| 1109 | 1126 1109 | 1112 | 1126 1109 | ν3 PO43− HPO4 incorporation | [46] | ||
| 1093 1073 1057 1025 | 1093 1074 1053 1025 | 1098 1077 1054 1028 1003 | 1093 1078 1052 1025 | 1094 1075 1053 1025 | 1098 1075 1052 1025 1003 | ν3 PO43− in Hap | [38,44,48,49,50,52] |
| 1039 | P-O-C/P-O bond vibrations | [39] | |||||
| 993 919 | 994 | 993 908 | ν3 PO43−/ν3-ν1 coupling/symmetric stretching of C-O | [53]/[8] | |||
| 945 | 945 | 943 | 945 | 945 | 943 | ν1 PO43− in Hap/stretching vibration of C-O | [45]/[42] |
| Wavenumber (cm−1) | FTIR Band Assignment | Ref. | ||
|---|---|---|---|---|
| CaP_1_CS | CaP_2_CS | CaP_3_CS | ||
| 3270 | 3251 | 3317 | polymeric O-H stretch or N-H | [39] |
| 2924 | 2918 | 2922 | C-H symmetric stretching | [8,41] |
| 2885 | 2884 | 2883 | C-H asymmetric stretching | [40,41] |
| 1713 | 1717 | 1712 | C=O | [33] |
| 1649 | 1654 | 1641 | stretching of (-C=O-) of amide I group | [8,41] |
| 1554 | 1554 | 1553 | bending of N-H | [39] |
| 1426 | 1428 | 1424 | bending of C-H in (-CH2)/asymmetric stretching of CO32− | [41]/[8] |
| 1377 | 1377 | 1372 | bending of C-H in (-CH3) | [8,41] |
| 1321 | 1318 | 1319 | C-N stretching of amide III | [41] |
3.3. X-ray Diffraction
3.4. Scanning Electron Microscopy
Surface
3.5. Energy Dispersive X-ray Spectroscopy
3.5.1. EDX Analysis of CaP Coatings
3.5.2. EDX Analysis of CaP_CS Coatings
3.6. SEM and EDX Analysis of CaP and CaP_CS Layers Adhesion by Tape Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium Phosphates in Biomedical Applications: Materials for the Future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; InTechOpen: London, UK, 2012. [Google Scholar]
- Brånemark, P.I.; Breine, U.; Adell, R.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-Osseous Anchorage of Dental Prostheses: I. Experimental Studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Duraisamy, R.; Ganapathy, D.; Shanmugam, R. Applications of Chitosan in Dental Implantology-a Literature Review. Int. J. Dent. Oral Sci. 2021, 8, 4140–4146. [Google Scholar] [CrossRef]
- Wang, J.; Van Apeldoorn, A.; De Groot, K. Electrolytic Deposition of Calcium Phosphate/Chitosan Coating on Titanium Alloy: Growth Kinetics and Influence of Current Density, Acetic Acid, and Chitosan. J. Biomed. Mater. Res. Part A 2006, 76A, 503–511. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Visan, A.; Stan, G.E.; Ristoscu, C.; Popescu-Pelin, G.; Sopronyi, M.; Besleaga, C.; Luculescu, C.; Chifiriuc, M.C.; Hussien, M.D.; Marsan, O.; et al. Combinatorial MAPLE Deposition of Antimicrobial Orthopedic Maps Fabricated from Chitosan and Biomimetic Apatite Powders. Int. J. Pharm. 2016, 511, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Neoh, K.G.; Lin, C.C.; Kishen, A. Biomimetic Deposition of Calcium Phosphate Minerals on the Surface of Partially Demineralized Dentine Modified with Phosphorylated Chitosan. J. Biomed. Mater. Res. -Part B Appl. Biomater. 2011, 98 B, 150–159. [Google Scholar] [CrossRef]
- Osman, R.B.; Swain, M.V. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Gao, Y.; Zhao, D.; Zhang, W.; Zhao, W.; Wu, M.; Cui, Y.; Li, Q.; Zhang, Z.; Ma, C. Titanium Implants and Local Drug Delivery Systems Become Mutual Promoters in Orthopedic Clinics. Nanomaterials 2022, 12, 47. [Google Scholar] [CrossRef]
- Groza, A.; Dreghici, D.B.; Ganciu, M. Calcium Phosphate Layers Deposited on Thermal Sensitive Polymer Substrates in Radio Frequency Magnetron Plasma Discharge. Coatings 2019, 9, 709. [Google Scholar] [CrossRef] [Green Version]
