A Comparative Study of PMETAC-Modified Mesoporous Silica and Titania Thin Films for Molecular Transport Manipulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Thin-Film Characterization
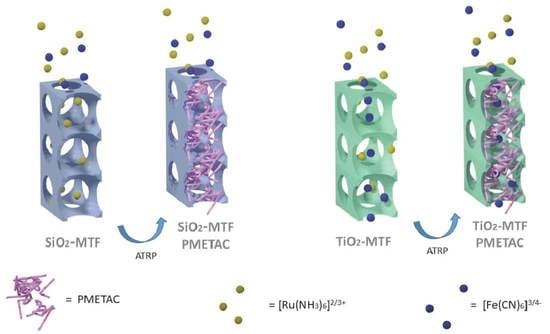
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mann, S.; Ozin, G.A. Synthesis of inorganic materials with complex form. Nature 1996, 382, 313–318. [Google Scholar] [CrossRef]
- Soler-Illia, G.J.A.A.; Azzaroni, O. Multifunctional hybrids by combining ordered mesoporous materials and macromolecular building blocks. Chem. Soc. Rev. 2011, 40, 1107. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Soler-Illia, G.J.A.A.; Azzaroni, O. Gated supramolecular chemistry in hybrid mesoporous silica nanoarchitectures: Controlled delivery and molecular transport in response to chemical, physical and biological stimuli. Chem. Commun. 2015, 51, 6050–6075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, D.L. Programmed Adsorption and Release of Proteins in a Microfluidic Device. Science 2003, 301, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Schoch, R.B.; Han, J.; Renaud, P. Transport phenomena in nanofluidics. Rev. Mod. Phys. 2008, 80, 839–883. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.; Arribart, H.; Guiraud-Guille, M.M. Biomimetism and bioinspiration as tools for the design of innovative materials and systems. Nat. Mater. 2005, 4, 277–288. [Google Scholar] [CrossRef]
- Soler-Illia, G.J.d.A.A.; Sanchez, C.; Lebeau, B.; Patarin, J. Chemical Strategies to Design Textured Materials: From Microporous and Mesoporous Oxides to Nanonetworks and Hierarchical Structures. Chem. Rev. 2002, 102, 4093–4138. [Google Scholar] [CrossRef]
- Azzaroni, O. Polymer Brushes Here, There, and Everywhere: Recent Advances in Their Practical Applications and Emerging Opportunities in Multiple Research Fields. Polym. Chem. 2012, 50, 3225–3258. [Google Scholar] [CrossRef]
- Brown, A.A.; Khan, N.S.; Steinbock, L.; Huck, W.T.S. Synthesis of oligo(ethylene glycol) methacrylate polymer brushes. Eur. Polym. J. 2005, 41, 1757–1765. [Google Scholar] [CrossRef]
- Fu, Q.; Rao, G.V.R.; Ista, L.K.; Wu, Y.; Andrzejewski, B.P.; Sklar, L.A.; Ward, T.L.; López, G.P. Control of Molecular Transport Through Stimuli-Responsive Ordered Mesoporous Materials. Adv. Mater. 2003, 15, 1262–1266. [Google Scholar] [CrossRef]
- Calvo, A.; Yameen, B.; Williams, F.J.; Soler-Illia, G.J.A.A.; Azzaroni, O. Mesoporous Films and Polymer Brushes Helping Each Other to Modulate Ionic Transport in Nanoconfined Environments. An Interesting Example of Synergism in Functional Hybrid Assemblies. J. Am. Chem. Soc. 2009, 131, 10866–10868. [Google Scholar] [CrossRef]
- Calvo, A.; Yameen, B.; Williams, F.J.; Azzaroni, O.; Soler-Illia, G.J.A.A. Facile molecular design of hybrid functional assemblies with controllable transport properties: Mesoporous films meet polyelectrolyte brushes. Chem. Commun. 2009, 18, 2553. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Lunn, J.D.; Shantz, D.F.; Jones, C.W. Poly(L-lysine) Brush-Mesoporous Silica Hybrid Material as a Biomolecule-Based Adsorbent for CO2 Capture from Simulated Flue Gas and Air. Chem.-A Eur. J. 2011, 17, 10556–10561. [Google Scholar] [CrossRef]
- Choi, M.; Kleitz, F.; Liu, D.; Lee, H.Y.; Ahn, W. Controlled Polymerization in Mesoporous Silica toward the Design of Organic—Inorganic Composite Nanoporous Materials. J. Am. Chem. Soc. 2005, 127, 1924–1932. [Google Scholar] [CrossRef]
- Lunn, J.D.; Shantz, D.F. Peptide Brush—Ordered Mesoporous Silica Nanocomposite Materials. Chem. Mater. 2009, 21, 3638–3648. [Google Scholar] [CrossRef]
