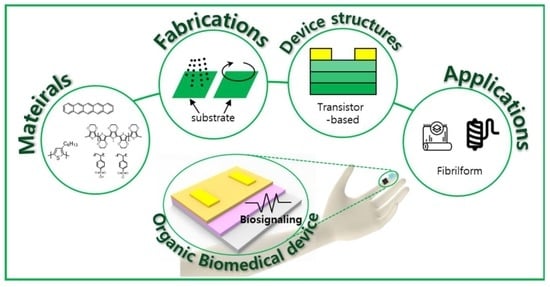
New Opportunities for Organic Semiconducting Polymers in Biomedical Applications
Abstract
:1. Introduction
2. Materials: Organic Semiconductors in Biomedical Engineering
2.1. p-Type Organic Semiconductors in Biomedical Engineering
2.2. n-Type Organic Semiconductors in Biomedical Engineering
3. Fabrication
3.1. Soft Lithography
3.2. Direct Writing Techniques
4. Device Structure
5. Applications
5.1. OFETs on a Flexible Substrate
5.2. OFETs on Unconventional Substrates
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, S.; Zhao, H.; Zhou, L.; Zhou, X.; Iyer, P.K.; Lv, F.; Liu, L.; Wang, S. An Optoelectronic Device for Rapid Monitoring of Creatine Kinase Using Cationic Conjugated Polyelectrolyte. Adv. Mater. Technol. 2019, 4, 1900361. [Google Scholar] [CrossRef]
- Giannini, S.; Blumberger, J. Charge Transport in Organic Semiconductors: The Perspective from Nonadiabatic Molecular Dynamics. Acc. Chem. Res. 2022, 55, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Paterson, A.F.; Singh, S.; Fallon, K.J.; Hodsden, T.; Han, Y.; Schroeder, B.C.; Bronstein, H.; Heeney, M.; McCulloch, I.; Anthopoulos, T.D. Recent Progress in High-Mobility Organic Transistors: A Reality Check. Adv. Mater. 2018, 30, 1801079. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, D.; Inal, S. Organic Bioelectronics: From Functional Materials to Next-Generation Devices and Power Sources. Adv. Mater. 2020, 32, 2001439. [Google Scholar] [CrossRef]
- Chen, X.; Hussain, S.; Hao, Y.; Tian, X.; Gao, R. Review—Recent Advances of Signal Amplified Smart Conjugated Polymers for Optical Detection on Solid Support. ECS J. Solid State Sci. Technol. 2021, 10, 037006. [Google Scholar] [CrossRef]
- Chen, X.; Hussain, S.; Abbas, A.; Hao, Y.; Malik, A.H.; Tian, X.; Song, H.; Gao, R. Conjugated polymer nanoparticles and their nanohybrids as smart photoluminescent and photoresponsive material for biosensing, imaging, and theranostics. Microchim. Acta 2022, 189, 83. [Google Scholar] [CrossRef]
- Roth, B.; Savagatrup, S.; de los Santos, N.V.; Hagemann, O.; Carlé, J.E.; Helgesen, M.; Livi, F.; Bundgaard, E.; Søndergaard, R.R.; Krebs, F.C.; et al. Mechanical Properties of a Library of Low-Band-Gap Polymers. Chem. Mater. 2016, 28, 2363–2373. [Google Scholar] [CrossRef] [Green Version]
- Root, S.E.; Savagatrup, S.; Printz, A.D.; Rodriquez, D.; Lipomi, D.J. Mechanical Properties of Organic Semiconductors for Stretchable, Highly Flexible, and Mechanically Robust Electronics. Chem. Rev. 2017, 117, 6467–6499. [Google Scholar] [CrossRef]
- Hopkins, J.; Fidanovski, K.; Lauto, A.; Mawad, D. All-Organic Semiconductors for Electrochemical Biosensors: An Overview of Recent Progress in Material Design. Front. Bioeng. Biotechnol. 2019, 7, 237. [Google Scholar] [CrossRef]