- Goreninskii, S.I.; Bogomolova, N.N.; Malchikhina, A.I.; Golovkin, A.S.; Bolbasov, E.N.; Safronova, T.V.; Putlyaev, V.I.; Tverdokhlebov, S.I. Biological Effect of the Surface Modification of the Fibrous Poly(L-Lactic Acid) Scaffolds by Radio Frequency Magnetron Sputtering of Different Calcium-Phosphate Targets. Bionanoscience 2017, 7, 50–57. [Google Scholar] [CrossRef]
- Surmenev, R.; Vladescu, A.; Surmeneva, M.; Ivanova, A.; Braic, M.; Grubova, I.; Cotrut, C.M. Radio Frequency Magnetron Sputter Deposition as a Tool for Surface Modification of Medical Implants. In Modern Technologies for Creating the Thin-film Systems and Coatings; IntechOpen: London, UK, 2017; ISBN 978-953-51-3004-8. [Google Scholar]
- Surmenev, R.A.; Surmeneva, M.A.; Grubova, I.Y.; Chernozem, R.V.; Krause, B.; Baumbach, T.; Loza, K.; Epple, M. RF Magnetron Sputtering of a Hydroxyapatite Target: A Comparison Study on Polytetrafluorethylene and Titanium Substrates. Appl. Surf. Sci. 2017, 414, 335–344. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Groza, A.; Gaiaschi, S.; Rokosz, K.; Raaen, S.; Negrila, C.C.; Prodan, A.M.; Costescu, A.; et al. Development of Cerium-Doped Hydroxyapatite Coatings with Antimicrobial Properties for Biomedical Applications. Coatings 2020, 10, 516. [Google Scholar] [CrossRef]
- Arnell, R.D.; Kelly, P.J. Recent Advances in Magnetron Sputtering. Surf. Coat. Technol. 1999, 112, 170–176. [Google Scholar] [CrossRef]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M.; Bîrcă, A.C.; Holban, A.M.; Urzica, I.; Avramescu, S.M.; Gălăţeanu, B.; Hudiţă, A. Nanostructured Thin Coatings Containing Anthriscus Sylvestris Extract with Dual Bioactivity. Molecules 2020, 25, 3866. [Google Scholar] [CrossRef]
- Mihailescu, N.; Stan, G.E.; Duta, L.; Chifiriuc, M.C.; Bleotu, C.; Sopronyi, M.; Luculescu, C.; Oktar, F.N.; Mihailescu, I.N. Structural, Compositional, Mechanical Characterization and Biological Assessment of Bovine-Derived Hydroxyapatite Coatings Reinforced with MgF2 or MgO for Implants Functionalization. Mater. Sci. Eng. C 2016, 59, 863–874. [Google Scholar] [CrossRef]
- Li, X.Y.; Nan, K.H.; Shi, S.; Chen, H. Preparation and Characterization of Nano-Hydroxyapatite/Chitosan Cross-Linking Composite Membrane Intended for Tissue Engineering. Int. J. Biol. Macromol. 2012, 50, 43–49. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J. Matrix-Assisted Pulsed Laser Evaporation (MAPLE) Technique for Deposition of Hybrid Nanostructures. Front. Nanosci. Nanotechnol. 2017, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Grumezescu, V.; Grumezescu, A.M.; Ficai, A.; Negut, I.; Vasile, B.Ș.; Gălățeanu, B.; Hudiță, A. Composite Coatings for Osteoblast Growth Attachment Fabricated by Matrix-Assisted Pulsed Laser Evaporation. Polymers 2022, 14, 2934. [Google Scholar] [CrossRef]
- Negut, I.; Ristoscu, C.; Tozar, T.; Dinu, M.; Parau, A.C.; Grumezescu, V.; Hapenciuc, C.; Popa, M.; Stan, M.S.; Marutescu, L.; et al. Implant Surfaces Containing Bioglasses and Ciprofloxacin as Platforms for Bone Repair and Improved Resistance to Microbial Colonization. Pharmaceutics 2022, 14, 1175. [Google Scholar] [CrossRef] [PubMed]
- Groza, A.; Surmeian, A. Characterization of the Oxides Present in a Polydimethylsiloxane Layer Obtained by Polymerisation of Its Liquid Precursor in Corona Discharge. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- ASTM D3359-09; Standard Test Methods for Measuring Adhesion by Tape Test. ASTM International: West Con-Shohocken, PA, USA, 2009. Available online: https://www.astm.org/d3359-09.html (accessed on 24 November 2022).