- Rafti, M.; Brunsen, A.; Fuertes, M.C.; Azzaroni, O.; Soler-Illia, G.J.A.A. Heterogeneous Catalytic Activity of Platinum Nanoparticles Hosted in Mesoporous Silica Thin Films Modified with Polyelectrolyte Brushes. ACS Appl. Mater. Interfaces 2013, 5, 8833–8840. [Google Scholar] [CrossRef]
- Wang, J.-S.; Matyjaszewski, K. Controlled/“living” radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Kato, M.; Kamigaito, M.; Sawamoto, M.; Higashimura, T. Polymerization of Methyl Methacrylate with the Carbon Tetrachloride/Dichlorotris-(triphenylphosphine)ruthenium(II)/Methylaluminum Bis(2,6-di-tert-butylphenoxide) Initiating System: Possibility of Living Radical Polymerization. Macromolecules 1995, 28, 1721–1723. [Google Scholar] [CrossRef]
- Patten, T.E.; Xia, J.; Abernathy, T.; Matyjaszewski, K. Polymers with Very Low Polydispersities from Atom Transfer Radical Polymerization. Science 1996, 272, 866–868. [Google Scholar] [CrossRef]
- Angelomé, P.C.; Soler-Illia, G.J.d.A.A. Organically Modified Transition-Metal Oxide Mesoporous Thin Films and Xerogels. Chem. Mater. 2005, 17, 322–331. [Google Scholar] [CrossRef]
- Kruk, M.; Dufour, B.; Celer, E.B.; Kowalewski, T.; Jaroniec, M.; Matyjaszewski, K. Synthesis of Mesoporous Carbons Using Ordered and Disordered Mesoporous Silica Templates and Polyacrylonitrile as Carbon Precursor. J. Phys. Chem. B 2005, 109, 9216–9225. [Google Scholar] [CrossRef]
- Kruk, M.; Dufour, B.; Celer, E.B.; Kowalewski, T.; Jaroniec, M.; Matyjaszewski, K. Grafting Monodisperse Polymer Chains from Concave Surfaces of Ordered Mesoporous Silicas. Macromolecules 2008, 41, 8584–8591. [Google Scholar] [CrossRef]
- Yu, F.; Tang, X.; Pei, M. Facile synthesis of PDMAEMA-coated hollow mesoporous silica nanoparticles and their pH-responsive controlled release. Microporous Mesoporous Mater. 2013, 173, 64–69. [Google Scholar] [CrossRef]
- Angelomé, P.C. Films Delgados Mesoporosos de Óxidos Metálicos, Mixtos e Híbridos. Hacia un Diseño Racional de Nanomateriales Funcionales. Ph.D. Thesis, Universidad de Buenos Aires, Buenos Aires, Argentina, 2008. [Google Scholar]
- Boissiere, C.; Grosso, D.; Lepoutre, S.; Nicole, L.; Bruneau, A.B.; Sanchez, C. Porosity and mechanical properties of mesoporous thin films assessed by environmental ellipsometric porosimetry. Langmuir 2005, 21, 12362–12371. [Google Scholar] [CrossRef]
- Brunsen, A.; Díaz, C.; Pietrasanta, L.I.; Yameen, B.; Ceolín, M.; Soler-Illia, G.J.; Azzaroni, O. Proton and calcium-gated ionic mesochannels: Phosphate-bearing polymer brushes hosted in mesoporous thin films as biomimetic interfacial architectures. Langmuir 2012, 28, 3583–3592. [Google Scholar] [CrossRef]
- Fattakhova-Rohlfing, D.; Wark, M.; Brezesinski, T.; Smarsly, B.M.; Rathouský, J. Highly Organized Mesoporous TiO2 Films with Controlled Crystallinity: A Li-Insertion Study. Adv. Funct. Mater. 2007, 17, 123–132. [Google Scholar] [CrossRef]
- Tagliazucchi, M.; Williams, F.J.; Calvo, E.J. Effect of acid-base equilibria on the donnan potential of layer-by-layer redox polyelectrolyte multilayers. J. Phys. Chem. B 2007, 111, 8105–8113. [Google Scholar] [CrossRef] [PubMed]
- Fattakhova-Rohlfing, D.; Wark, M.; Rathouský, J. Ion-Permselective pH-Switchable Mesoporous Silica Thin Layers. Chem. Mater. 2007, 19, 1640–1647. [Google Scholar] [CrossRef]
