Emerging Applications of Versatile Polyaniline-Based Polymers in the Food Industry
Abstract
:1. Introduction
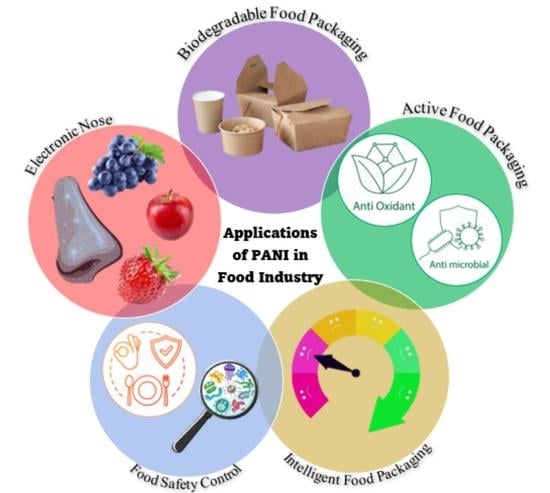
2. Applications of PANI in the Food Industry
2.1. Biodegradable Food Packaging
| Matrix | Preparation | Results | Ref |
|---|---|---|---|
| Chitosan | In situ polymerisation |
| [78] |
| [79] | ||
| WPI | Ex situ polymerisation |
| [80] |
| Starch | Ex situ polymerisation |
| [6] |
| Starch/ZnO NPs | Impregnation on ZnO NP |
| [85] |
| CNF | In situ polymerisation |
| [81] |
| Cellulose acetate | Ex situ polymerisation |
| [86] |
| CNWs | In situ polymerisation |
| [82] |
| PLA | Ex situ polymerisation |
| [29] |
| [83] |
2.2. Active Food Packaging
2.3. Intelligent Food Packaging
2.4. Food Safety Control
| Contaminant | Device | Target | Food | Ref |
|---|---|---|---|---|
| Antibiotic | β-Lactamase immobilised on PANI-coated optical fibre | β-lactam antibiotics | Packaged milk, cow milk, buffalo milk | [147] |
| SPME based on Cu/PANI/GO coupled with HPLC-UV | DOX, OXY, TET | Water, pasteurised bovine milk | [150] | |
| MSPE based on RA/MMPANI/HM/CAS coupled with HPLC | DOX, OXY, TPM, PEN-G | Milk | [149] | |
| PANI NW-based MIP deposited on a gold electrode | CHL | - | [148] | |
| PANI/GO/QD-based MIP probe | LOM | Milk, chicken meat, egg | [167] | |
| NSAID | SPE based on PANI NFM coupled with UPLC-MS/MS | IBU, NAP, DC, CPF, KTP, TLF, SA | Meat, egg | [152] |
| CS/PANI/ZnAl-LDH | NAP | - | [154] | |
| Au/PANI-based MIP membrane | IBU | - | [153] | |
| SPME based on graphene/PANI coupled with IMS | MFA, IBU | - | [168] | |
| Heavy metal ions | MSPE based on PANI-coated magnetic NPs coupled with FAAS | Co(II) | Soft drinks, spices, vegetables, water | [169] |
| PAN/PANI membrane | Pb(II), Cr(VI) | Water | [163] | |
| Electrochemically assisted SPE based on PANI NF/GO coupled with FAAS | Pb(II) | Juices (peach, orange, grape), water (tap, mineral) | [162] | |
| MSPE based on SiO2-magnetic GO/PANI/PPy coupled with ICP-MS | Cr(III), Pb(II) | Water, rice, milk, wine | [156] | |
| SPE based on nanostructured PANI coupled with FAAS | Cu(II), Pb(II) | Shrimp, crab, fish, apple, tomato, mushroom, potato, water | [170] |
2.5. Electronic Noses
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gouveia Alonso, P.E. Alternative Methods for the Synthesis of Electrically Conductive Bacterial Cellulose-Polyaniline Composites for Potential Drug Delivery Application. Master Dissertation, Universidade da Madeira, Funchal, Portugal, 2017. [Google Scholar]
- Saeb, M.R.; Zarrintaj, P.; Khandelwal, P.; Chauhan, N.P.S. Synthetic Route of Polyaniline (I): Conventional Oxidative Polymerization; Elsevier Inc.: Oxford, UK, 2019. [Google Scholar]
- Giacobbe, S.; Pezzella, C.; Della Ventura, B.; Giacobelli, V.G.; Rossi, M.; Fontanarosa, C.; Amoresano, A.; Sannia, G.; Velotta, R.; Piscitelli, A. Green synthesis of conductive polyaniline by Trametes versicolor laccase using a DNA template. Eng. Life Sci. 2019, 19, 631–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapurina, I.; Shishov, M.A. The mechanism of the oxidative polymerization of aniline and the formation of supramolecular polyaniline structures. In New Polymers for Special Applications; InTechOpen: Rijeka, Croatia, 2012; Volume 9, pp. 251–312. [Google Scholar]
- Ramakrishnaiah, T.; Gunderi Dhananjaya, P.; Vakwadi Sainagesh, C.; Reddy, S.; Kumaraswamy, S.; Chikkahanumajja Surendranatha, N. A review: Electrical and gas sensing properties of polyaniline/ferrite nanocomposites. Sens. Rev. 2022, 42, 164–175. [Google Scholar] [CrossRef]
- Chia, M.; Ahmad, I.; Phang, S. Starch/Polyaniline Biopolymer Film as Potential Intelligent Food Packaging with Colourimetric Ammonia Sensor. Polymers 2022, 14, 1122. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, A.; Ramedani, A.; Abrishamkar, A.; Milan, P.B.; Moghadan, Z.S.; Chauhan, N.P.S.; Sefat, F.; Mozafari, M. Synthetic route of PANI (V): Electrochemical polymerization. In Fundamentals and Emerging Applications of Polyaniline; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 105–119. [Google Scholar]
- Gandara, M.; Dalmolin, C.; Gonçalves, E.S. Physicochemical interactions between polyaniline and graphene oxide: The reasons for the stability of their chemical structure and thermal properties. Mater. Today Chem. 2021, 22, 100627. [Google Scholar] [CrossRef]
- Zhou, K.L.; Wang, H.; Jiu, J.T.; Liu, J.B.; Yan, H.; Suganuma, K. Polyaniline films with modified nanostructure for bifunctional flexible multicolor electrochromic and supercapacitor applications. Chem. Eng. J. 2018, 345, 290–299. [Google Scholar] [CrossRef]
- Mažeikienė, R.; Niaura, G.; Malinauskas, A. Study of redox and protonation processes of polyaniline by the differential multiwavelength Raman spectroelectrochemistry. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2019, 221, 117147. [Google Scholar] [CrossRef]
- Mažeikienė, R.; Niaura, G.; Malinauskas, A. A comparative multiwavelength Raman spectroelectrochemical study of polyaniline: A review. J. Solid State Electrochem. 2019, 23, 1631–1640. [Google Scholar] [CrossRef]
- Walde, P.; Kashima, K.; Ćirić-Marjanović, G. Synthesizing polyaniline with laccase/O2 as catalyst. Front. Bioeng. Biotechnol. 2019, 7, 165. [Google Scholar] [CrossRef] [PubMed]
- Mello, H.J.N.P.D.; Mulato, M. Enzymatically functionalized polyaniline thin films produced with one-step electrochemical immobilization and its application in glucose and urea potentiometric biosensors. Biomed. Microdevices 2020, 22, 22. [Google Scholar] [CrossRef]
- Al-Thani, N.; Hassan, M.K.; Bhadra, J. Polyaniline/Polystyrene Blends: In-Depth Analysis of the Effect of Sulfonic Acid Dopant Concentration on AC Conductivity Using Broadband Dielectric Spectroscopy. Int. J. Polym. Sci. 2018, 2018, 1416531. [Google Scholar] [CrossRef]