- López, E.O.; Mello, A.; Sendão, H.; Costa, L.T.; Rossi, A.L.; Ospina, R.O.; Borghi, F.F.; Silva Filho, J.G.; Rossi, A.M. Growth of Crystalline Hydroxyapatite Thin Films at Room Temperature by Tuning the Energy of the RF-Magnetron Sputtering Plasma. ACS Appl. Mater. Interfaces 2013, 5, 9435–9445. [Google Scholar] [CrossRef]
- Major, G.H.; Fairley, N.; Sherwood, P.M.A.; Linford, M.R.; Terry, J.; Fernandez, V.; Artyushkova, K. Practical Guide for Curve Fitting in X-Ray Photoelectron Spectroscopy. J. Vac. Sci. Technol. A 2020, 38, 061203. [Google Scholar] [CrossRef]
- Boyd, A.R.; O’Kane, C.; Meenan, B.J. Control of Calcium Phosphate Thin Film Stoichiometry Using Multi-Target Sputter Deposition. Surf. Coat. Technol. 2013, 233, 131–139. [Google Scholar] [CrossRef]
- Uskoković, V. X-Ray Photoelectron and Ion Scattering Spectroscopic Surface Analyses of Amorphous and Crystalline Calcium Phosphate Nanoparticles with Different Chemical Histories. Phys. Chem. Chem. Phys. 2020, 22, 5531–5547. [Google Scholar] [CrossRef] [PubMed]
- Okawa, S.; Homma, K.; Kanatani, M.; Watanabe, K. Characterization of Calcium Phosphate Deposited on Valve Metal by Anodic Oxidation with Polarity Inversion. Dent. Mater. J. 2009, 28, 513–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectrsocopy: A Reference Book of Standard Spectra for Identification and Interpretation of Xps Data; Chastain, J., Ed.; Perkin-Elmer Corporation and Physical Electronics Division: Chichester, UK, 1992; ISBN 9780470014226. [Google Scholar]
- Praserthdam, S.; Rittiruam, M.; Maungthong, K.; Saelee, T.; Somdee, S.; Praserthdam, P. Performance Controlled via Surface Oxygen-Vacancy in Ti-Based Oxide Catalyst during Methyl Oleate Epoxidation. Sci. Rep. 2020, 10, 18952. [Google Scholar] [CrossRef]
- Dreghici, D.B.; Butoi, B.; Predoi, D.; Iconaru, S.L.; Stoican, O.; Groza, A. Chitosan–Hydroxyapatite Composite Layers Generated in Radio Frequency Magnetron Sputtering Discharge: From Plasma to Structural and Morphological Analysis of Layers. Polymers 2020, 12, 3065. [Google Scholar] [CrossRef]
- Bensalem, S.; Hamdi, B.; Del Confetto, S.; Iguer-Ouada, M.; Chamayou, A.; Balard, H.; Calvet, R. Characterization of Chitosan/Montmorillonite Bionanocomposites by Inverse Gas Chromatography. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 516, 336–344. [Google Scholar] [CrossRef]
- Saman, N.; Johari, K.; Kong, H.; Mohtar, S.S.; Hassan, O.; Ali, N.; Mat, H. Enhanced Elemental Mercury Removal by Facile Sulfurization of Agrowaste Chars. Chem. Eng. Res. Des. 2019, 144, 198–208. [Google Scholar] [CrossRef]
- Vandecandelaere, N.; Rey, C.; Drouet, C. Biomimetic Apatite-Based Biomaterials: On the Critical Impact of Synthesis and Post-Synthesis Parameters. J. Mater. Sci. Mater. Med. 2012, 23, 2593–2606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.K.; Leoni, M.; Maniglio, D.; Migliaresi, C. Hydroxyapatite Nanorods: Soft-Template Synthesis, Characterization and Preliminary in Vitro Tests. J. Biomater. Appl. 2013, 28, 49–61. [Google Scholar] [CrossRef]