- Takahashi, S.; Watahiki, R.; Tomida, K.; Wang, B.; Anzai, J. Voltammetric Studies on Gold Electrodes Coated with Chitosan-Containing Layer-by-Layer Films. Materials 2013, 6, 5427–5439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amenitsch, H.; Bernstorff, S.; Kriechbaum, M.; Lombardo, D.; Mio, H.; Rappolt, M.; Laggner, P. Performance and First Results of the ELETTRA High-Flux Beamline for Small-Angle X-ray Scattering. J. Appl. Crystallogr. 1997, 30, 872–876. [Google Scholar] [CrossRef]
- Brinker, C.J.; Lu, Y.; Sellinger, A.; Fan, H. Evaporation-induced self-assembly: Functional nanostructures made easy. Adv. Mater. 1999, 11, 579–585. [Google Scholar] [CrossRef]
- MH Lim, A.S. Comparative studies of grafting and direct syntheses of inorganic− organic hybrid mesoporous materials. Chem. Mater. 1999, 11, 3285–3295. [Google Scholar] [CrossRef]
- Elbert, J.; Krohm, F.; Rüttiger, C.; Kienle, S.; Didzoleit, H.; Balzer, B.N.; Hugel, T.; Stühn, B.; Gallei, M.; Brunsen, A. Polymer-Modifi ed Mesoporous Silica Thin Films for Redox-Mediated Selective Membrane Gating. Adv. Funct. Mater. 2014, 24, 1591–1601. [Google Scholar] [CrossRef]
- Krohm, F.; Didzoleit, H.; Schulze, M.; Dietz, C.; Stark, R.W.; Hess, C.; Stühn, B.; Brunsen, A. Controlling polymerization initiator concentration in mesoporous silica thin films. Langmuir 2014, 30, 369–379. [Google Scholar] [CrossRef]
- Braun, K.; Pochert, A.; Beck, M.; Fiedler, R. Dissolution kinetics of mesoporous silica nanoparticles in different simulated body fluids. J. Sol-Gel Sci. Technol. 2016, 79, 319–327. [Google Scholar] [CrossRef]
- Alberti, S.; Steinberg, P.Y.; Giménez, G.; Amenitsch, H.; Ybarra, G.; Azzaroni, O.; Angelomé, P.C.; Soler-Illia, G.J.A.A. Chemical Stability of Mesoporous Oxide Thin Film Electrodes under Electrochemical Cycling: From Dissolution to Stabilization. Langmuir 2019, 35, 6279–6287. [Google Scholar] [CrossRef]
- Calvo, A.; Fuertes, M.C.; Yameen, B.; Williams, F.J.; Azzaroni, O.; Soler-illia, G.J.A.A. Nanochemistry in Confined Environments: Polyelectrolyte Brush-Assisted Synthesis of Gold Nanoparticles inside Ordered Mesoporous Thin Films. Langmuir 2010, 26, 5559–5567. [Google Scholar] [CrossRef]
- Silies, L.; Gonzalez Solveyra, E.; Szleifer, I.; Andrieu-Brunsen, A. Insights into the Role of Counterions on Polyelectrolyte-Modified Nanopore Accessibility. Langmuir 2018, 34, 5943–5953. [Google Scholar] [CrossRef]
- Crepaldi, E.L.; Soler-Illia, G.J.d.A.A.; Grosso, D.; Cagnol, F.; Ribot, F.; Sanchez, C. Controlled Formation of Highly Organized Mesoporous Titania Thin Films: From Mesostructured Hybrids to Mesoporous Nanoanatase TiO2. J. Am. Chem. Soc. 2003, 125, 9770–9786. [Google Scholar] [CrossRef]
- Silies, L.; Didzoleit, H.; Hess, C.; Stühn, B.; Andrieu-Brunsen, A. Mesoporous thin films, zwitterionic monomers, and iniferter-initiated polymerization: Polymerization in a confined space. Chem. Mater. 2015, 27, 1971–1981. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, G.; Sun, B.; Chen, F.; Zhao, M.; Li, T. Tunable Shell Thickness in Silica Nanospheres Functionalized by a Hydrophobic PMMA-PSt Diblock Copolymer Brush via Activators Generated by Electron Transfer for Atom Transfer Radical Polymerization. Macromol. Chem. Phys. 2013, 214, 1602–1611. [Google Scholar] [CrossRef]
- Ogieglo, W.; Wormeester, H.; Eichhorn, K.-J.; Wessling, M.; Benes, N.E. In situ ellipsometry studies on swelling of thin polymer films: A review. Prog. Polym. Sci. 2015, 42, 42–78. [Google Scholar] [CrossRef]
- Kostruba, A.; Stetsyshyn, Y.; Vlokh, R. Method for determination of the parameters of transparent ultrathin films deposited on transparent substrates under conditions of low optical contrast. Appl. Opt. 2015, 54, 6208. [Google Scholar] [CrossRef] [PubMed]
