Swelling and Collapse of Cylindrical Polyelectrolyte Microgels
Abstract
:1. Introduction
2. Model and Simulation Methods
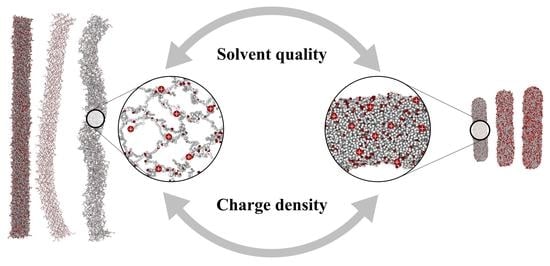
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plamper, F.A.; Richtering, W. Functional Microgels and Microgel Systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Senff, H.; Richtering, W. Temperature Sensitive Microgel Suspensions: Colloidal Phase Behavior and Rheology of Soft Spheres. J. Chem. Phys. 1999, 111, 1705–1711. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, R.; Rudov, A.A.; Lyon, L.A.; Richtering, W.; Pich, A.; Potemkin, I.I. Electrostatic Interactions and Osmotic Pressure of Counterions Control the PH-Dependent Swelling and Collapse of Polyampholyte Microgels with Random Distribution of Ionizable Groups. Macromolecules 2015, 48, 5914–5927. [Google Scholar] [CrossRef]
- Backes, S.; Witt, M.U.; Roeben, E.; Kuhrts, L.; Aleed, S.; Schmidt, A.M.; von Klitzing, R. Loading of PNIPAM Based Microgels with CoFe2O4 Nanoparticles and Their Magnetic Response in Bulk and at Surfaces. J. Phys. Chem. B 2015, 119, 12129–12137. [Google Scholar] [CrossRef] [PubMed]
- Gelissen, A.P.H.; Scotti, A.; Turnhoff, S.K.; Janssen, C.; Radulescu, A.; Pich, A.; Rudov, A.A.; Potemkin, I.I.; Richtering, W. An Anionic Shell Shields a Cationic Core Allowing for Uptake and Release of Polyelectrolytes within Core–Shell Responsive Microgels. Soft Matter 2018, 14, 4287–4299. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, M.; Dargel, C.; Meier, L.; Brändel, T.; Hellweg, T. Smart Microgels as Drug Delivery Vehicles for the Natural Drug Aescin: Uptake, Release and Interactions. Colloid Polym. Sci. 2020, 298, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Wiese, S.; Tsvetkova, Y.; Daleiden, N.J.E.; Spieß, A.C.; Richtering, W. Microgel Stabilized Emulsions: Breaking on Demand. Colloids Surf. A Physicochem. Eng. Asp. 2016, 495, 193–199. [Google Scholar] [CrossRef]
- Zhang, T.; Ngai, T. One-Step Formation of Double Emulsions Stabilized by PNIPAM-Based Microgels: The Role of Co-Monomer. Langmuir 2021, 37, 1045–1053. [Google Scholar] [CrossRef]
- Yang, L.Q.; Hao, M.M.; Wang, H.Y.; Zhang, Y. Amphiphilic Polymer-Ag Composite Microgels with Tunable Catalytic Activity and Selectivity. Colloid Polym. Sci. 2015, 293, 2405–2417. [Google Scholar] [CrossRef]
- Sabadasch, V.; Dirksen, M.; Fandrich, P.; Cremer, J.; Biere, N.; Anselmetti, D.; Hellweg, T. Pd Nanoparticle-Loaded Smart Microgel-Based Membranes as Reusable Catalysts. ACS Appl. Mater. Interfaces 2022, 14, 49181–49188. [Google Scholar] [CrossRef]
- Berger, S.; Singh, R.; Sudha, J.D.; Adler, H.J.; Pich, A. Microgel/Clay Nanohybrids as Responsive Scavenger Systems. Polymer 2010, 51, 3829–3835. [Google Scholar] [CrossRef]
- Saha, P.; Santi, M.; Emondts, M.; Roth, H.; Rahimi, K.; Großkurth, J.; Ganguly, R.; Wessling, M.; Singha, N.K.; Pich, A. Stimuli-Responsive Zwitterionic Core–Shell Microgels for Antifouling Surface Coatings. ACS Appl. Mater. Interfaces 2020, 12, 58223–58238. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.C.; Gehlen, D.B.; Haraszti, T.; Köhler, J.; Licht, C.J.; De Laporte, L. Biofunctionalized Aligned Microgels Provide 3D Cell Guidance to Mimic Complex Tissue Matrices. Biomaterials 2018, 163, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Balaceanu, A.; Verkh, Y.; Kehren, D.; Tillmann, W.; Pich, A. Thermoresponsive Core-Shell Microgels. Synthesis and Characterisation. Z. für Phys. Chem. 2014, 228, 253–267. [Google Scholar] [CrossRef]
- Crespy, D.; Zuber, S.; Turshatov, A.; Landfester, K.; Popa, A.M. A Straightforward Synthesis of Fluorescent and Temperature-Responsive Nanogels. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1043–1048. [Google Scholar] [CrossRef]