- Delgado-López, J.M.; Iafisco, M.; Rodríguez, I.; Tampieri, A.; Prat, M.; Gómez-Morales, J. Crystallization of Bioinspired Citrate-Functionalized Nanoapatite with Tailored Carbonate Content. Acta Biomater. 2012, 8, 3491–3499. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Drabczyk, A.; Kudłacik-Kramarczyk, S.; Głab, M.; Kedzierska, M.; Jaromin, A.; Mierzwiński, D.; Tyliszczak, B. Physicochemical Investigations of Chitosan-Based Hydrogels Containing Aloe Vera Designed for Biomedical Use. Materials 2020, 13, 3073. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the Use of Chitosan Contribute to Oxalate Kidney Stone Formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.D.S.; Perim, R.B.; de Alvarenga, E.S.; Guimarães, L.D.M.; Teixeira, A.V.N.D.C.; dos Reis Coimbra, J.S.; de Oliveira, E.B. Insights on Physicochemical Aspects of Chitosan Dispersion in Aqueous Solutions of Acetic, Glycolic, Propionic or Lactic Acid. Int. J. Biol. Macromol. 2019, 128, 140–148. [Google Scholar] [CrossRef]
- Radwan-Pragłowska, J.; Janus, Ł.; Piątkowski, M.; Sierakowska, A.; Matysek, D. ZnO Nanorods Functionalized with Chitosan Hydrogels Crosslinked with Azelaic Acid for Transdermal Drug Delivery. Colloids Surf. B Biointerfaces 2020, 194, 111170. [Google Scholar] [CrossRef]
- Li, J. Structural Characterisation of Apatite-like Materials. Master’s Thesis, University of Birmingham, Birmingham, UK, 2009. [Google Scholar]
- Albu, P.; Vlase, G.; Vlase, T. Study of Bioactive Hydroxyapatite/Gelatin Composite. Part I - Synthesis and Characterization of the Material. New Front. Chem. 2015, 24, 47–53. [Google Scholar]
- Butler, D.H.; Shahack-Gross, R. Formation of Biphasic Hydroxylapatite-Beta Magnesium Tricalcium Phosphate in Heat Treated Salmonid Vertebrae. Sci. Rep. 2017, 7, 3610. [Google Scholar] [CrossRef] [Green Version]
- Samantaray, S.K.; Parida, K.M. Effect of Anions on the Textural and Catalytic Activity of Titania. J. Mater. Sci. 2003, 38, 1835–1848. [Google Scholar] [CrossRef]
- Iconaru, S.L.; Predoi, D.; Ciobanu, C.S.; Motelica-Heino, M.; Guegan, R.; Bleotu, C. Development of Silver Doped Hydroxyapatite Thin Films for Biomedical Applications. Coatings 2022, 12, 341. [Google Scholar] [CrossRef]
- Popa, C.L.; Ciobanu, C.S.; Voicu, G.; Vasile, E.; Chifiriuc, M.C.; Iconaru, S.L.; Predoi, D. Influence of Thermal Treatment on the Antimicrobial Activity of Silver-Doped Biological Apatite. Nanoscale Res. Lett. 2015, 10, 502. [Google Scholar] [CrossRef] [Green Version]
- Asaduzzaman, S.M. Extraction of Hydroxyapatite from Bovine and Human Cortical Bone by Thermal Decomposition and Effect of Gamma Radiation: A Comparative Study. Int. J. Complement. Altern. Med. 2017, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Stanislavov, A.S.; Sukhodub, L.F.; Sukhodub, L.B.; Kuznetsov, V.N.; Bychkov, K.L.; Kravchenko, M.I. Structural Features of Hydroxyapatite and Carbonated Apatite Formed under the Influence of Ultrasound and Microwave Radiation and Their Effect on the Bioactivity of the Nanomaterials. Ultrason. Sonochem. 2018, 42, 84–96. [Google Scholar] [CrossRef]
- Mróz, W.; Bombalska, A.; Budner, B.; Burdyńska, S.; Jedyński, M.; Prokopiuk, A.; Menaszek, E.; Ścisłowska-Czarnecka, A.; Niedzielska, A.; Niedzielski, K. Comparative Study of Hydroxyapatite and Octacalcium Phosphate Coatings Deposited on Metallic Implants by PLD Method. Appl. Phys. A Mater. Sci. Process. 2010, 101, 713–716. [Google Scholar] [CrossRef] [Green Version]