- Karaoğlan, N.; Bindal, C. Synthesis and optical characterization of benzene sulfonic acid doped polyaniline. Eng. Sci. Technol. Int. J. 2018, 21, 1152–1158. [Google Scholar] [CrossRef]
- Sharma, A.; Goyal, P.K.; Rawal, I.; Rajpal, A.; Khokhar, A.; Kumar, V.; Dahiya, S. Structural characteristics and opto-electrical properties of in-situ synthesized polyaniline films. Opt. Mater. 2022, 131, 112712. [Google Scholar] [CrossRef]
- Mahato, N.; Mohapatra, D.; Cho, M.H.; Ahn, K.S. Semi-Polycrystalline–Polyaniline Empowered Electrochemical Capacitor. Energies 2022, 15, 2001. [Google Scholar] [CrossRef]
- Hosseini, H.; Mousavi, S.M. Density functional theory simulation for Cr(VI) removal from wastewater using bacterial cellulose/polyaniline. Int. J. Biol. Macromol. 2020, 165, 883–901. [Google Scholar] [CrossRef] [PubMed]
- Ciric-Marjanovic, G. Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications. Synth. Met. 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Ugraskan, V.; Karaman, F. Polyaniline/Graphitic Carbon Nitride Nanocomposites with Improved Thermoelectric Properties. J. Electron. Mater. 2021, 50, 3455–3461. [Google Scholar] [CrossRef]
- Toušek, J.; Rutsch, R.; Toušková, J. Explanation of the high conductivity of HCl protonated polyaniline films. Mater. Chem. Phys. 2021, 260, 124153. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.; Zeng, G.; Gong, X.; Xue, W.; Li, J.; Yang, Y.; Zhou, C.; Li, Z.; Yan, X.; et al. Modifying delafossite silver ferrite with polyaniline: Visible-light-response Z-scheme heterojunction with charge transfer driven by internal electric field. Chem. Eng. J. 2019, 370, 1087–1100. [Google Scholar] [CrossRef]
- Hamdy, M.S.; Abd-Rabboh, H.S.M.; Benaissa, M.; Al-Metwaly, M.G.; Galal, A.H.; Ahmed, M.A. Fabrication of novel polyaniline/ZnO heterojunction for exceptional photocatalytic hydrogen production and degradation of fluorescein dye through direct Z-scheme mechanism. Opt. Mater. 2021, 117, 111198. [Google Scholar] [CrossRef]
- Ali Mohsin, M.E.; Shrivastava, N.K.; Arsad, A.; Basar, N.; Hassan, A. The Effect of pH on the Preparation of Electrically Conductive and Physically Stable PANI/Sago Blend Film via in situ Polymerization. Front. Mater. 2020, 7, 20. [Google Scholar] [CrossRef]
- Mirmohseni, A.; Azizi, M.; Dorraji, M.S.S. Cationic graphene oxide nanosheets intercalated with polyaniline nanofibers: A promising candidate for simultaneous anticorrosion, antistatic, and antibacterial applications. Prog. Org. Coat. 2020, 139, 105419. [Google Scholar] [CrossRef]
- Shim, G.; Tran, M.X.; Liu, G.; Byun, D.; Lee, J.K. Flexible, fiber-shaped, quasi-solid-state Zn-polyaniline batteries with methanesulfonic acid-doped aqueous gel electrolyte. Energy Storage Mater. 2021, 35, 739–749. [Google Scholar] [CrossRef]
- Ahn, S.; Park, M.H.; Jeong, S.H.; Kim, Y.H.; Park, J.; Kim, S.; Kim, H.; Cho, H.; Wolf, C.; Pei, M.; et al. Fine Control of Perovskite Crystallization and Reducing Luminescence Quenching Using Self-Doped Polyaniline Hole Injection Layer for Efficient Perovskite Light-Emitting Diodes. Adv. Funct. Mater. 2019, 29, 1807535. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Jiang, Y.; Duan, Z.; Liu, B.; Zhao, Q.; Wang, S.; Yuan, Z.; Tai, H. Ultrasensitive flexible NH3 gas sensor based on polyaniline/SrGe4O9 nanocomposite with ppt-level detection ability at room temperature. Sens. Actuators B Chem. 2020, 319, 128293. [Google Scholar] [CrossRef]
- Wong, P.Y.; Phang, S.W.; Baharum, A. Effects of synthesised polyaniline (PAni) contents on the anti-static properties of PAni-based polylactic acid (PLA) films. RSC Adv. 2020, 10, 39693–39699. [Google Scholar] [CrossRef] [PubMed]
- Zailan, F.D.; Chen, R.S.; Shahdan, D.; Ahmad, S. Effect of conductive polyaniline in thermoplastic natural rubber blends on the mechanical, thermal stability, and electrical conductivity properties. J. Appl. Polym. Sci. 2019, 136, 47525. [Google Scholar] [CrossRef]
- Nepomuceno, N.C.; Seixas, A.A.A.; Medeiros, E.S.; Mélo, T.J.A. Evaluation of conductivity of nanostructured polyaniline/cellulose nanocrystals (PANI/CNC) obtained via in situ polymerization. J. Solid State Chem. 2021, 302, 122372. [Google Scholar] [CrossRef]
- Zailan, F.D.; Chen, R.S.; Ahmad, S.; Shahdan, D.; Ali, A.M.; Mohd Ruf, M.F.H. Blends of linear low-density polyethylene, natural rubber and polyaniline: Tensile properties and thermal stability. Malays. J. Anal. Sci. 2018, 22, 999–1006. [Google Scholar]
- Tanguy, N.R.; Thompson, M.; Yan, N. A review on advances in application of polyaniline for ammonia detection. Sens. Actuators B Chem. 2018, 257, 1044–1064. [Google Scholar] [CrossRef]
- Kumari Jangid, N.; Jadoun, S.; Kaur, N. A review on high-throughput synthesis, deposition of thin films and properties of polyaniline. Eur. Polym. J. 2020, 125, 109485. [Google Scholar] [CrossRef]
- Laourari, I.; Lakhdari, N.; Belgherbi, O.; Medjili, C.; Berkani, M.; Vasseghian, Y.; Golzadeh, N.; Lakhdari, D. Antimicrobial and antifungal properties of NiCu-PANI/PVA quaternary nanocomposite synthesized by chemical oxidative polymerization of polyaniline. Chemosphere 2022, 291, 132696. [Google Scholar] [CrossRef]
- Mišurović, J.; Mojović, M.; Marjanović, B.; Vulić, P.; Ćirić-Marjanović, G. Magnetite nanoparticles-catalysed synthesis of conductive polyaniline. Synth. Met. 2019, 257, 116174. [Google Scholar] [CrossRef]
- Gizdavic-Nikolaidis, M.; Pupe, J.M.; Silva, L.P.; Stanisavljev, D.; Svirskis, D.; Swift, S. Composition tuning of scalable antibacterial polyaniline/chitosan composites through rapid enhanced microwave synthesis. Mater. Chem. Phys. 2022, 278, 125676. [Google Scholar] [CrossRef]
- Sapurina, I.; Bubulinca, C.; Trchová, M.; Prokeš, J.; Stejskal, J. Solid manganese dioxide as heterogeneous oxidant of aniline in the preparation of conducting polyaniline or polyaniline/manganese dioxide composites. Colloids Surf. A Physicochem. Eng. Asp. 2022, 638, 128298. [Google Scholar] [CrossRef]