- Bonaventura, G.; Iemmolo, R.; La Cognata, V.; Zimbone, M.; La Via, F.; Fragalà, M.E.; Barcellona, M.L.; Pellitteri, R.; Cavallaro, S. Biocompatibility between Silicon or Silicon Carbide surface and Neural Stem Cells. Sci. Rep. 2019, 9, 11540. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Malugin, A.; Ghandehari, H. Impact of silica nanoparticle design on cellular toxicity and hemolytic activity. ACS Nano 2011, 5, 5717–5728. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, I.L.; Fritsch-Decker, S.; Leidner, A.; Al-Rawi, M.; Hug, V.; Diabaté, S.; Grage, S.L.; Meffert, M.; Stoeger, T.; Gerthsen, D.; et al. Biocompatibility of Amine-Functionalized Silica Nanoparticles: The Role of Surface Coverage. Small 2019, 15, 1805400. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.S.; Adamcakova-Dodd, A.; Lehman, S.E.; Wongrakpanich, A.; Thorne, P.S.; Larsen, S.C.; Salem, A.K. Amine modification of nonporous silica nanoparticles reduces inflammatory response following intratracheal instillation in murine lungs. Toxicol. Lett. 2015, 241, 207–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šafaříková, E.; Šindlerová, L.Š.; Stříteský, S.; Kubala, L.; Vala, M.; Weiter, M.; Víteček, J. Evaluation and improvement of organic semiconductors’ biocompatibility towards fibroblasts and cardiomyocytes. Sens. Actuators B Chem. 2018, 260, 418–425. [Google Scholar] [CrossRef]
- Roh, J.; Lee, T.; Kang, C.-M.; Kwak, J.; Lang, P.; Horowitz, G.; Kim, H.; Lee, C. Injection-modulated polarity conversion by charge carrier density control via a self-assembled monolayer for all-solution-processed organic field-effect transistors. Sci. Rep. 2017, 7, 46365. [Google Scholar] [CrossRef]
- Zeglio, E.; Rutz, A.L.; Winkler, T.E.; Malliaras, G.G.; Herland, A. Conjugated Polymers for Assessing and Controlling Biological Functions. Adv. Mater. 2019, 31, 1806712. [Google Scholar] [CrossRef]
- Dong, H.; Fu, X.; Liu, J.; Wang, Z.; Hu, W. 25th anniversary article: Key points for high-mobility organic field-effect transistors. Adv. Mater. 2013, 25, 6158–6183. [Google Scholar] [CrossRef]
- Kang, B.; Jang, M.; Chung, Y.; Kim, H.; Kwak, S.K.; Oh, J.H.; Cho, K. Enhancing 2D growth of organic semiconductor thin films with macroporous structures via a small-molecule heterointerface. Nat. Commun. 2014, 5, 4752. [Google Scholar] [CrossRef] [Green Version]
- Jurchescu, O.D.; Popinciuc, M.; van Wees, B.J.; Palstra, T. Interface-Controlled, High-Mobility Organic Transistors. Adv. Mater. 2007, 19, 688–692. [Google Scholar] [CrossRef]
- Ebata, H.; Izawa, T.; Miyazaki, E.; Takimiya, K.; Ikeda, M.; Kuwabara, H.; Yui, T. Highly soluble [1]benzothieno[3,2-b]benzothiophene (BTBT) derivatives for high-performance, solution-processed organic field-effect transistors. J. Am. Chem. Soc. 2007, 129, 15732–15733. [Google Scholar] [CrossRef]
- Minemawari, H.; Yamada, T.; Matsui, H.; Tsutsumi, J.Y.; Haas, S.; Chiba, R.; Kumai, R.; Hasegawa, T. Inkjet printing of single-crystal films. Nature 2011, 475, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Giri, G.; Ayzner, A.L.; Zoombelt, A.P.; Mannsfeld, S.C.B.; Chen, J.; Nordlund, D.; Toney, M.F.; Huang, J.; Bao, Z. Ultra-high mobility transparent organic thin film transistors grown by an off-centre spin-coating method. Nat. Commun. 2014, 5, 3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeya, J.; Yamagishi, M.; Tominari, Y.; Hirahara, R.; Nakazawa, Y.; Nishikawa, T.; Kawase, T.; Shimoda, T.; Ogawa, S. Very high-mobility organic single-crystal transistors with in-crystal conduction channels. Appl. Phys. Lett. 2007, 90, 102120. [Google Scholar] [CrossRef]
