Novel Hybrid Nanomaterials Based on Poly-N-Phenylanthranilic Acid and Magnetic Nanoparticles with Enhanced Saturation Magnetization
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Fe3O4/P-N-PAA
2.3. Synthesis of MNP/P-N-PAA
2.4. Characterization
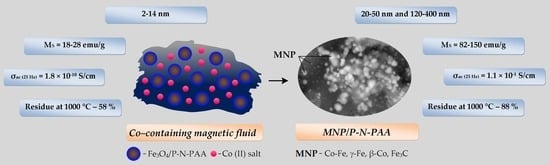
3. Results and Discussion
3.1. Characterization of Nanomaterials
3.2. Magnetic Properties of Nanomaterials
3.3. Electrical Properties of Nanomaterials
3.4. Thermal Properties of Nanomaterials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Godovsky, D.Y. Device applications of polymer-nanocomposites. Adv. Polym. Sci. 2000, 153, 163–205. [Google Scholar] [CrossRef]
- Karpacheva, G.P. Hybrid magnetic nanocomposites including polyconjugated polymers. Polym. Sci. Ser. C 2016, 58, 131–146. [Google Scholar] [CrossRef]
- Idumah, C.I. Novel trends in conductive polymeric nanocomposites, and bionanocomposites. Synth. Met. 2021, 273, 116674. [Google Scholar] [CrossRef]
- Ravindra, S.; Behzad, Y.; Kitirote, S.; Harikaranahalli, W.; Shivaraju, P. Potentiality of polymer nanocomposites for sustainable environmental applications: A review of recent advances. Polymer 2021, 233, 124184. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Sánchez-Marcos, J.; Herrasti, P. Magnetic nanoparticles-based conducting polymer nanocomposites. In Conducting Polymer Hybrids; Kumar, V., Kalia, S., Swart, H.C., Eds.; Springer Series on Polymer and Composite Materials; Chapter 2; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 45–80. ISSN1 2364-1878. ISSN2 2364-1886 (electronic). ISBN1 978-3-319-46456-5. ISBN2 978-3-319-46458-9 (eBook). [Google Scholar] [CrossRef]
- Al Betar, A.-R.F. Enhanced electrocatalytic water oxidation using cobalt-based polyaniline hybrid assembly. Synth. Met. 2021, 275, 116738. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Luo, X.; Guo, H.; Wang, N.; Su, D.; Zhang, C.; Liu, T.; Wang, G.; Cui, L. Polyaniline engineering defect-induced nitrogen doped carbon-supported Co3O4 hybrid composite as a high-efficiency electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2020, 526, 146626. [Google Scholar] [CrossRef]
- Shambharkar, B.H.; Umare, S.S. Production and characterization of polyaniline/Co3O4 nanocomposite as a cathode of Zn–polyaniline battery. Mater. Sci. Eng. B 2010, 175, 120–128. [Google Scholar] [CrossRef]
- Talooki, E.F.; Ghorbani, M.; Rahimnejad, M.; Lashkenari, M.S. Investigating the effects of in-situ fabrication of a binder-free Co3O4-polyaniline cathode towards enhanced oxygen reduction reaction and power generation of microbial fuel cells. Synth. Met. 2019, 258, 116225. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Chang, M.-Y.; Wang, Y.-Z.; Huang, Y.-C.; Ho, K.-S.; Hsieh, T.-H.; Kuo, Y.-C. Polyaniline based Pt-electrocatalyst for a proton exchanged membrane fuel cell. Polymers 2020, 12, 617. [Google Scholar] [CrossRef] [Green Version]
- Sadegh, F.; Modarresi-Alam, A.R.; Noroozifar, M.; Kerman, K. A facile and green synthesis of superparamagnetic Fe3O4@PANI nanocomposite with a core–shell structure to increase of triplet state population and efficiency of the solar cells. J. Env. Chem. Eng. 2021, 9, 104942. [Google Scholar] [CrossRef]