- Kumar, A.; Mudila, H. Structural characterization of polyaniline. Plant Arch. 2020, 20, 2670–2672. [Google Scholar]
- Shim, E.; Noro, J.; Cavaco-Paulo, A.; Silva, C.; Kim, H.R. Effect of additives on the in situ laccase-catalyzed polymerization of aniline onto bacterial cellulose. Front. Bioeng. Biotechnol. 2019, 7, 264. [Google Scholar] [CrossRef] [PubMed]
- Abalyaeva, V.V.; Dremova, N.N. Electrochemical Behavior of Polyaniline in the Presence of the Vanadate Anion. Russ. J. Electrochem. 2019, 55, 850–859. [Google Scholar] [CrossRef]
- Mezhuev, Y.O.; Korshak, Y.V. Theory of chain growth in chemical oxidative polymerization of aniline derivatives. Synth. Met. 2020, 267, 116445. [Google Scholar] [CrossRef]
- Anjalin, M.; Kanagathara, N.; Baby Suganthi, A.R. A brief review on aniline and its derivatives. Mater. Today Proc. 2020, 33, 4751–4755. [Google Scholar] [CrossRef]
- Vujković, M.J.; Etinski, M.; Vasić, B.; Kuzmanović, B.; Bajuk-Bogdanović, D.; Dominko, R.; Mentus, S. Polyaniline as a charge storage material in an aqueous aluminum-based electrolyte: Can aluminum ions play the role of protons? J. Power Sources 2021, 482, 228937. [Google Scholar] [CrossRef]
- Prasutiyo, Y.J.; Manaf, A.; Hafizah, M.A.E. Synthesis of polyaniline by chemical oxidative polymerization and characteristic of conductivity and reflection for various strong acid dopants. J. Phys. Conf. Ser. 2020, 1442, 012003. [Google Scholar] [CrossRef]
- Ingle, R.V.; Shaikh, S.F.; Bhujbal, P.K.; Pathan, H.M.; Tabhane, V.A. Polyaniline Doped with Protonic Acids: Optical and Morphological Studies. ES Mater. Manuf. 2020, 8, 54–59. [Google Scholar] [CrossRef]
- Monicka, K.; Polpaya, I.C.; Varughese, S. Sulphosuccinic acid as a novel dopant for solution processable polyaniline. Mater. Chem. Phys. 2018, 217, 357–364. [Google Scholar] [CrossRef]
- Korent, A.; Žagar Soderžnik, K.; Šturm, S.; Žužek Rožman, K. A Correlative Study of Polyaniline Electropolymerization and its Electrochromic Behavior. J. Electrochem. Soc. 2020, 167, 106504. [Google Scholar] [CrossRef]
- Kalotra, S.; Mehta, R. Synthesis of polyaniline/clay nanocomposites by in situ polymerization and its application for the removal of Acid Green 25 dye from wastewater. Polym. Bull. 2021, 78, 2439–2463. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Humpolíček, P.; Kašpárková, V.; Pacherník, J.; Stejskal, J.; Bober, P.; Capáková, Z.; Radaszkiewicz, K.A.; Junkar, I.; Lehocký, M. The biocompatibility of polyaniline and polypyrrole: A comparative study of their cytotoxicity, embryotoxicity and impurity profile. Mater. Sci. Eng. C 2018, 91, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Kašpárková, V.; Humpoliček, P.; Stejskal, J.; Capáková, Z.; Bober, P.; Skopalová, K.; Lehocký, M. Exploring the critical factors limiting polyaniline biocompatibility. Polymers 2019, 11, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhou, M.; Dou, C.; Ma, G.; Wang, Y.; Feng, N.; Wang, W.; Fang, L. Synthesis and biocompatibility assessment of polyaniline nanomaterials. J. Bioact. Compat. Polym. 2019, 34, 16–24. [Google Scholar] [CrossRef]
- Wibowo, A.; Vyas, C.; Cooper, G.; Qulub, F.; Suratman, R.; Mahyuddin, A.I.; Dirgantara, T.; Bartolo, P. 3D printing of polycaprolactone-polyaniline electroactive Scaffolds for bone tissue engineering. Materials 2020, 13, 512. [Google Scholar] [CrossRef] [Green Version]
- Kašpárková, V.; Jasenská, D.; Capáková, Z.; Maráková, N.; Stejskal, J.; Bober, P.; Lehocký, M.; Humpolíček, P. Polyaniline colloids stabilized with bioactive polysaccharides: Non-cytotoxic antibacterial materials. Carbohydr. Polym. 2019, 219, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Humpolíček, P.; Radaszkiewicz, K.A.; Capáková, Z.; Pacherník, J.; Bober, P.; Kašpárková, V.; Rejmontová, P.; Lehocký, M.; Ponížil, P.; Stejskal, J. Polyaniline cryogels: Biocompatibility of novel conducting macroporous material. Sci. Rep. 2018, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Omran, K.A. Bioactivation of Polyaniline for Biomedical Applications and Metal Oxide Composites. J. Chem. 2022, 2022, 9328512. [Google Scholar] [CrossRef]
- Gonçalves, J.P.; de Oliveira, C.C.; da Silva Trindade, E.; Riegel-Vidotti, I.C.; Vidotti, M.; Simas, F.F. In vitro biocompatibility screening of a colloidal gum Arabic-polyaniline conducting nanocomposite. Int. J. Biol. Macromol. 2021, 173, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Benson, N.U.; Bassey, D.E.; Palanisami, T. COVID pollution: Impact of COVID-19 pandemic on global plastic waste footprint. Heliyon 2021, 7, e06343. [Google Scholar] [CrossRef] [PubMed]
- Meys, R.; Frick, F.; Westhues, S.; Sternberg, A.; Klankermayer, J.; Bardow, A. Towards a circular economy for plastic packaging wastes—The environmental potential of chemical recycling. Resour. Conserv. Recycl. 2020, 162, 105010. [Google Scholar] [CrossRef]
- Moeini, A.; Germann, N.; Malinconico, M.; Santagata, G. Formulation of secondary compounds as additives of biopolymer-based food packaging: A review. Trends Food Sci. Technol. 2021, 114, 342–354. [Google Scholar] [CrossRef]
- Barra, A.; Santos, J.D.C.; Silva, M.R.F.; Nunes, C.; Ruiz-Hitzky, E.; Gonçalves, I.; Yildirim, S.; Ferreira, P.; Marques, P.A.A.P. Graphene derivatives in biopolymer-based composites for food packaging applications. Nanomaterials 2020, 10, 2077. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.Y.; Arif, Z.U. Novel biopolymer-based sustainable composites for food packaging applications: A narrative review. Food Packag. Shelf Life 2022, 33, 100892. [Google Scholar] [CrossRef]