- Krüger, A.J.D.; Köhler, J.; Cichosz, S.; Rose, J.C.; Gehlen, D.B.; Haraszti, T.; Möller, M.; De Laporte, L. A Catalyst-Free, Temperature Controlled Gelation System for in-Mold Fabrication of Microgels. Chem. Commun. 2018, 54, 6943–6946. [Google Scholar] [CrossRef]
- Seiffert, S.; Weitz, D.A. Microfluidic Fabrication of Smart Microgels from Macromolecular Precursors. Polymer 2010, 51, 5883–5889. [Google Scholar] [CrossRef] [Green Version]
- Rudyak, V.Y.; Kozhunova, E.Y.; Chertovich, A.V. Towards the Realistic Computer Model of Precipitation Polymerization Microgels. Sci. Rep. 2019, 9, 13052. [Google Scholar] [CrossRef] [Green Version]
- Krüger, A.J.D.; Bakirman, O.; Guerzoni, L.P.B.; Jans, A.; Gehlen, D.B.; Rommel, D.; Haraszti, T.; Kuehne, A.J.C.; De Laporte, L. Compartmentalized Jet Polymerization as a High-Resolution Process to Continuously Produce Anisometric Microgel Rods with Adjustable Size and Stiffness. Adv. Mater. 2019, 31, 1903668. [Google Scholar] [CrossRef] [Green Version]
- Crassous, J.J.; Mihut, A.M.; Månsson, L.K.; Schurtenberger, P. Anisotropic Responsive Microgels with Tuneable Shape and Interactions. Nanoscale 2015, 7, 15971–15982. [Google Scholar] [CrossRef]
- Nickel, A.C.; Scotti, A.; Houston, J.E.; Ito, T.; Crassous, J.; Pedersen, J.S.; Richtering, W. Anisotropic Hollow Microgels That Can Adapt Their Size, Shape, and Softness. Nano Lett. 2019, 19, 8161–8170. [Google Scholar] [CrossRef] [PubMed]
- Nickel, A.C.; Kratzenberg, T.; Bochenek, S.; Schmidt, M.M.; Rudov, A.A.; Falkenstein, A.; Potemkin, I.I.; Crassous, J.J.; Richtering, W. Anisotropic Microgels Show Their Soft Side. Langmuir 2022, 38, 5063–5080. [Google Scholar] [CrossRef] [PubMed]
- Wolff, H.J.M.; Linkhorst, J.; Göttlich, T.; Savinsky, J.; Krüger, A.J.D.; De Laporte, L.; Wessling, M. Soft Temperature-Responsive Microgels of Complex Shape in Stop-Flow Lithography. Lab Chip 2020, 20, 285–295. [Google Scholar] [CrossRef]
- Rose, J.C.; Cámara-Torres, M.; Rahimi, K.; Köhler, J.; Möller, M.; De Laporte, L. Nerve Cells Decide to Orient inside an Injectable Hydrogel with Minimal Structural Guidance. Nano Lett. 2017, 17, 3782–3791. [Google Scholar] [CrossRef] [PubMed]
- Zholudev, S.I.; Gumerov, R.A.; Larina, A.A.; Potemkin, I.I. Swelling, Collapse and Ordering of Rod-like Microgels in Solution: Computer Simulation Studies. J. Colloid Interface Sci. 2023, 629, 270–278. [Google Scholar] [CrossRef]
- Toxvaerd, S.; Dyre, J.C. Role of the First Coordination Shell in Determining the Equilibrium Structure and Dynamics of Simple Liquids. J. Chem. Phys. 2011, 135, 134501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumyantsev, A.M.; Rudov, A.A.; Potemkin, I.I. Communication: Intraparticle Segregation of Structurally Homogeneous Polyelectrolyte Microgels Caused by Long-Range Coulomb Repulsion. J. Chem. Phys. 2015, 142, 171105. [Google Scholar] [CrossRef] [Green Version]
- Stevens, M.J.; Kremer, K. The Nature of Flexible Linear Polyelectrolytes in Salt Free Solution: A Molecular Dynamics Study. J. Chem. Phys. 1995, 103, 1669–1690. [Google Scholar] [CrossRef]
- Jeon, J.; Dobrynin, A.V. Molecular Dynamics Simulations of Polyelectrolyte-Polyampholyte Complexes. Effect of Solvent Quality and Salt Concentration. J. Phys. Chem. B 2006, 110, 24652–24665. [Google Scholar] [CrossRef]
- Tiwari, R.; Hönders, D.; Schipmann, S.; Schulte, B.; Das, P.; Pester, C.W.; Klemradt, U.; Walther, A. A Versatile Synthesis Platform to Prepare Uniform, Highly Functional Microgels via Click-Type Functionalization of Latex Particles. Macromolecules 2014, 47, 2257–2267. [Google Scholar] [CrossRef]
- Kremer, K.; Grest, G.S. Dynamics of Entangled Linear Polymer Melts: A Molecular-dynamics Simulation. J. Chem. Phys. 1990, 92, 5057–5086. [Google Scholar] [CrossRef]
- LAMMPS Molecular Dynamics Simulator. Available online: http://Lammps.Sandia.Gov/ (accessed on 20 October 2022).