- Poimanova, E.Y.; Shaposhnik, P.A.; Anisimov, D.S.; Zavyalova, E.G.; Trul, A.A.; Skorotetcky, M.S.; Borshchev, O.V.; Vinnitskiy, D.Z.; Polinskaya, M.S.; Krylov, V.B.; et al. Biorecognition Layer Based On Biotin-Containing [1]Benzothieno[3,2-b][1]benzothiophene Derivative for Biosensing by Electrolyte-Gated Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces 2022, 14, 16462–16476. [Google Scholar] [CrossRef] [PubMed]
- Khodagholy, D.; Rivnay, J.; Sessolo, M.; Gurfinkel, M.; Leleux, P.; Jimison, L.H.; Stavrinidou, E.; Hervé, T.; Sanaur, S.; Owens, R.M.; et al. High transconductance organic electrochemical transistors. Nat. Commun. 2013, 4, 2133. [Google Scholar] [CrossRef]
- Nguyen-Dang, T.; Chae, S.; Harrison, K.; Llanes, L.C.; Yi, A.; Kim, H.J.; Biswas, S.; Visell, Y.; Bazan, G.C.; Nguyen, T.-Q. Efficient Fabrication of Organic Electrochemical Transistors via Wet Chemical Processing. ACS Appl. Mater. Interfaces 2022, 14, 12469–12478. [Google Scholar] [CrossRef]
- Tang, K.; Miao, W.; Guo, S. Crosslinked PEDOT:PSS Organic Electrochemical Transistors on Interdigitated Electrodes with Improved Stability. ACS Appl. Polym. Mater. 2021, 3, 1436–1444. [Google Scholar] [CrossRef]
- Oh, J.H.; Lee, H.W.; Mannsfeld, S.C.B.; Stoltenberg, R.M.; Jung, E.; Jin, Y.W.; Kim, J.M.; Yoo, J.-B.; Bao, Z. Solution-processed, high-performance n-channel organic microwire transistors. Proc. Natl. Acad. Sci. USA 2009, 106, 6065–6070. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.; Oh, J.H.; Sun, Y.S.; Deppisch, M.; Krause, A.M.; Radacki, K.; Braunschweig, H.; Könemann, M.; Erk, P.; Bao, Z.; et al. High-Performance Air-Stable n-Channel Organic Thin Film Transistors Based on Halogenated Perylene Bisimide Semiconductors. J. Am. Chem. Soc. 2009, 131, 6215–6228. [Google Scholar] [CrossRef]
- Minder, N.A.; Ono, S.; Chen, Z.; Facchetti, A.; Morpurgo, A.F. Band-Like Electron Transport in Organic Transistors and Implication of the Molecular Structure for Performance Optimization. Adv. Mater. 2011, 24, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Laquindanum, J.G.; Katz, H.E.; Dodabalapur, A.; Lovinger, A.J. n-Channel Organic Transistor Materials Based on Naphthalene Frameworks. J. Am. Chem. Soc. 1996, 118, 11331–11332. [Google Scholar] [CrossRef]
- Shukla, D.; Nelson, S.F.; Freeman, D.C.; Rajeswaran, M.; Ahearn, W.G.; Meyer, D.M.; Carey, J.T. Thin-Film Morphology Control in Naphthalene-Diimide-Based Semiconductors: High Mobility n-Type Semiconductor for Organic Thin-Film Transistors. Chem. Mater. 2008, 20, 7486–7491. [Google Scholar] [CrossRef]
- He, T.; Stolte, M.; Würthner, F. Air-Stable n-Channel Organic Single Crystal Field-Effect Transistors Based on Microribbons of Core-Chlorinated Naphthalene Diimide. Adv. Mater. 2013, 25, 6951–6955. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tee, B.C.K.; Cha, J.J.; Cui, Y.; Chung, J.W.; Lee, S.Y.; Bao, Z. High-Mobility Field-Effect Transistors from Large-Area Solution-Grown Aligned C60 Single Crystals. J. Am. Chem. Soc. 2012, 134, 2760–2765. [Google Scholar] [CrossRef] [PubMed]