- Wang, K.; Yi, C.; Liu, C.; Hu, X.; Chuang, S.; Gong, X. Effects of magnetic nanoparticles and external magnetostatic field on the bulk heterojunction polymer solar cells. Sci. Rep. 2015, 5, 9265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, L.; Watson, B.W., II; Qin, Y. Hybrid conjugated polymer/magnetic nanoparticle composite nanofibers through cooperative non-covalent interactions. Nanoscale Adv. 2020, 2, 2462–2470. [Google Scholar] [CrossRef]
- Lv, H.; Pan, Q.; Song, Y.; Liu, X.-X.; Liu, T. A review on nano-/microstructured materials constructed by electrochemical technologies for supercapacitors. Nano-Micro Lett. 2020, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Verma, B. Synthesis of polymer composite based on polyaniline-acetylene black-copper ferrite for supercapacitor electrodes. Polymer 2019, 168, 61–69. [Google Scholar] [CrossRef]
- Kumar, M.P.; Lathika, L.M.; Mohanachandran, A.P.; Rakhi, R.B. A high-performance flexible supercapacitor anode based on polyaniline/Fe3O4 composite@carbon cloth. ChemistrySelect 2018, 3, 3234–3240. [Google Scholar] [CrossRef]
- Alsulami, Q.A.; Alharbi, L.M.; Keshk, S.M.A.S. Synthesis of a graphene oxide/ZnFe2O4/polyaniline nanocomposite and its structural and electrochemical characterization for supercapacitor application. Int. J. Energy Res. 2021, 46, 2438–2445. [Google Scholar] [CrossRef]
- Rajkumar, S.; Elanthamilan, E.; Merlin, J.P.; Sathiyan, A. Enhanced electrochemical behaviour of FeCo2O4/PANI electrode material for supercapacitors. J. Alloys Compd. 2021, 874, 159876. [Google Scholar] [CrossRef]
- Govan, J. Recent advances in magnetic nanoparticles and nanocomposites for the remediation of water resources. Magnetochemistry 2020, 6, 49. [Google Scholar] [CrossRef]
- Hlongwane, G.N.; Sekoai, P.T.; Meyyappan, M.; Moothi, K. Simultaneous removal of pollutants from water using nanoparticles: A shift from single pollutant control to multiple pollutant control. Sci. Total Environ. 2019, 656, 808–833. [Google Scholar] [CrossRef]
- Tahoon, M.A.; Siddeeg, S.M.; Salem Alsaiari, N.S.; Mnif, W.; Ben Rebah, F. Effective heavy metals removal from water using nanomaterials: A review. Processes 2020, 8, 645. [Google Scholar] [CrossRef]
- Rahdar, A.; Rahdar, S.; Labuto, G. Environmentally friendly synthesis of Fe2O3@SiO2 nanocomposite: Characterization and application as an adsorbent to aniline removal from aqueous solution. Environ. Sci. Pollut. Res. 2020, 27, 9181–9191. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.H.; Hassan, A.A.; Amr, A.E.-G.E.; El-Shalakany, H.H.; Al-Omar, A.M. Synthesis and characterization of CuFe2O4 nanoparticles modified with polythiophene: Applications to mercuric ions removal. Nanomaterials 2020, 10, 586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momina, K.A. Study of different polymer nanocomposites and their pollutant removal efficiency: Review. Polymer 2021, 217, 123453. [Google Scholar] [CrossRef]