- Boudjelida, S.; Souad, D.; Hana, F.; Benguerba, Y.; Imane, C.; Mauro, C. Physicochemical Properties and Atomic-Scale Interactions in Polyaniline (Emeraldine Base)/Starch Bio-Based Composites: Experimental and Computational Investigations. Polymers 2022, 14, 1505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, G.H.; Gu, W.; Zhao, Y.; Tang, S.; Ji, G. A breathable and flexible fiber cloth based on cellulose/polyaniline cellular membrane for microwave shielding and absorbing applications. J. Colloid Interface Sci. 2022, 605, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Shahadat, M.; Jha, A.; Shahid-ul-Islam; Adnan, R.; Ali, S.W.; Ismail, I.M.I.; Oves, M.; Ahammad, S.Z. Recent advances in chitosan-polyaniline based nanocomposites for environmental applications: A review. Polymer 2022, 254, 124975. [Google Scholar] [CrossRef]
- Abou Hammad, A.B.; Abd El-Aziz, M.E.; Hasanin, M.S.; Kamel, S. A novel electromagnetic biodegradable nanocomposite based on cellulose, polyaniline, and cobalt ferrite nanoparticles. Carbohydr. Polym. 2019, 216, 54–62. [Google Scholar] [CrossRef]
- Shahadat, M.; Khan, M.Z.; Rupani, P.F.; Embrandiri, A.; Sultana, S.; Ahammad, S.Z.; Wazed Ali, S.; Sreekrishnan, T.R. A critical review on the prospect of polyaniline-grafted biodegradable nanocomposite. Adv. Colloid Interface Sci. 2017, 249, 2–16. [Google Scholar] [CrossRef]
- Wong, P.Y.; Takeno, A.; Takahashi, S.; Phang, S.W.; Baharum, A. Crazing effect on the bio-based conducting polymer film. Polymers 2021, 13, 3425. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Yuan, X.; Tao, J.; Peng, S.; Yang, W.; Dong, X.; Zhang, C.; Luo, Y. Polyaniline modified mesoporous titanium dioxide that enhances oxo-biodegradation of polyethylene films for agricultural plastic mulch application. Polym. Int. 2019, 68, 1332–1340. [Google Scholar] [CrossRef]
- Anisimov, Y.A.; Evitts, R.W.; Cree, D.E.; Wilson, L.D. Polyaniline/biopolymer composite systems for humidity sensor applications: A review. Polymers 2021, 13, 2722. [Google Scholar] [CrossRef] [PubMed]
- Aswathy, N.R.; Kumar, S.A.; Mohanty, S.; Nayak, S.K.; Palai, A.K. Polyaniline/multi-walled carbon nanotubes filled biopolymer based flexible substrate electrodes for supercapacitor applications. J. Energy Storage 2021, 35, 102256. [Google Scholar] [CrossRef]
- Arrieta-Almario, A.A.; Mendoza-Fandino, J.M.; Palencia, M.S. Composite material elaborated from conducting biopolymer cassava starch and polyaniline. Rev. Mex. Ing. Química 2020, 19, 707–715. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Z.; Wang, X.; Yang, G.; Liu, Y. Adsorption-photocatalysis performance of polyaniline/dicarboxyl acid cellulose@graphene oxide for dye removal. Int. J. Biol. Macromol. 2021, 182, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.; Alotaibi, B.M.; Abdelhamied, M.M. Structural characteristics and optical properties of methylcellulose/polyaniline films modified by low energy oxygen irradiation. Inorg. Chem. Commun. 2022, 141, 109502. [Google Scholar] [CrossRef]
- Minisy, I.M.; Salahuddin, N.A.; Ayad, M.M. Chitosan/polyaniline hybrid for the removal of cationic and anionic dyes from aqueous solutions. J. Appl. Polym. Sci. 2019, 136, 47056. [Google Scholar] [CrossRef]
- Kumar, A.M.; Jose, J.; Hussein, M.A. Novel polyaniline/chitosan/reduced graphene oxide ternary nanocomposites: Feasible reinforcement in epoxy coatings on mild steel for corrosion protection. Prog. Org. Coat. 2022, 163, 106678. [Google Scholar] [CrossRef]
- Pirsa, S.; Mohammadi, B. Conducting/biodegradable chitosan-polyaniline film; Antioxidant, color, solubility and water vapor permeability properties. Main Group Chem. 2021, 20, 133–147. [Google Scholar] [CrossRef]
- Mohammadi, B.; Pirsa, S.; Alizadeh, M. Preparing chitosan–polyaniline nanocomposite film and examining its mechanical, electrical, and antimicrobial properties. Polym. Polym. Compos. 2019, 27, 507–517. [Google Scholar] [CrossRef]
- de Oliveira, A.C.S.; Ugucioni, J.C.; da Rocha, R.A.; Borges, S.V. Development of whey protein isolate/polyaniline smart packaging: Morphological, structural, thermal, and electrical properties. J. Appl. Polym. Sci. 2019, 136, 47316. [Google Scholar] [CrossRef]
- He, W.; Tian, J.; Li, J.; Jin, H.; Li, Y. Characterization and Properties of Cellulose Nanofiber/Polyaniline Film Composites Synthesized through in Situ Polymerization. BioResources 2016, 11, 8535–8547. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.Y.; Sui, G.X.; Bhattacharyya, D. Synthesis and characterisation of nanocellulose-based polyaniline conducting films. Compos. Sci. Technol. 2014, 99, 31–36. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Y.; Zhu, X.; Zhou, Y.; Hong, X. Preparation and characterization of polylactic acid/polyaniline/nanocrystalline cellulose nanocomposite films. Int. J. Biol. Macromol. 2020, 146, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Visakh, P.M. Polyaniline-Based Blends, Composites, and Nanocomposites: State of the Art, New Challenges, and Opportunities. In Polyaniline Blends, Composites, and Nanocomposites; Vikash, P.M., Pina, C.D., Falletta, E., Eds.; Elsevier Inc.: Oxford, UK, 2018; pp. 1–22. [Google Scholar]
- Charoensri, K.; Rodwihok, C.; Wongratanaphisan, D.; Ko, J.A.; Chung, J.S.; Park, H.J. Investigation of functionalized surface charges of thermoplastic starch/zinc oxide nanocomposite films using polyaniline: The potential of improved antibacterial properties. Polymers 2021, 13, 425. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Seong, E.S. Characterization of Biodegradable Conductive Composite Films with Polyaniline (2). J. Korean Oil Chem. Soc. 2015, 32, 85–92. [Google Scholar] [CrossRef]
- Azmi, N.S.; Kadir Basha, R.; Othman, S.H.; Mohammed, M.A.P. Characterization of antioxidant tapioca starch/polyaniline composites film prepared using solution casting method. Food Res. 2019, 3, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Manomaisantiphap, S.; Takahashi, K.; Goto, T.; Tsushima, N.; Takahashi, T.; Yokozeki, T. Cationic scavenging by polyaniline: Boon or bane from synthesis point of view of its nanocomposites. Polymer 2018, 149, 169–177. [Google Scholar] [CrossRef]