- Hahm, S.G.; Rho, Y.; Jung, J.; Kim, H.; Sajoto, T.; Kim, F.S.; Barlow, S.; Park, C.E.; Jenekhe, S.A.; Marder, S.R.; et al. High-Performance n-Channel Thin-Film Field-Effect Transistors Based on a Nanowire-Forming Polymer. Adv. Funct. Mater. 2012, 23, 2060–2071. [Google Scholar] [CrossRef]
- Liu, C.; Jang, J.; Xu, Y.; Kim, H.J.; Khim, D.; Park, W.-T.; Noh, Y.-Y.; Kim, J.-J. Effect of Doping Concentration on Microstructure of Conjugated Polymers and Characteristics in N-Type Polymer Field-Effect Transistors. Adv. Funct. Mater. 2014, 25, 758–767. [Google Scholar] [CrossRef]
- Kim, R.; Amegadze, P.S.K.; Kang, I.; Yun, H.-J.; Noh, Y.-Y.; Kwon, S.-K.; Kim, Y.-H. High-Mobility Air-Stable Naphthalene Diimide-Based Copolymer Containing Extended π-Conjugation for n-Channel Organic Field Effect Transistors. Adv. Funct. Mater. 2013, 23, 5719–5727. [Google Scholar] [CrossRef]
- Ding, L.; Wang, Z.-Y.; Wang, J.-Y.; Pei, J. Organic Semiconducting Materials Based on BDOPV: Structures, Properties, and Applications. Chin. J. Chem. 2019, 38, 13–24. [Google Scholar] [CrossRef]
- Kim, G.; Han, A.R.; Lee, H.R.; Lee, J.; Oh, J.H.; Yang, C. Acceptor–acceptor type isoindigo-based copolymers for high-performance n-channel field-effect transistors. Chem. Commun. 2014, 50, 2180–2183. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.L.; Oh, J.H.; Reichardt, A.D.; Bao, Z. Chlorination: A general route toward electron transport in organic semiconductors. J. Am. Chem. Soc. 2009, 131, 3733–3740. [Google Scholar] [CrossRef]
- Lee, J.Y.; Roth, S.; Park, Y.W. Anisotropic field effect mobility in single crystal pentacene. Appl. Phys. Lett. 2006, 88, 252106. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.-G.; Kim, J.; Jo, S.; Kang, J.; Jo, J.-W.; Lee, W.; Hwang, C.; Moon, J.; Yang, L.; et al. Scalable Sub-micron Patterning of Organic Materials Toward High Density Soft Electronics. Sci. Rep. 2015, 5, 14520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khim, D.; Baeg, K.-J.; Kim, J.; Kang, M.; Lee, S.-H.; Chen, Z.; Facchetti, A.; Kim, D.-Y.; Noh, Y.-Y. High performance and stable N-channel organic field-effect transistors by patterned solvent-vapor annealing. ACS Appl. Mater. Interfaces 2013, 5, 10745–10752. [Google Scholar] [CrossRef]
- He, Z.; Chen, J.; Sun, Z.; Szulczewski, G.; Li, D. Air-flow navigated crystal growth for TIPS pentacene-based organic thin-film transistors. Org. Electron. 2012, 13, 1819–1826. [Google Scholar] [CrossRef]
- Sakamoto, K.; Ueno, J.; Bulgarevich, K.D.; Miki, K. Anisotropic charge transport and contact resistance of 6,13-bis(triisopropylsilylethynyl) pentacene field-effect transistors fabricated by a modified flow-coating method. Appl. Phys. Lett. 2012, 100, 123301. [Google Scholar] [CrossRef]
- Giri, G.; Park, S.; Vosgueritchian, M.; Shulaker, M.M.; Bao, Z. High-mobility, aligned crystalline domains of TIPS-pentacene with metastable polymorphs through lateral confinement of crystal growth. Adv. Mater. 2013, 26, 487–493. [Google Scholar] [CrossRef]