- Rezvani, M.; Asgharinezhad, A.A.; Ebrahimzadeh, H.; Shekari, N. A polyaniline-magnetite nanocomposite as an anion exchange sorbent for solid-phase extraction of chromium (VI) ions. Microchim. Acta 2014, 181, 1887–1895. [Google Scholar] [CrossRef]
- Lei, C.; Wang, C.; Chen, W.; He, M.; Huang, B. Polyaniline@magnetic chitosan nanomaterials for highly efficient simultaneous adsorption and in-situ chemical reduction of hexavalent chromium: Removal efficacy and mechanisms. Sci. Total Environ. 2020, 733, 139316. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Almesfer, M.K.; Elkhaleefa, A.; Shigidi, I.; Shamim, M.Z.; Ali, I.H.; Rehan, M. Conductive polymers and their nanocomposites as adsorbents in environmental applications. Polymers 2021, 13, 3810. [Google Scholar] [CrossRef]
- Bhaumik, M.; Leswifi, T.Y.; Maity, A.; Shrinivasu, V.V.; Onyango, M.S. Removal of fluoride from aqueous solution by polypyrrole/Fe3O4 magnetic nanocomposite. J. Hasardous Mater. 2011, 186, 150–159. [Google Scholar] [CrossRef]
- Jokar, M.; Foroutani, R.; Safaralizadeh, M.H.; Farhadi, K. Synthesis and characterization of polyaniline/Fe3O4 magnetic nanocomposite as practical approach for fluoride removal process. Ann. Res. Rev. Biol. 2014, 4, 3262–3273. [Google Scholar] [CrossRef]
- Bober, P.; Minisy, I.M.; Acharya, U.; Pfleger, J.; Babayan, V.; Kazantseva, N.; Hodan, J.; Stejskal, J. Conducting polymer composite aerogel with magnetic properties for organic dye removal. Synth. Met. 2020, 260, 116266. [Google Scholar] [CrossRef]
- Zare, E.N.; Motahari, A.; Sillanpää, M. Nanoadsorbents based on conducting polymer nanocomposites with main focus on polyaniline and its derivatives for removal of heavy metal ions/dyes: A review. Environ. Res. 2018, 162, 173–195. [Google Scholar] [CrossRef]
- Sen, T.; Shimpi, N.G.; Mishra, S.; Sharma, R. Polyaniline/γ-Fe2O3 nanocomposite for room temperature LPG sensing. Sens. Actuators B 2014, 190, 120–126. [Google Scholar] [CrossRef]
- Alharthy, R.D.; Saleh, A. A novel trace-level ammonia gas sensing based on flexible PAni-CoFe2O4 nanocomposite film at room temperature. Polymers 2021, 13, 3077. [Google Scholar] [CrossRef] [PubMed]
- Batool, R.; Akhtar, M.A.; Hayat, A.; Han, D.; Niu, L.; Ahmad, M.A.; Nawaz, M.H. A nanocomposite prepared from magnetite nanoparticles, polyaniline and carboxy-modified graphene oxide for non-enzymatic sensing of glucose. Microchim. Acta 2019, 186, 267. [Google Scholar] [CrossRef] [PubMed]
- Bandgar, D.K.; Navale, S.T.; Naushad, M.; Mane, R.S.; Stadler, F.J.; Patil, V.B. Ultra-sensitive polyaniline–iron oxide nanocomposite room temperature flexible ammonia sensor. RSC Adv. 2015, 5, 68964–68971. [Google Scholar] [CrossRef]
- Malook, K.; Khan, H.; Shah, M. Ammonia sensing behavior of polypyrrole-bimetallic oxide composites. Polym. Composite. 2020, 41, 2610–2615. [Google Scholar] [CrossRef]
- Umare, S.S.; Shambharkar, B.H. Synthesis, characterization, and corrosion inhibition study of polyaniline-α-Fe2O3 nanocomposite. J. Appl. Polym. Sci. 2013, 127, 3349–3355. [Google Scholar] [CrossRef]