- Tsiourvas, D.; Tsetsekou, A.; Arkas, M.; Diplas, S.; Mastrogianni, E. Covalent Attachment of a Bioactive Hyperbranched Polymeric Layer to Titanium Surface for the Biomimetic Growth of Calcium Phosphates. J. Mater. Sci. Mater. Med. 2011, 22, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szatkowski, T.; Kołodziejczak-Radzimska, A.; Zdarta, J.; Szwarc-Rzepka, K.; Paukszta, D.; Wysokowski, M.; Ehrlich, H.; Jesionowski, T. Synthesis and Characterization of Hydroxyapatite/Chitosan Composites. Physicochem. Probl. Miner. Process. 2015, 51, 575–585. [Google Scholar] [CrossRef]
- Danilchenko, S.N. Chitosan–Hydroxyapatite Composite Biomaterials Made by a One Step Co-Precipitation Method: Preparation, Characterization and in Vivo Tests. J. Biol. Phys. Chem. 2009, 9, 119–126. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, E.S.M.; Omar, A.; Ibrahim, M.; Abdel-Fattah, W.I. On the Structural Analysis and Electronic Properties of Chitosan/Hydroxyapatite Interaction. J. Comput. Theor. Nanosci. 2009, 6, 1663–1669. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abdel-Fattah, W.I.; El-Sayed, E.-S.M.; Omar, A. A Novel Model for Chitosan/Hydroxyapatite Interaction. Quantum Matter 2013, 2, 234–237. [Google Scholar] [CrossRef]
- Xianmiao, C.; Yubao, L.; Yi, Z.; Li, Z.; Jidong, L.; Huanan, W. Properties and in Vitro Biological Evaluation of Nano-Hydroxyapatite/Chitosan Membranes for Bone Guided Regeneration. Mater. Sci. Eng. C 2009, 29, 29–35. [Google Scholar] [CrossRef]
- Ansari, Z.; Kalantar, M.; Soriente, A.; Fasolino, I.; Kharaziha, M.; Ambrosio, L.; Raucci, M.G. In-Situ Synthesis and Characterization of Chitosan/Hydroxyapatite Nanocomposite Coatings to Improve the Bioactive Properties of Ti6Al4V Substrates. Materials 2020, 13, 3772. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Kim, S.K. Chitosan Composites for Bone Tissue Engineering—An Overview. Mar. Drugs 2010, 8, 2252–2266. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, S.; Gupta, A.; Mohanta, T.; Mitra, R.; Samanta, D.; Mandal, A.B.; Majumder, M.; Rawat, R.; Singha, N.R. Scalable Synthesis of Hide Substance-Chitosan-Hydroxyapatite: Novel Biocomposite from Industrial Wastes and Its Efficiency in Dye Removal. ACS Omega 2018, 3, 11486–11496. [Google Scholar] [CrossRef] [Green Version]
- Surmeneva, M.A.; Surmenev, R.A.; Nikonova, Y.A.; Selezneva, I.I.; Ivanova, A.A.; Putlyaev, V.I.; Prymak, O.; Epple, M. Fabrication, Ultra-Structure Characterization and in Vitro Studies of RF Magnetron Sputter Deposited Nano-Hydroxyapatite Thin Films for Biomedical Applications. Appl. Surf. Sci. 2014, 317, 172–180. [Google Scholar] [CrossRef]
- Pichugin, V.F.; Surmenev, R.A.; Shesterikov, E.V.; Ryabtseva, M.A.; Eshenko, E.V.; Tverdokhlebov, S.I.; Prymak, O.; Epple, M. The Preparation of Calcium Phosphate Coatings on Titanium and Nickel–Titanium by Rf-Magnetron-Sputtered Deposition: Composition, Structure and Micromechanical Properties. Surf. Coat. Technol. 2008, 202, 3913–3920. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Chaikina, M.V.; Zaikovskiy, V.I.; Pichugin, V.F.; Buck, V.; Prymak, O.; Epple, M.; Surmenev, R.A. The Structure of an RF-Magnetron Sputter-Deposited Silicate-Containing Hydroxyapatite-Based Coating Investigated by High-Resolution Techniques. Surf. Coat. Technol. 2013, 218, 39–46. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Ryabtseva, M.A.; Shesterikov, E.V.; Pichugin, V.F.; Peitsch, T.; Epple, M. The Release of Nickel from Nickel–Titanium (NiTi) Is Strongly Reduced by a Sub-Micrometer Thin Layer of Calcium Phosphate Deposited by Rf-Magnetron Sputtering. J. Mater. Sci. Mater. Med. 2010, 21, 1233–1239. [Google Scholar] [CrossRef]