- Hockney, R.; Eastwood, J. Computer Simulation Using Particles; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Kobayashi, H.; Halver, R.; Sutmann, G.; Winkler, R.G. Polymer Conformations in Ionic Microgels in the Presence of Salt: Theoretical and Mesoscale Simulation Results. Polymers 2017, 9, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Monte, G.; Ninarello, A.; Camerin, F.; Rovigatti, L.; Gnan, N.; Zaccarelli, E. Numerical Insights on Ionic Microgels: Structure and Swelling Behaviour. Soft Matter 2019, 15, 8113–8128. [Google Scholar] [CrossRef] [Green Version]
- Kratz, K.; Hellweg, T.; Eimer, W. Influence of Charge Density on the Swelling of Colloidal Poly(N-Isopropylacrylamide-Co-Acrylic Acid) Microgels. Colloids Surfaces A Physicochem. Eng. Asp. 2000, 170, 137–149. [Google Scholar] [CrossRef]
- Su, W.; Yang, M.; Zhao, K.; Ngai, T. Influence of Charged Groups on the Structure of Microgel and Volume Phase Transition by Dielectric Analysis. Macromolecules 2016, 49, 7997–8008. [Google Scholar] [CrossRef]
- Quesada-Pérez, M.; Maroto-Centeno, J.A.; Forcada, J.; Hidalgo-Alvarez, R. Gel Swelling Theories: The Classical Formalism and Recent Approaches. Soft Matter 2011, 7, 10536. [Google Scholar] [CrossRef]
- Potemkin, I.I.; Khokhlov, A.R. Nematic Ordering in Dilute Solutions of Rodlike Polyelectrolytes. J. Chem. Phys. 2004, 120, 10848–10851. [Google Scholar] [CrossRef] [PubMed]
- Kundagrami, A.; Kumar, R.; Muthukumar, M. Simulations and Theories of Single Polyelectrolyte Chains. In Modeling and Simulation in Polymers; Wiley: Hoboken, NJ, USA, 2010; pp. 247–341. [Google Scholar] [CrossRef]
- Noguchi, H.; Yoshikawa, K. Morphological Variation in a Collapsed Single Homopolymer Chain. J. Chem. Phys. 1998, 109, 5070–5077. [Google Scholar] [CrossRef]
- Khokhlov, A.R.; Yu, A.; Grosberg, V.S.P. Statistical Physics of Macromolecules; AIP series in polymers and complex materials; AIP Press: Melville, NY, USA, 1994. [Google Scholar]
- Voevodin, V.V.; Antonov, A.S.; Nikitenko, D.A.; Shvets, P.A.; Sobolev, S.I.; Sidorov, I.Y.; Stefanov, K.S.; Voevodin, V.V.; Zhumatiy, S.A. Supercomputer Lomonosov-2: Large Scale, Deep Monitoring and Fine Analytics for the User Community. Supercomput. Front. Innov. 2019, 6, 4–11. [Google Scholar]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Portnov, I.V.; Larina, A.A.; Gumerov, R.A.; Potemkin, I.I. Swelling and Collapse of Cylindrical Polyelectrolyte Microgels. Polymers 2022, 14, 5031. https://doi.org/10.3390/polym14225031
Portnov IV, Larina AA, Gumerov RA, Potemkin II. Swelling and Collapse of Cylindrical Polyelectrolyte Microgels. Polymers. 2022; 14(22):5031. https://doi.org/10.3390/polym14225031
Chicago/Turabian StylePortnov, Ivan V., Alexandra A. Larina, Rustam A. Gumerov, and Igor I. Potemkin. 2022. "Swelling and Collapse of Cylindrical Polyelectrolyte Microgels" Polymers 14, no. 22: 5031. https://doi.org/10.3390/polym14225031