- Kumar, H.; Luthra, M.; Punia, M.; Singh, R.M. Co3O4/PANI nanocomposites as a photocatalytic, antibacterial and anticorrosive agent: Experimental and theoretical approach. Colloid Interface Sci. Commun. 2021, 45, 100512. [Google Scholar] [CrossRef]
- Kumar, H.; Boora, A.; Yadav, A.; Rajni; Rahul. Polyaniline-metal oxide-nano-composite as a nano-electronics, optoelectronics, heat resistance, and anticorrosive material. Results Chem. 2020, 2, 100046. [Google Scholar] [CrossRef]
- Zadeh, M.K.; Yeganeh, M.; Shoushtari, M.T.; Esmaeilkhanian, A. Corrosion performance of polypyrrole-coated metals: A review of perspectives and recent advances. Synth. Met. 2021, 274, 116723. [Google Scholar] [CrossRef]
- Zeng, X.J.; Cheng, X.Y.; Yu, R.H.; Stucky, G.D. Electromagnetic microwave absorption theory and recent achievements in microwave absorbers. Carbon 2020, 168, 606–623. [Google Scholar] [CrossRef]
- Luo, X.; Li, H.; Deng, D.; Zheng, L.; Wu, Y.; Luo, W.; Zhang, M.; Gong, R. Preparation and excellent electromagnetic absorption properties of dendritic structured Fe3O4@PANI composites. J. Alloys Compd. 2022, 891, 161922. [Google Scholar] [CrossRef]
- Mallikarjuna, N.N.; Manohar, S.K.; Kulkarni, P.V.; Venkataraman, A.; Aminabhavi, T.M. Novel high dielectric constant nanocomposites of polyaniline dispersed with γ-Fe2O3 nanoparticles. J. Appl. Polym. Sci. 2005, 97, 1868–1874. [Google Scholar] [CrossRef]
- Janem, N.; Azizi, Z.S.; Tehranchi, M.M. Microwave absorption and magnetic properties of thin-film Fe3O4@polypyrrole nanocomposites: The synthesis method effect. Synth. Met. 2021, 282, 116948. [Google Scholar] [CrossRef]
- Maruthi, N.; Faisal, M.; Raghavendra, N. Conducting polymer based composites as efficient EMI shielding materials: A comprehensive review and future prospects. Synth. Met. 2021, 272, 116664. [Google Scholar] [CrossRef]
- Xu, F.; Ma, L.; Huo, Q.; Gan, M.; Tang, J. Microwave absorbing properties and structural design of microwave absorbers based on polyaniline and polyaniline/magnetite nanocomposite. J. Magn. Magn. Mater. 2015, 374, 311–316. [Google Scholar] [CrossRef]
- Zhang, B.; Du, Y.; Zhang, P.; Zhao, H.; Kang, L.; Han, X.; Xu, P. Microwave absorption enhancement of Fe3O4/polyaniline core/shell hybrid microspheres with controlled shell thickness. J. Appl. Polym. Sci. 2013, 130, 1909–1916. [Google Scholar] [CrossRef]
- Wu, Z.C.; Tan, D.G.; Tian, K.; Hu, W.; Wang, J.J.; Su, M.X.; Li, L. Facile preparation of core-shell Fe3O4@polypyrrole composites with superior electromagnetic wave absorption properties. J. Phys. Chem. C 2017, 121, 15784–15792. [Google Scholar] [CrossRef]
- Tran, N.; Lee, M.Y.; Jeong, W.H.; Phan, T.L.; Tuan, N.Q.; Lee, B.W. Thickness independent microwave absorption performance of La-doped BaFe12O19 and polyaniline composites. J. Magn. Magn. Mater. 2021, 538, 168299. [Google Scholar] [CrossRef]
- Jia, H.; Xing, H.; Ji, X.; Gao, S. Synergistic effect of hexagonal flake Co3O4@PANI core–shell composites with excellent microwave-absorbing properties. J. Mater. Sci. Mater. Electron. 2019, 30, 3386–3395. [Google Scholar] [CrossRef]