- Feng, L.; Xia, W.; Wang, T.; Jiang, C.; Gong, H.; Gao, B.; Jiang, Z.; Liu, X.; He, J. Structure stability of polyaniline/graphene nanocomposites in gamma-ray environment. J. Radioanal. Nucl. Chem. 2018, 315, 627–638. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Luo, X.; Guo, H.; Wang, N.; Su, D.; Zhang, C.; Liu, T.; Wang, G.; Cui, L. Polyaniline engineering defect-induced nitrogen doped carbon-supported Co3O4 hybrid composite as a high-efficiency electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2020, 526, 146626. [Google Scholar] [CrossRef]
- Norouzian, R.S.; Lakouraj, M.M. Polyaniline-thiacalix[4]arene metallopolymer, self-doped, and externally doped conductive polymers. Prog. Org. Coat. 2020, 146, 105731. [Google Scholar] [CrossRef]
- Rana, A.K.; Scarpa, F.; Kumar, V. Industrial Crops & Products Cellulose/polyaniline hybrid nanocomposites: Design, fabrication, and emerging multidimensional applications. Ind. Crops Prod. 2022, 187, 115356. [Google Scholar]
- Thong, N.M.; Vo, Q.V.; Le Huyen, T.; Van Bay, M.; Dung, N.N.; Thu Thao, P.T.; Nam, P.C. Functionalization and antioxidant activity of polyaniline-fullerene hybrid nanomaterials: A theoretical investigation. RSC Adv. 2020, 10, 14595–14605. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhu, L.H.; Li, J.; Tang, H.Q. Antioxidant activity of polyaniline nanofibers. Chin. Chem. Lett. 2007, 18, 1005–1008. [Google Scholar] [CrossRef]
- Gizdavic-Nikolaidis, M.; Travas-Sejdic, J.; Kilmartin, P.A.; Bowmaker, G.A.; Cooney, R.P. Evaluation of antioxidant activity of aniline and polyaniline. Curr. Appl. Phys. 2004, 4, 343–346. [Google Scholar] [CrossRef]
- Aizamddin, M.F.; Mahat, M.M.; Ariffin, Z.Z.; Nawawi, M.A.; Jani, N.A.; Nor Amdan, N.A.; Sadasivuni, K.K. Antibacterial Performance of Protonated Polyaniline-Integrated Polyester Fabrics. Polymers 2022, 14, 2617. [Google Scholar] [CrossRef] [PubMed]
- Zujovic, Z.; Kilmartin, P.A.; Travas-sejdic, J. The Applications of Solid-State NMR to Conducting Polymers. The Special Case on Polyaniline. Molecules 2020, 25, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boomi, P.; Poorani, G.P.; Palanisamy, S.; Selvam, S.; Ramanathan, G.; Ravikumar, S.; Barabadi, H.; Prabu, H.G.; Jeyakanthan, J.; Saravanan, M. Evaluation of Antibacterial and Anticancer Potential of Polyaniline-Bimetal Nanocomposites Synthesized from Chemical Reduction Method. J. Clust. Sci. 2019, 30, 715–726. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Luong, J.H.T.; Gedanken, A. Antimicrobial properties of the polyaniline composites against pseudomonas aeruginosa and klebsiella pneumoniae. J. Funct. Biomater. 2020, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Switha, D.; Basha, S.K.; Kumari, V.S. A novel, biocompatible nanostarch incorporated Polyaniline-Polyvinyl alcohol-Nanostarch hybrid scaffold for tissue engineering applications. Eur. Polym. J. 2022, 178, 111448. [Google Scholar] [CrossRef]
- Robertson, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Swift, S. The antimicrobial action of polyaniline involves production of oxidative stress while functionalisation of polyaniline introduces additional mechanisms. PeerJ 2018, 6, e5135. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, C.; Vandarkuzhali, S.A.A.; Radha, N. Antimicrobial activities of nanostructured polyanilines doped with aromatic nitro compounds. Arab. J. Chem. 2019, 12, 3785–3798. [Google Scholar] [CrossRef] [Green Version]
- Andriianova, A.N.; Latypova, L.R.; Vasilova, L.Y.; Kiseleva, S.V.; Zorin, V.V.; Abdrakhmanov, I.B.; Mustafin, A.G. Antibacterial properties of polyaniline derivatives. J. Appl. Polym. Sci. 2021, 138, 51397. [Google Scholar] [CrossRef]
- Mahdavi, B.; Farbodi, M. Investigation of Morphology and Antibacterial Properties of Nylon 6, 6/PANI/ZnO Nanocomposite. Int. J. New Chem. 2022, 9, 282–290. [Google Scholar]
- Fenniche, F.; Henni, A.; Khane, Y.; Aouf, D.; Harfouche, N.; Bensalem, S.; Zerrouki, D.; Belkhalfa, H. Electrochemical Synthesis of Reduced Graphene Oxide–Wrapped Polyaniline Nanorods for Improved Photocatalytic and Antibacterial Activities. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1011–1025. [Google Scholar] [CrossRef]
- Abdel Rehim, M.H.; Yassin, M.A.; Zahran, H.; Kamel, S.; Moharam, M.E.; Turky, G. Rational design of active packaging films based on polyaniline-coated polymethyl methacrylate/nanocellulose composites. Polym. Bull. 2020, 77, 2485–2499. [Google Scholar] [CrossRef]
- Abdolsattari, P.; Rezazadeh-bari, M. Smart Film Based on Polylactic Acid, Modified with Polyaniline/ZnO/CuO: Investigation of Physicochemical Properties and its use of Intelligent Packaging of Orange Juice. Food Bioprocess Technol. 2022, 1–30. [Google Scholar] [CrossRef]
- Shalini, A.; Nishanthi, R.; Palani, P.; Jaisankar, V. One pot synthesis, characterization of polyaniline and cellulose/polyaniline nanocomposites: Application towards in vitro measurements of antibacterial activity. Mater. Today Proc. 2016, 3, 1633–1642. [Google Scholar] [CrossRef]
- Pandiselvi, K.; Thambidurai, S. Synthesis, characterization, and antimicrobial activity of chitosan-zinc oxide/polyaniline composites. Mater. Sci. Semicond. Process. 2015, 31, 573–581. [Google Scholar] [CrossRef]
- Zare, E.N.; Lakouraj, M.M. Biodegradable polyaniline/dextrin conductive nanocomposites: Synthesis, characterization, and study of antioxidant activity and sorption of heavy metal ions. Iran. Polym. J. 2014, 23, 257–266. [Google Scholar] [CrossRef]
- Wu, J.C.C.; Ray, S.; Gizdavic-Nikolaidis, M.; Uy, B.; Swift, S.; Jin, J.; Cooney, R.P. Nanostructured bioactive material based on polycaprolactone and polyaniline fiber-scaffolds. Synth. Met. 2014, 198, 41–50. [Google Scholar] [CrossRef]
- Nand, A.V.; Swift, S.; Uy, B.; Kilmartin, P.A. Evaluation of antioxidant and antimicrobial properties of biocompatible low density polyethylene/polyaniline blends. J. Food Eng. 2013, 116, 422–429. [Google Scholar] [CrossRef]