- Chang, J.; Chi, C.; Zhang, J.; Wu, J. Controlled growth of large-area high-performance small-molecule organic single-crystalline transistors by slot-die coating using a mixed solvent system. Adv. Mater. 2013, 25, 6442–6447. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Tee, B.C.K.; Giri, G.; Xu, J.; Kim, H.; Becerril, H.A.; Stoltenberg, R.M.; Lee, T.H.; Xue, G.; Mannsfeld, S.C.B.; et al. Solution coating of large-area organic semiconductor thin films with aligned single-crystalline domains. Nat. Mater. 2013, 12, 665–671. [Google Scholar] [CrossRef]
- Bae, I.; Kang, S.J.; Shin, Y.J.; Park, Y.J.; Kim, R.H.; Mathevet, F.; Park, C. Tailored Single Crystals of Triisopropylsilylethynyl Pentacene by Selective Contact Evaporation Printing. Adv. Mater. 2011, 23, 3398–3402. [Google Scholar] [CrossRef]
- Dickey, K.C.; Subramanian, S.; Anthony, J.E.; Han, L.-H.; Chen, S.; Loo, Y.-L. Large-area patterning of a solution-processable organic semiconductor to reduce parasitic leakage and off currents in thin-film transistors. Appl. Phys. Lett. 2007, 90, 244103. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Jang, M.; Lee, M.; An, T.K.; Anthony, J.E.; Kim, H.; Yang, H.; Park, C.E. Unified film patterning and annealing of an organic semiconductor with micro-grooved wet stamps. J. Mater. Chem. C 2016, 4, 6996–7003. [Google Scholar] [CrossRef]
- Suh, K.Y.; Kim, Y.S.; Lee, H. Capillary Force Lithography. Adv. Mater. 2001, 13, 1386–1389. [Google Scholar] [CrossRef]
- Jo, P.S.; Vailionis, A.; Park, Y.M.; Salleo, A. Scalable fabrication of strongly textured organic semiconductor micropatterns by capillary force lithography. Adv. Mater. 2012, 24, 3269–3274. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Cho, B.; Baek, J.; Hwang, J.K.; Lee, H.; Sung, M.M. Single-Crystal Organic Nanowire Electronics by Direct Printing from Molecular Solutions. Adv. Funct. Mater. 2013, 23, 4776–4784. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.K.; Cho, S.; Dang, J.M.; Kwak, E.B.; Song, K.-K.; Moon, J.; Sung, M.M. Direct nanoprinting by liquid-bridge-mediated nanotransfer moulding. Nat. Nanotechnol. 2010, 5, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Rho, Y.; Kim, Y.; Kim, H.; Hahm, S.G.; Park, C.E. A Lattice-Strained Organic Single-Crystal Nanowire Array Fabricated via Solution-Phase Nanograting-Assisted Pattern Transfer for Use in High-Mobility Organic Field-Effect Transistors. Adv. Mater. 2016, 28, 3209–3215. [Google Scholar] [CrossRef]
- Felmet, K.; Loo, Y.-L.; Sun, Y. Patterning conductive copper by nanotransfer printing. Appl. Phys. Lett. 2004, 85, 3316–3318. [Google Scholar] [CrossRef]
- Kang, B.; Min, H.; Seo, U.; Lee, J.; Park, N.; Cho, K.; Lee, H.S. Directly Drawn Organic Transistors by Capillary Pen: A New Facile Patterning Method using Capillary Action for Soluble Organic Materials. Adv. Mater. 2013, 25, 4117–4122. [Google Scholar] [CrossRef]
- Kim, H.; Hong, K.; Lee, K.H.; Frisbie, C.D. Performance and stability of aerosol-jet-printed electrolyte-gated transistors based on poly(3-hexylthiophene). ACS Appl. Mater. Interfaces 2013, 5, 6580–6585. [Google Scholar] [CrossRef]