- Nelea, V.; Morosanu, C.; Iliescu, M.; Mihailescu, I.N. Hydroxyapatite Thin Films Grown by Pulsed Laser Deposition and Radio-Frequency Magnetron Sputtering: Comparative Study. Appl. Surf. Sci. 2004, 228, 346–356. [Google Scholar] [CrossRef]
- Fraile-martínez, O.; García-montero, C.; Coca, A.; Álvarez-mon, M.A.; Monserrat, J.; Gómez-lahoz, A.M.; Coca, S.; Álvarez-mon, M.; Acero, J.; Bujan, J.; et al. Applications of Polymeric Composites in Bone Tissue Engineering and Jawbone Regeneration. Polymers 2021, 13, 3429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, X.; Mao, M.; Li, J.; Wei, T.; Sun, H. Chitosan/Hydroxyapatite Composite Coatings on Porous Ti6Al4V Titanium Implants: In Vitro and in Vivo Studies. J. Periodontal Implant Sci. 2020, 50, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xia, X.; Guo, M.; Jiang, Y.; Li, Y.; Zhang, Z.; Liu, S.; Li, H.; Liang, C.; Wang, H. Biological and Antibacterial Properties of the Micro-Nanostructured Hydroxyapatite/Chitosan Coating on Titanium. Sci. Rep. 2019, 9, 14052. [Google Scholar] [CrossRef] [PubMed]


















| XPS Peaks | CaP_1 (eV) | CaP_2 (eV) | CaP_3 (eV) |
|---|---|---|---|
| Ca 2p3/2 | 347.2; 348.4 | 347; 348.3 | 347; 348.3 |
| Ca 2p1/2 | 350.7; 351.9 | 350.5; 351.8 | 350.5; 351.8 |
| P 2p3/2 | 133.1 | 132.9 | 132.9 |
| P 2p1/2 | 134 | 133.75 | 133.8 |
| O 1s | 530.4 | 530.4 | 530.4 |
| 531.2 | 531.1 | 531 | |
| 532.6 | 532.6 | 532.7 | |
| 534.1 | 534.3 | 534.6 | |
| C 1s | 284.6 | 284.6 | 284.6 |
| 286.3 | 286.3 | 286.3 | |
| 288.5 | 288.1 | 288.5 | |
| Ti 2p | 461.9 | 461.9 | 461.9 |
| XPS Lines | CaP_1_CS (eV) | CaP_2_CS (eV) | CaP_3_CS (eV) |
|---|---|---|---|
| Ca 2p | 350.7; 354.2 | - | - |
| P 2p | 135.3 | - | - |
| O 1s | 531.2 | 531.1 | 531 |
| 532.3 | 532.3 | 532.1 | |
| 533.2 | 533.2 | 532.7 | |
| C 1s | 284.6 | 284.6 | 284.6 |
| 285.7 | 285.9 | 285.9 | |
| 286.8 | 286.7 | 286.9 | |
| 288.8 | 288.3 | 288.7 | |
| N 1s | 399 | 399.1 | 399.1 |
| 400 | 400 | 400.5 | |
| 402.5 | 402.9 | 404.5 | |
| Ti 2p | 460.4; 466.2 | - | - |
| CaP_1 | CaP_2 | CaP_3 |
| 1.69 ± 0.02 | 1.86 ± 0.04 | 1.93 ± 0.09 |
| CaP_1_CS | CaP_2_CS | CaP_3_CS |
| 1.57 ± 0.02 | 1.70 ± 0.05 | 1.97± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarif, M.E.; Yehia-Alexe, S.A.; Bita, B.; Negut, I.; Locovei, C.; Groza, A. Calcium Phosphates–Chitosan Composite Layers Obtained by Combining Radio-Frequency Magnetron Sputtering and Matrix-Assisted Pulsed Laser Evaporation Techniques. Polymers 2022, 14, 5241. https://doi.org/10.3390/polym14235241
Zarif ME, Yehia-Alexe SA, Bita B, Negut I, Locovei C, Groza A. Calcium Phosphates–Chitosan Composite Layers Obtained by Combining Radio-Frequency Magnetron Sputtering and Matrix-Assisted Pulsed Laser Evaporation Techniques. Polymers. 2022; 14(23):5241. https://doi.org/10.3390/polym14235241
Chicago/Turabian StyleZarif, Maria Elena, Sasa Alexandra Yehia-Alexe, Bogdan Bita, Irina Negut, Claudiu Locovei, and Andreea Groza. 2022. "Calcium Phosphates–Chitosan Composite Layers Obtained by Combining Radio-Frequency Magnetron Sputtering and Matrix-Assisted Pulsed Laser Evaporation Techniques" Polymers 14, no. 23: 5241. https://doi.org/10.3390/polym14235241