- Hsu, C.F.; Kilmartin, P.A. Antioxidant capacity of robust polyaniline-ethyl cellulose films. React. Funct. Polym. 2012, 72, 814–822. [Google Scholar] [CrossRef]
- Nand, A.V.; Ray, S.; Travas-Sejdic, J.; Kilmartin, P.A. Characterization of polyethylene terephthalate/polyaniline blends as potential antioxidant materials. Mater. Chem. Phys. 2012, 134, 443–450. [Google Scholar] [CrossRef]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria—Problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Torre, R.; Costa-Rama, E.; Nouws, H.P.A.; Delerue-Matos, C. Screen-printed electrode-based sensors for food spoilage control: Bacteria and biogenic amines detection. Biosensors 2020, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ran, X.; Tang, H.; Cao, D. Recent advances on reaction-based amine fluorescent probes. Dyes Pigment. 2021, 194, 109634. [Google Scholar] [CrossRef]
- Franceschelli, L.; Berardinelli, A.; Dabbou, S.; Ragni, L.; Tartagni, M. Sensing Technology for Fish Freshness and Safety: A Review. Sensors 2021, 21, 1373. [Google Scholar] [CrossRef]
- Cai, C.; Mo, J.; Lu, Y.; Zhang, N.; Wu, Z.; Wang, S.; Nie, S. Integration of a porous wood-based triboelectric nanogenerator and gas sensor for real-time wireless food-quality assessment. Nano Energy 2021, 83, 105833. [Google Scholar] [CrossRef]
- Jung, Y.; Min, J.; Choi, J.; Bang, J.; Jeong, S.; Rok, K.; Ahn, J.; Cho, Y.; Hong, S.; Hong, S.; et al. Smart paper electronics by laser-induced graphene for biodegradable real-time food spoilage monitoring. Appl. Mater. Today 2022, 29, 101589. [Google Scholar] [CrossRef]
- Herath, I.S.; O’Donnell, T.E.; Pavlov, J.; Attygalle, A.B. Screening freshness of seafood by measuring trimethylamine (TMA) levels using helium-plasma ionization mass spectrometry (HePI-MS). J. Anal. Sci. Technol. 2019, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Bariya, M.; Fahad, H.M.; Wu, J.; Han, R.; Gupta, N.; Javey, A. Trace-Level, Multi-Gas Detection for Food Quality Assessment Based on Decorated Silicon Transistor Arrays. Adv. Mater. 2020, 32, 1908385. [Google Scholar] [CrossRef]
- Sánchez-García, F.; Hernández, I.; Palacios, V.M.; Roldán, A.M. Freshness quality and shelf life evaluation of the seaweed Ulva rigida through physical, chemical, microbiological, and sensory methods. Foods 2021, 10, 181. [Google Scholar] [CrossRef]
- Zhao, J.; Madni, G.R.; Anwar, M.A. Exploring rural inhabitants’ perceptions towards food wastage during COVID-19 lockdowns: Implications for food security in Pakistan. PLoS ONE 2022, 17, e0264534. [Google Scholar] [CrossRef]
- Ocampo, A.M.; Santos, L.R.; Julian, S.; Bailon, M.X.; Bautista, J. Polyaniline-based cadaverine sensor through digital image colorimetry. E-Polymers 2018, 18, 465–471. [Google Scholar] [CrossRef]
- Maravillas, M.J.; Mendenilla, A.M.; Merino, A.F.; Quinto, E. Development of a method through digital image analysis for analyzing polyaniline based sensors for total volatile basic nitrogen (tvbn) as fish freshness indicators. ASEAN J. Chem. Eng. 2018, 18, 7–12. [Google Scholar]
- Shao, P.; Liu, L.; Yu, J.; Lin, Y.; Gao, H.; Chen, H.; Sun, P. An overview of intelligent freshness indicator packaging for food quality and safety monitoring. Trends Food Sci. Technol. 2021, 118, 285–296. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Zhu, L.; Tang, Y.; Gao, X.; Tang, L.; Li, X.; Li, J. Dual-mode smart packaging based on tetraphenylethylene-functionalized polyaniline sensing label for monitoring the freshness of fish. Sens. Actuators B Chem. 2020, 323, 128694. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Li, H.; Liu, X.; Guo, T.; Zhang, G.; Xiong, Y. A renewable intelligent colorimetric indicator based on polyaniline for detecting freshness of tilapia. Packag. Technol. Sci. 2017, 31, 133–140. [Google Scholar] [CrossRef]
- Anjali, M.K.; Bharath, G.; Rashmi, H.M.; Avinash, J.; Naresh, K.; Raju, P.N.; Raghu, H.V. Polyaniline-Pectin nanoparticles immobilized paper based colorimetric sensor for detection of Escherichia coli in milk and milk products. Curr. Res. Food Sci. 2022, 5, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Abutalib, M.M.; Rajeh, A. Preparation and characterization of polyaniline/sodium alginate-doped TiO2 nanoparticles with promising mechanical and electrical properties and antimicrobial activity for food packaging applications. J. Mater. Sci. Mater. Electron. 2020, 31, 9430–9442. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Shi, J.; Li, Z.; Wang, X.; Hu, X.; Gong, Y.; Zou, X. A Novel Gas Sensor for Detecting Pork Freshness Based on PANI/AgNWs/Silk. Foods 2022, 11, 2372. [Google Scholar] [CrossRef]
- Kazemi, F.; Naghib, S.M.; Zare, Y.; Rhee, K.Y. Biosensing Applications of Polyaniline (PANI)-Based Nanocomposites: A Review. Polym. Rev. 2021, 61, 553–597. [Google Scholar] [CrossRef]
- Dodero, A.; Escher, A.; Bertucci, S.; Castellano, M.; Lova, P. Intelligent packaging for real-time monitoring of food-quality: Current and future developments. Appl. Sci. 2021, 11, 3532. [Google Scholar] [CrossRef]
- Poyatos-Racionero, E.; Ros-Lis, J.V.; Vivancos, J.L.; Martínez-Máñez, R. Recent advances on intelligent packaging as tools to reduce food waste. J. Clean. Prod. 2018, 172, 3398–3409. [Google Scholar] [CrossRef]
- Thakur, B.; Amarnath, C.A.; Mangoli, S.H.; Sawant, S.N. Polyaniline nanoparticle based colorimetric sensor for monitoring bacterial growth. Sens. Actuators B Chem. 2015, 207, 262–268. [Google Scholar] [CrossRef]
- Li, L.; Ferng, L.H.; Wei, Y.; Yang, C.; Ji, H.F. Highly stable polyaniline-poly(sodium 4-styrenesulfonate) nanoparticles for sensing of amines. J. Nanosci. Nanotechnol. 2014, 14, 6593–6598. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Jayus; Restyana, A.; Abdullah, A.; Heng, L.Y.; Ahmad, M. A novel colorimetric food package label for fish spoilage based on polyaniline film. Food Control 2012, 25, 184–189. [Google Scholar] [CrossRef]