- Manzoor, S.; Yasmin, G.; Raza, N.; Fernandez, J.; Atiq, R.; Chohan, S.; Iqbal, A.; Manzoor, S.; Malik, B.; Winter, F.; et al. Synthesis of polyaniline coated magnesium and cobalt oxide nanoparticles through eco-friendly approach and their application as antifungal agents. Polymers 2021, 13, 2669. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, X.; Song, L.; Cui, H.; Myers, J.; Tingting, B.; Zhou, Y.; Chen, Z.; Ning, G. Controlled drug release and hydrolysis mechanism of polymer–magnetic nanoparticle composite. ACS Appl. Mater. Interfaces 2015, 7, 9410–9419. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-H.; Ho, L.-C.; Wang, Y.-Z.; Ho, K.-S.; Tsai, C.-H.; Hung, L.-F. New inverse emulsion-polymerized iron/polyaniline composites for permanent, highly magnetic iron compounds via calcination. Polymers 2021, 13, 3240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Z.; Cheng, Y.-W.; Ho, L.-C.; Huang, W.-Y.; Ho, K.-S.; Syu, Y.-T. Superparamagnetic, high magnetic α-Fe & α″-Fe16N2 mixture prepared from inverse suspension-polymerized Fe3O4@polyaniline composite. Polymers 2021, 13, 2380. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, A.C.V.; de Oliveira, R.J.; Alves Junior, S.; Rodrigues, A.R.; Machado, F.L.A.; Cabral, F.A.O.; de Azevedo, W.M. Synthesis, characterization and magnetic properties of polyaniline-magnetite nanocomposites. Synth. Met. 2010, 160, 685–690. [Google Scholar] [CrossRef]
- Aphesteguy, J.C.; Jacobo, S.E. Synthesis of a soluble polyaniline–ferrite composite: Magnetic and electric properties. J. Mater. Sci. 2007, 42, 7062–7068. [Google Scholar] [CrossRef]
- Sapurina, I.; Bubulinca, C.; Trchova, M.; Prokes, J.; Stejskal, J. Conducting polypyrrole and polypyrrole/manganese dioxide composites prepared with a solid sacrificial oxidant of pyrrole. Synth. Met. 2021, 278, 116807. [Google Scholar] [CrossRef]
- Khan, A.; Aldwayan, A.S.; Alhoshan, M.; Alsalhi, M. Synthesis by in situ chemical oxidative polymerization and characterization of polyaniline/iron oxide nanoparticle composite. Polym. Int. 2010, 59, 1690–1694. [Google Scholar] [CrossRef]
- Tanriverdi, E.E.; Uzumcu, A.T.; Kavas, H.; Demir, A.; Baykal, A. Conductivity study of polyaniline-cobalt ferrite (PANI-CoFe2O4) nanocomposite. Nano-Micro Lett. 2011, 3, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Karpacheva, G.P.; Ozkan, S.Z.; Eremeev, I.S.; Bondarenko, G.N.; Dzidziguri, E.L.; Chernavskii, P.A. Synthesis of hybrid magnetic nanomaterial based on polydiphenylamine-2-carboxylic acid and Fe3O4 in the interfacial process. Eur. Chem. Bull. 2014, 3, 1001–1007. [Google Scholar] [CrossRef]
- Ozkan, S.Z.; Dzidziguri, E.L.; Chernavskii, P.A.; Karpacheva, G.P.; Efimov, M.N.; Bondarenko, G.N. Metal-polymer nanocomposites based on polydiphenylamine and cobalt nanoparticles. Nanotechnol. Russ. 2013, 8, 452–460. [Google Scholar] [CrossRef]
- Karpacheva, G.P.; Ozkan, S.Z.; Dzidziguri, E.L.; Chernavskii, P.A.; Eremeev, I.S.; Efimov, M.N.; Ivantsov, M.I.; Bondarenko, G.N. Hybrid metal-polymer nanocomposites based on polyphenoxazine and cobalt nanoparticles. Eur. Chem. Bull. 2015, 4, 135–141. [Google Scholar] [CrossRef]