- Min, S.-Y.; Kim, T.S.; Kim, B.J.; Cho, H.; Noh, Y.-Y.; Yang, H.; Cho, J.H.; Lee, T.-W. Large-scale organic nanowire lithography and electronics. Nat. Commun. 2013, 4, 1773. [Google Scholar] [CrossRef] [Green Version]
- Park, S.J.; Lee, J.; Seo, S.E.; Kim, K.H.; Park, C.S.; Lee, S.H.; Ban, H.S.; Lee, B.D.; Song, H.S.; Kim, J.; et al. High-Performance Conducting Polymer Nanotube-based Liquid-Ion Gated Field-Effect Transistor Aptasensor for Dopamine Exocytosis. Sci. Rep. 2020, 10, 3772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Lee, S.; Yokota, T.; Jimbo, Y.; Wang, Y.; Nayeem, O.G.; Nishinaka, M.; Someya, T. Nanomesh Organic Electrochemical Transistor for Comfortable On-Skin Electrodes with Local Amplifying Function. ACS Appl. Electron. Mater. 2020, 2, 3601–3609. [Google Scholar] [CrossRef]
- Li, Z.; Xu, B.; Han, J.; Huang, J.; Fu, H. A Polycation-Modified Nanofillers Tailored Polymer Electrolytes Fiber for Versatile Biomechanical Energy Harvesting and Full-Range Personal Healthcare Sensing. Adv. Funct. Mater. 2021, 32, 2106731. [Google Scholar] [CrossRef]
- Parlak, O.; Keene, S.T.; Marais, A.; Curto, V.F.; Salleo, A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci. Adv. 2018, 4, eaar2904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, J.H.; Kim, Y.M.; Moon, H.C. Porous Ion Gel: A Versatile Ionotronic Sensory Platform for High-Performance, Wearable Ionoskins with Electrical and Optical Dual Output. ACS Nano 2021, 15, 15132–15141. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, K.; Sasaki, Y.; Zhou, Q.; Lyu, X.; Yamanashi, Y.; Nakahara, K.; Nagaoka, H.; Minami, T. Oxytocin detection at ppt level in human saliva by an extended-gate-type organic field-effect transistor. Analyst 2022, 147, 1055–1059. [Google Scholar] [CrossRef]
- Lai, S.; Viola, F.A.; Cosseddu, P.; Bonfiglio, A. Floating Gate, Organic Field-Effect Transistor-Based Sensors towards Biomedical Applications Fabricated with Large-Area Processes over Flexible Substrates. Sensors 2018, 18, 688. [Google Scholar] [CrossRef]
- Baek, S.; Kwon, J.; Mano, T.; Tokito, S.; Jung, S. A Flexible 3D Organic Preamplifier for a Lactate Sensor. Macromol. Biosci. 2020, 20, 2000144. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Park, S.; Yi, Y.; Kim, H.; Lim, J.A. Highly Sensitive Flexible Pressure Sensors Based on Printed Organic Transistors with Centro-Apically Self-Organized Organic Semiconductor Microstructures. ACS Appl. Mater. Interfaces 2017, 9, 42996–43003. [Google Scholar] [CrossRef]
- Jang, J.; Nam, S.; Hwang, J.; Park, J.-J.; Im, J.; Park, C.-E.; Kim, J.M. Photocurable polymer gate dielectrics for cylindrical organic field-effect transistors with high bending stability. J. Mater. Chem. 2012, 22, 1054–1060. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.M.; Kang, H.W.; Hwang, K.; Lim, H.S.; Ju, B.K.; Lim, J.A. Metal–Insulator–Semiconductor Coaxial Microfibers Based on Self-Organization of Organic Semiconductor:Polymer Blend for Weavable, Fibriform Organic Field-Effect Transistors. Adv. Funct. Mater. 2016, 26, 2706–2714. [Google Scholar] [CrossRef]