- Ma, Z.; Chen, P.; Cheng, W.; Yan, K.; Pan, L.; Shi, Y.; Yu, G. Highly Sensitive, Printable Nanostructured Conductive Polymer Wireless Sensor for Food Spoilage Detection. Nano Lett. 2018, 18, 4570–4575. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, Z.; Shi, J.; Zhang, F.; Zhou, X.; Li, Y.; Holmes, M.; Zhang, W.; Zou, X. Titanium dioxide-polyaniline/silk fibroin microfiber sensor for pork freshness evaluation. Sens. Actuators B Chem. 2018, 260, 465–474. [Google Scholar] [CrossRef]
- Matindoust, S.; Farzi, A.; Baghaei, M. Ammonia gas sensor based on flexible polyaniline films for rapid detection of spoilage in protein-rich foods. J. Mater. Sci. Mater. Electron. 2017, 28, 7760–7768. [Google Scholar] [CrossRef]
- Park, H.J.; Yoon, J.H.; Lee, K.G.; Choi, B.G. Potentiometric performance of flexible pH sensor based on polyaniline nanofiber arrays. Nano Converg. 2019, 6, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustapha, N.; Khairuddin, N.; Muhamad, I.I.; Hashim, S.; Siddique, M.B.M. Characterization of HEC/PANI film as a potential electroactive packaging with pH Sensor. Sains Malays. 2016, 45, 1169–1176. [Google Scholar]
- Yang, Y.; Qiu, W.; Li, Y.; Liu, L. Antibiotic residues in poultry food in Fujian Province of China. Food Addit. Contam. Part B Surveill. 2020, 13, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ying, G.G.; Deng, W.J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef] [PubMed]
- Menkem, Z.E.; Ngangom, B.L.; Tamunjoh, S.S.A.; Boyom, F.F. Antibiotic residues in food animals: Public health concern. Acta Ecol. Sin. 2019, 39, 411–415. [Google Scholar] [CrossRef]
- Nag, P.; Sadani, K.; Mohapatra, S.; Mukherji, S.; Mukherji, S. Evanescent Wave Optical Fiber Sensors Using Enzymatic Hydrolysis on Nanostructured Polyaniline for Detection of β-Lactam Antibiotics in Food and Environment. Anal. Chem. 2021, 93, 2299–2308. [Google Scholar] [CrossRef] [PubMed]
- Vu, V.-P.; Tran, Q.-T.; Pham, D.-T.; Tran, P.-D.; Thierry, B.; Chu, T.-X.; Mai, A.-T. Possible detection of antibiotic residue using molecularly imprinted polyaniline-based sensor. Vietnam. J. Chem. 2019, 57, 328–333. [Google Scholar] [CrossRef] [Green Version]
- Florez, D.H.A.; Dutra, F.V.A.; Borges, K.B. Magnetic solid phase extraction employing a novel restricted access material based on mesoporous polyaniline coated with hydrophilic monomers and casein for determination of antibiotics in milk samples. Microchem. J. 2019, 150, 104145. [Google Scholar] [CrossRef]
- Sereshti, H.; Karami, F.; Nouri, N.; Farahani, A. Electrochemically controlled solid phase microextraction based on a conductive polyaniline-graphene oxide nanocomposite for extraction of tetracyclines in milk and water. J. Sci. Food Agric. 2021, 101, 2304–2311. [Google Scholar] [CrossRef]
- Rana, M.S.; Lee, S.Y.; Kang, H.J.; Hur, S.J. Reducing veterinary drug residues in animal products: A review. Food Sci. Anim. Resour. 2019, 39, 687–703. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Jian, N.; Cao, J.; Zhang, H.; Li, J.; Xu, Q.; Wang, C. Rapid, simple and green solid phase extraction based on polyaniline nanofibers-mat for detecting non-steroidal anti-inflammatory drug residues in animal-origin food. Food Chem. 2020, 328, 127097. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, Y.; Dong, H.; Zhao, J.; Wang, C.; Zhou, S.; Lu, J.; Yan, Y.; Li, H. Accelerating the design of molecularly imprinted nanocomposite membranes modified by Au@polyaniline for selective enrichment and separation of ibuprofen. Appl. Surf. Sci. 2018, 428, 555–565. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, S.; Xia, M.; Wang, F.; Ju, X. Three-dimension hierarchical composite via in-situ growth of Zn/Al layered double hydroxide plates onto polyaniline-wrapped carbon sphere for efficient naproxen removal. J. Hazard. Mater. 2022, 423, 127192. [Google Scholar] [CrossRef] [PubMed]
- Jian, N.; Qian, L.; Wang, C.; Li, R.; Xu, Q.; Li, J. Novel nanofibers mat as an efficient, fast and reusable adsorbent for solid phase extraction of non-steroidal anti-inflammatory drugs in environmental water. J. Hazard. Mater. 2019, 363, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Suo, L.; Zhao, J.; Dong, X.; Gao, X.; Li, X.; Xu, J.; Lu, X.; Zhao, L. Functionalization of a SiO2-coated magnetic graphene oxide composite with polyaniline-polypyrrole for magnetic solid phase extraction of ultra-trace Cr(iii) and Pb(ii) in water and food samples using a Box-Behnken design. New J. Chem. 2019, 43, 12126–12136. [Google Scholar] [CrossRef]
- Akhtar, M.; Tahir, A.; Zulfiqar, S.; Hanif, F.; Warsi, M.F.; Agboola, P.O.; Shakir, I. Ternary hybrid of polyaniline-alanine-reduced graphene oxide for electrochemical sensing of heavy metal ions. Synth. Met. 2020, 265, 116410. [Google Scholar] [CrossRef]
- Sonone, S.S.; Jadhav, S.V.; Sankhla, M.S.; Kumar, R. Water Contamination by Heavy Metals and their Toxic Effect on Aquaculture and Human Health through Food Chain. Lett. Appl. Nanobiosci. 2020, 10, 2148–2166. [Google Scholar]
- Eskandari, E.; Kosari, M.; Davood Abadi Farahani, M.H.; Khiavi, N.D.; Saeedikhani, M.; Katal, R.; Zarinejad, M. A review on polyaniline-based materials applications in heavy metals removal and catalytic processes. Sep. Purif. Technol. 2020, 231, 115901. [Google Scholar] [CrossRef]
- Senguttuvan, S.; Senthilkumar, P.; Janaki, V.; Kamala-Kannan, S. Significance of conducting polyaniline based composites for the removal of dyes and heavy metals from aqueous solution and wastewaters—A review. Chemosphere 2021, 267, 129201. [Google Scholar] [CrossRef]