- Ozkan, S.Z.; Karpacheva, G.P.; Dzidziguri, E.L.; Chernavskii, P.A.; Bondarenko, G.N.; Efimov, M.N.; Pankina, G.V. One step synthesis of hybrid magnetic material based on polyphenoxazine and bimetallic Co-Fe nanoparticles. Polym. Bull. 2017, 74, 3043–3060. [Google Scholar] [CrossRef]
- Ozkan, S.Z.; Karpacheva, G.P.; Dzidziguri, E.L.; Efimov, M.N.; Bondarenko, G.N.; Shandryuk, G.A.; Chernavskii, P.A.; Pankina, G.V. Iron-containing magnetic nanocomposites based on polyphenoxazine. J. Polym. Res. 2019, 26, 176. [Google Scholar] [CrossRef]
- Ozkan, S.Z.; Karpacheva, G.P.; Efimov, M.N.; Vasilev, A.A.; Muratov, D.G.; Petrov, V.A.; Chernavskii, P.A.; Pankina, G.V. One-step synthesis, characterization and properties of novel hybrid electromagnetic nanomaterials based on polydiphenylamine and Co-Fe particles in the absence and presence of single-walled carbon nanotubes. RSC Adv. 2021, 11, 24772–24786. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, S.Z.; Kostev, A.I.; Karpacheva, G.P. RU Patent for the Invention of “Nanocomposite Magnetic Material Based on a Polyconjugated Polymer and a Mixture of Magnetic Nanoparticles, and a Method for Production Thereof”. Russian Patent Application NO. 2768158 C1, 23 March 2022. [Google Scholar]
- Chernavskii, P.A.; Pankina, G.V.; Lunin, V.V. Magnetometric methods of investigation of supported catalysts. Russ. Chem. Rev. 2011, 80, 579–604. [Google Scholar] [CrossRef]
- Soloveva, A.Y.; Ioni, Y.V.; Gubin, S.P. Synthesis of Fe3O4 nanoparticles on the surface of graphene. Mendeleev Commun. 2016, 26, 38–39. [Google Scholar] [CrossRef]
- Jonscher, A.K. The universal dielectric response. Nature 1977, 267, 673–679. [Google Scholar] [CrossRef]
- Dyre, J.C.; Schrøder, T.B. Universality of ac conduction in disordered solids. Rev. Mod. Phys. 2000, 72, 873–892. [Google Scholar] [CrossRef] [Green Version]
- Xie, P.; Li, Y.; Hou, Q.; Sui, K.; Liu, C.; Fu, X.; Zhang, J.; Murugadoss, V.; Fan, J.; Wang, Y.; et al. Tunneling-induced negative permittivity in Ni/MnO nanocomposites by a bio-gel derived strategy. J. Mater. Chem. C 2020, 8, 3029–3039. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Ohashi, F.; Kameyama, T. Simple preparation of sulfate anion-doped polyaniline-clay nanocomposites by an environmentally friendly mechanochemical synthesis route. Macromol. Rapid Commun. 2004, 25, 1687–1691. [Google Scholar] [CrossRef]


















| Nanomaterials | T, °C | Co, wt% | Fe, wt% | Nano-Particles Size, nm | Me Phase Composition | HC, Oe | MS, emu/g | MR, emu/g | MR/MS |
|---|---|---|---|---|---|---|---|---|---|
| Co/PDPA [61] | 450 | 10 | - | 2–8 | α-Co, β-Co | 145 | 22.23 | 0.69 | 0.03 |
| Co/PPOA [62] | 500 | 10 | - | 4–14 | β-Co | 134 | 26.33 | 3.05 | 0.116 |
| Co-Fe/PDPA [65] | 600 | 5 | 10 | 8–30, 400–800 | Co-Fe | 5 | 20.43 | 0.06 | 0.003 |
| Co-Fe/PPOA [63] | 600 | 5 | 10 | 4–24, 400–1400 | Co-Fe | 55 | 27.28 | 0.7 | 0.025 |
| Materials | [Co] *, wt% | Co, % | Fe, % | C, % | N, % | H, % | O, % | C/N | C/H |
|---|---|---|---|---|---|---|---|---|---|
| P-N-PAA | - | - | - | 60.7 | 8.2 | 5.8 | 25.3 | 7.7 | 10.5 |