- Kostruba, A.; Stetsyshyn, Y.; Mayevska, S.; Yakovlev, M.; Vankevych, P.; Nastishin, Y.; Kravets, V. Composition, thickness and properties of grafted copolymer brush coatings determined by ellipsometry: Calculation and prediction. Soft Matter 2018, 14, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Andrieu-Brunsen, A.; Micoureau, S.; Tagliazucchi, M.; Szleifer, I.; Azzaroni, O.; Soler-Illia, G.J.A.A. Mesoporous hybrid thin film membranes with PMETAC@Silica architectures: Controlling ionic gating through the tuning of polyelectrolyte density. Chem. Mater. 2015, 27, 808–821. [Google Scholar] [CrossRef]
- Muthusubramanian, N. Stimuli Responsive Polymer Actuated Microstructures Using Si-Atrp. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2013. [Google Scholar]
- Saint-André, S.; Albanese, F.; Soler-Illia, G.J.A.A.; Tagliazucchi, M. Charge percolation in redox-active thin membrane hybrids of mesoporous silica and poly(viologens). Phys. Chem. Chem. Phys. 2019, 21, 2743–2754. [Google Scholar] [CrossRef]
- Walcarius, A. Mesoporous Materials-Based Electrochemical Sensors. Electroanalysis 2015, 27, 1303–1340. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH-dependent surface charging and points of zero charge. V. Update. J. Colloid Interface Sci. 2011, 353, 120519. [Google Scholar] [CrossRef]
- Mueller, R.; Kammler, H.K.; Wegner, K.; Pratsinis, S.E. OH Surface Density of SiO2 and TiO2 by Thermogravimetric Analysis. Langmuir 2003, 19, 160–165. [Google Scholar] [CrossRef]
- Kanta, A.; Sedev, R.; Ralston, J. Thermally- and Photoinduced Changes in the Water Wettability of Low-Surface-Area Silica and Titania. Langmuir 2005, 21, 2400–2407. [Google Scholar] [CrossRef]
- Silies, L.; Andrieu-Brunsen, A. Programming Ionic Pore Accessibility in Zwitterionic Polymer Modified Nanopores. Langmuir 2018, 34, 807–816. [Google Scholar] [CrossRef]
- Piccinini, E.; Alberti, S.; Longo, G.S.; Berninger, T.; Breu, J.; Dostalek, J.; Azzaroni, O.; Knoll, W. Pushing the Boundaries of Interfacial Sensitivity in Graphene FET Sensors: Polyelectrolyte Multilayers Strongly Increase the Debye Screening Length. J. Phys. Chem. C 2018, 122, 10181–10188. [Google Scholar] [CrossRef]
- Calvo, A.; Joselevich, M.; Soler-Illia, G.J.A.A.; Williams, F.J. Chemical reactivity of amino-functionalized mesoporous silica thin films obtained by co-condensation and post-grafting routes. Microporous Mesoporous Mater. 2009, 121, 67–72. [Google Scholar] [CrossRef]
- Calvo, A.; Angelomé, P.C.; Sánchez, V.M.; Scherlis, D.A.; Williams, F.J.; Soler-Illia, G.J.A.A. Mesoporous Aminopropyl-Functionalized Hybrid Thin Films with Modulable Surface and Environment-Responsive Behavior. Chem. Mater. 2008, 20, 4661–4668. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alberti, S.; Giussi, J.; Azzaroni, O.; Soler-Illia, G.J.A.A. A Comparative Study of PMETAC-Modified Mesoporous Silica and Titania Thin Films for Molecular Transport Manipulation. Polymers 2022, 14, 4823. https://doi.org/10.3390/polym14224823
Alberti S, Giussi J, Azzaroni O, Soler-Illia GJAA. A Comparative Study of PMETAC-Modified Mesoporous Silica and Titania Thin Films for Molecular Transport Manipulation. Polymers. 2022; 14(22):4823. https://doi.org/10.3390/polym14224823
Chicago/Turabian StyleAlberti, Sebastian, Juan Giussi, Omar Azzaroni, and Galo J. A. A. Soler-Illia. 2022. "A Comparative Study of PMETAC-Modified Mesoporous Silica and Titania Thin Films for Molecular Transport Manipulation" Polymers 14, no. 22: 4823. https://doi.org/10.3390/polym14224823