- Zare, E.N.; Motahari, A.; Sillanpää, M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review. Environ. Res. 2018, 162, 173–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, W.; Zhang, T.; Zhang, L.; Du, T.; Zhang, W.; Zhang, D.; Sun, J.; Yue, T.; Wang, Y.C.; et al. Conductive polyaniline-graphene oxide sorbent for electrochemically assisted solid-phase extraction of lead ions in aqueous food samples. Anal. Chim. Acta 2020, 1100, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.; Atassi, Y. Enhancement of removal efficiency of heavy metal ions by polyaniline deposition on electrospun polyacrylonitrile membranes. Water Sci. Eng. 2021, 14, 129–138. [Google Scholar] [CrossRef]
- Chen, J.; Wang, N.; Liu, Y.; Zhu, J.; Feng, J.; Yan, W. Synergetic effect in a self-doping polyaniline/TiO2 composite for selective adsorption of heavy metal ions. Synth. Met. 2018, 245, 32–41. [Google Scholar] [CrossRef]
- Gapusan, R.B.; Balela, M.D.L. Adsorption of anionic methyl orange dye and lead(II) heavy metal ion by polyaniline-kapok fiber nanocomposite. Mater. Chem. Phys. 2020, 243, 122682. [Google Scholar] [CrossRef]
- Abu Taleb, M.; Kumar, R.; Al-Rashdi, A.A.; Seliem, M.K.; Barakat, M.A. Fabrication of SiO2/CuFe2O4/polyaniline composite: A highly efficient adsorbent for heavy metals removal from aquatic environment. Arab. J. Chem. 2020, 13, 7533–7543. [Google Scholar] [CrossRef]
- Orachorn, N.; Bunkoed, O. A nanocomposite fluorescent probe of polyaniline, graphene oxide and quantum dots incorporated into highly selective polymer for lomefloxacin detection. Talanta 2019, 203, 261–268. [Google Scholar] [CrossRef]
- Abedi, H.; Ebrahimzadeh, H. Electromembrane-surrounded solid-phase microextraction coupled to ion mobility spectrometry for the determination of nonsteroidal anti-inflammatory drugs: A rapid screening method in complicated matrices. J. Sep. Sci. 2015, 38, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Elci, S.G. Determination of cobalt in food by magnetic solid-phase extraction (MSPE) preconcentration by polyaniline (PANI) and polythiophene (PTH) coated magnetic nanoparticles (MNPs) and microsample injection system–flame atomic absorption spectrometry (MIS-FAAS). Instrum. Sci. Technol. 2021, 49, 258–275. [Google Scholar] [CrossRef]
- Behbahani, M.; Bide, Y.; Salarian, M.; Niknezhad, M.; Bagheri, S.; Bagheri, A.; Nabid, M.R. The use of tetragonal star-like polyaniline nanostructures for efficient solid phase extraction and trace detection of Pb(II) and Cu(II) in agricultural products, sea foods, and water samples. Food Chem. 2014, 158, 14–19. [Google Scholar] [CrossRef]
- Tiggemann, L.; Ballen, S.C.; Bocalon, C.M.; Graboski, A.M.; Manzoli, A.; Steffens, J.; Valduga, E.; Steffens, C. Electronic nose system based on polyaniline films sensor array with different dopants for discrimination of artificial aromas. Innov. Food Sci. Emerg. Technol. 2017, 43, 112–116. [Google Scholar] [CrossRef]
- Graboski, A.M.; Galvagni, E.; Manzoli, A.; Shimizu, F.M.; Zakrzevski, C.A.; Weschenfelder, T.A.; Steffens, J.; Steffens, C. Lab-made electronic-nose with polyaniline sensor array used in classification of different aromas in gummy candies. Food Res. Int. 2018, 113, 309–315. [Google Scholar] [CrossRef]
- Graboski, A.M.; Ballen, S.C.; Galvagni, E.; Lazzari, T.; Manzoli, A.; Shimizu, F.M.; Steffens, J.; Steffens, C. Aroma detection using a gas sensor array with different polyaniline films. Anal. Methods 2019, 11, 654–660. [Google Scholar] [CrossRef]
- Ballen, S.C.; Graboski, A.M.; Manzoli, A.; Steffens, J.; Steffens, C. Monitoring Aroma Release in Gummy Candies During The Storage Using Electronic Nose. Food Anal. Methods 2020, 13, 3–12. [Google Scholar] [CrossRef]
- Swe, M.M.; Eamsa-Ard, T.; Srikhirin, T.; Kerdcharoen, T. Monitoring the Freshness Level of Beef Using Nanocomposite Gas Sensors in Electronic nose. In Proceedings of the 2019 IEEE International Conference on Consumer Electronics—Asia, ICCE-Asia 2019, Bangkok, Thailand, 12–14 June 2019; pp. 100–103. [Google Scholar]










| Composites | Antioxidant Properties | Antimicrobial Towards | Food | Ref |
|---|---|---|---|---|
| Chitosan/PANI | √ | - | - | [78] |
| CS/PANI-ZnO NP | - | S. aureus E. coli | - | [85] |
| PANI-(PMMA/CNC) | √ | B. cereus S. typhimurium | - | [106] |
| Chitosan/PANI | - | A. niger E. coli | - | [79] |
| PANI | - | S. aureus E.coli | - | [108] |
| Cellulose/PANI | ||||
| Chitosan-ZnO/PANI | - | S. aureus P. aeruginosa | - | [109] |
| Dextrin/PANI | √ | - | - | [110] |
| PCL/NF PANI | √ | S. aureus E. coli | - | [111] |
| LLDPE/NR PANI | √ | S. aureus | Fish oil | [112] |
| EC/PANI | √ | - | Fish oil | [113] |
| PET/NR PANI | √ | - | - | [114] |
| PLA/PANI/ZnO/CuO | √ | S. aureus E. coli | Orange juice | [107] |
| Method | Label | Target | Food | Ref |
|---|---|---|---|---|
| Colourimetric | PANI-Pec | E. coli | Milk and butter | [130] |
| Starch/PANI | NH3 | - | [6] | |
| PANI/TPE | Spoilage gas | Red drum fish | [128] | |
| PANI | TMA | Blue marlin fish | [126] | |
| PANI | NH3 and DMA | Tilapia | [129] | |
| PANI-Pec NP | E. coli | Tap water | [136] | |
| PANI-PSS | TEA | - | [137] | |
| PANI | NH3 | Milkfish | [138] | |
| Resistometric | PLA/PANI/CuO/ZnO | Spoilage gas | Orange juice | [107] |
| PANI/Ag NW/Silk | TMA | Pork | [132] | |
| PTS-PANI | NH3, putrescine, and cadaverine | Beef, pork, fish, and chicken meat | [139] | |
| TiO2-PANI/SFF | NH3 | Pork | [140] | |
| PANI-PI | NH3 | Meat | [141] | |
| Potentiometric | PANI NF/PET | pH | Milk & apple | [142] |
| Conductometric | HEC/PANI | pH | Milk | [143] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chia, M.-R.; Phang, S.-W.; Ahmad, I. Emerging Applications of Versatile Polyaniline-Based Polymers in the Food Industry. Polymers 2022, 14, 5168. https://doi.org/10.3390/polym14235168
Chia M-R, Phang S-W, Ahmad I. Emerging Applications of Versatile Polyaniline-Based Polymers in the Food Industry. Polymers. 2022; 14(23):5168. https://doi.org/10.3390/polym14235168
Chicago/Turabian StyleChia, Min-Rui, Sook-Wai Phang, and Ishak Ahmad. 2022. "Emerging Applications of Versatile Polyaniline-Based Polymers in the Food Industry" Polymers 14, no. 23: 5168. https://doi.org/10.3390/polym14235168