| Fe3O4/P-N-PAA | - | - | 38.5 | 23.58 | 2.75 | 1.88 | 33.3 | 8.6 | 12.5 |
| MNP/P-N-PAA | 20 | 29.1 | 58.3 | 8.48 | 0.94 | 0.12 | 3.1 | 9.0 | 70.7 |
| Nanomaterials | T, °C | [Co] *, wt% | [Fe] **, % | Co ***, % | Fe ***, % | **** MNP Phase Composition | HC, Oe | MS, emu/g | MR, emu/g | MR/MS |
|---|---|---|---|---|---|---|---|---|---|---|
| Fe3O4/P-N-PAA | 0 0 | - - | 16.4 38.5 | - - | 16.4 38.5 | Fe3O4 Fe3O4 | 0 0 | 18.41 27.58 | 0 0 | 0 0 |
| Fe3O4/P-N-PAA | 600 800 | - - | 16.4 38.5 | - - | 16.9 47.2 | Fe3O4 Fe3O4, FeO, α-Fe, γ-Fe, Fe4N | 0 25 | 17.02 12.41 | 0 0.28 | 0 0.022 |
| MNP/P-N-PAA | 800 800 800 700 800 | 5 10 20 20 30 | 16.4 16.4 16.4 38.5 16.4 | 8.6 13.6 28.3 29.1 38.0 | 19.2 17.4 14.2 58.3 17.7 | Co-Fe, γ-Fe, Fe3C Co-Fe, γ-Fe, β-Co Co-Fe, β-Co Co-Fe, β-Co Co-Fe, β-Co | 128 176 170 45 200 | 35.22 99.86 81.58 149.67 95.70 | 3.00 16.00 12.80 6.0 20.00 | 0.085 0.160 0.156 0.040 0.209 |
| Nanomaterials | MNP Phase Composition | * Polymer Component, % | Co | Fe | C | N | O | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt% | at% | wt% | at% | wt% | at% | wt% | at% | wt% | at% | |||
| Fe3O4/P-N-PAA | Fe3O4 | 28 | - | - | 12.99 | 3.43 | 49.45 | 60.76 | 8.84 | 9.32 | 28.72 | 26.49 |
| MNP/P-N-PAA | Co-Fe, β-Co | 16 | 72.44 | 55.26 | 19.63 | 15.80 | 7.05 | 26.39 | 0.19 | 0.62 | 0.68 | 1.92 |
| Materials | * σac, S/cm | σdc, S/cm | n | A | |
|---|---|---|---|---|---|
| P-N-PAA | 8.8 × 10−11 | 1.1 × 10−7 | 2.8 × 10−12 | 0.75 | 8.5 × 10−12 |
| Fe3O4/P-N-PAA | 1.8 × 10−10 | 6.7 × 10−6 | 1.3 × 10−10 | 0.99 | 1.3 × 10−12 |
| MNP/P-N-PAA | 1.1 × 10−1 | 1.2 × 10−1 | 1.1 × 10−1 | 0.98 | 6.5 × 10−10 |
| Materials | [Co] *, wt% | [Fe] **, % | Co ***, % | Fe ***, % | **** MNP Phase Composition | ^ T5%, °C | ^* T20%, °C | ^^ T50%, °C | ^^^ Residue, % |
|---|---|---|---|---|---|---|---|---|---|
| P-N-PAA | - | - | - | - | - | 185/205 | 357/299 | 523/663 | 0/20 |
| Fe3O4/P-N-PAA | - | 38.5 | - | 38.5 | Fe3O4 | 258/230 | 405/557 | >1000/>1000 | 72/58 |
| MNP/P-N-PAA | 5 | 16.4 | 8.6 | 19.2 | Co-Fe, γ-Fe, Fe3C | 102/111 | 459/>1000 | 579/>1000 | 40/77 |
| 30 | 16.4 | 38.0 | 17.7 | Co-Fe, β-Co | 108/371 | 507/>1000 | >1000/>1000 | 71/88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozkan, S.Z.; Kostev, A.I.; Chernavskii, P.A.; Karpacheva, G.P. Novel Hybrid Nanomaterials Based on Poly-N-Phenylanthranilic Acid and Magnetic Nanoparticles with Enhanced Saturation Magnetization. Polymers 2022, 14, 2935. https://doi.org/10.3390/polym14142935
Ozkan SZ, Kostev AI, Chernavskii PA, Karpacheva GP. Novel Hybrid Nanomaterials Based on Poly-N-Phenylanthranilic Acid and Magnetic Nanoparticles with Enhanced Saturation Magnetization. Polymers. 2022; 14(14):2935. https://doi.org/10.3390/polym14142935
Chicago/Turabian StyleOzkan, Sveta Zhiraslanovna, Aleksandr Ivanovich Kostev, Petr Aleksandrovich Chernavskii, and Galina Petrovna Karpacheva. 2022. "Novel Hybrid Nanomaterials Based on Poly-N-Phenylanthranilic Acid and Magnetic Nanoparticles with Enhanced Saturation Magnetization" Polymers 14, no. 14: 2935. https://doi.org/10.3390/polym14142935