- Kim, H.; Kang, T.-H.; Ahn, J.; Han, H.; Park, S.; Kim, S.J.; Park, M.-C.; Paik, S.-H.; Hwang, K.; Yi, H.; et al. Spirally Wrapped Carbon Nanotube Microelectrodes for Fiber Optoelectronic Devices beyond Geometrical Limitations toward Smart Wearable E-Textile Applications. ACS Nano 2020, 14, 17213–17223. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, H.; Ahn, J.; Hwang, K.; Ju, H.; Park, M.-C.; Yang, H.; Kim, H.; Jang, H.W.; Lim, J.A. A New Architecture for Fibrous Organic Transistors Based on a Double-Stranded Assembly of Electrode Microfibers for Electronic Textile Applications. Adv. Mater. 2019, 31, 1900564. [Google Scholar] [CrossRef] [PubMed]













| Materials | Examples | Key Advantages | Challenges |
|---|---|---|---|
| Organic semiconductor | TIPS-pentacene P3HT PEDOT:PSS C10-DNTT C8-BTBT | Low cost, low temperature, large area solution process, light weight, flexibility and stretchability, tunable optical and electrical properties by synthetic routes. | Generally lower conductivity, lower field-effect mobility, lower thermal stability, lower lifetime. |
| Inorganic semiconductor | Si, Ge Oxide (e.g., In-Ga-Zn-O) III-V (e.g., GaAs, GaN, InN, AlN) II-VI (e.g., CdSe, CdS, ZnSe, ZnS, ZnTe) | Better conductivity, better field-effect mobility, thermal stability, long lifetime. | Hard, heavy, high-cost vacuum process. |
| Material | Device Structure | Representative Method | Application | Refs. |
|---|---|---|---|---|
| Carbon dots/ polyvinyl alcohol | Fiber triboelectric nanogenerator | Microwave-assisted pyrolytic reaction | Monitoring of physiological signals | [63] |
| Carboxylated polypyrrole nanotubes | Liquid-ion-gated FET | Reverse microemulsion polymerization | Dopamine detection | [61] |
| PEDOT:PSS on nanomesh | Organic electrochemical transistor | Spray coating of PEDOT:PSS | On-skin ECG signal detection | [62] |
| Porous PEA-r-PS-r-PDVB | Porous ion gel | Use of sugar template | Monitoring of human motions | [65] |
| PEDOT:PSS | Organic electrochemical transistor | Laser-patterned microcapillary | Cortisol sensing | [64] |
| PEDOT:PSS/ TIPS-Pentacene | Floating-gate transistor | Inkjet printing | Temperature sensing | [67] |
| DPP-DTT/ P[NDI2OD-T2] | Shared-gate structure | Spin-coating | Lactate sensing | [68] |
| C6-DNT-VW | Extended-gate transistor | Printing | Oxytocin sensing | [66] |
| diF-TES-ADT | Vertical transistor | Printing | Monitoring of the radial artery pulse | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Yoo, H.; Lee, E.K. New Opportunities for Organic Semiconducting Polymers in Biomedical Applications. Polymers 2022, 14, 2960. https://doi.org/10.3390/polym14142960
Kim K, Yoo H, Lee EK. New Opportunities for Organic Semiconducting Polymers in Biomedical Applications. Polymers. 2022; 14(14):2960. https://doi.org/10.3390/polym14142960
Chicago/Turabian StyleKim, Kyunghun, Hocheon Yoo, and Eun Kwang Lee. 2022. "New Opportunities for Organic Semiconducting Polymers in Biomedical Applications" Polymers 14, no. 14: 2960. https://doi.org/10.3390/polym14142960