Review on Modification of Glucomannan as an Excipient in Solid Dosage Forms
Abstract
:1. Introduction
2. Methodology
3. Polysaccharides from Different Sources
3.1. Natural Polysaccharides
3.2. Petrochemical Synthesis
4. Structure and Physicochemical Properties of GM
5. Extraction Optimization
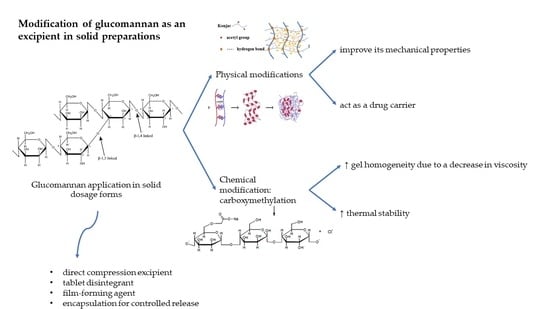
6. Chemical Modification
6.1. Increased Solubility
6.2. Reduced Viscosity
6.3. Increased Tensile Strength
6.4. Improved Thermal Stability
7. Physical Modification
8. GM application as an Excipient for Solid Dosage Forms
8.1. Direct Compression Excipient
8.2. Tablet Disintegrants
8.3. Film-Forming Agent
8.4. Sustained Release Agent
9. Discussion
10. Future Recommendations
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lajoinie, A.; Henin, E.; Kassai, B.; Terry, D. Solid oral forms availability in children: A cost saving investigation. Br. J. Clin. Pharmacol. 2014, 78, 1080–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.H.; Huang, G.Q.; Xu, T.C.; Xiao, J.X. Characterization of carboxymethylated konjac glucomannan for potential application in colon-targeted delivery. Food Hydrocoll. 2019, 94, 354–362. [Google Scholar] [CrossRef]
- Wu, C.; Sun, J.; Jiang, H.; Li, Y.; Pang, J. Construction of carboxymethyl konjac glucomannan/chitosan complex nanogels as potential delivery vehicles for curcumin. Food Chem. 2021, 362, 130242. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, J.; Lei, J.; Li, Y.; Chen, T.; Duan, T.; Yao, W.; Zhou, J.; Yu, Y.; Liu, Y. Silver nanoparticles incorporated konjac glucomannan-montmorillonite nacre-like composite films for antibacterial applications. Carbohydr. Polym. 2018, 197, 253–259. [Google Scholar] [CrossRef]
- Xiao, C.; Weng, L.; Zhang, L. Improvement of physical properties of crosslinked alginate and carboxymethyl konjac glucomannan blend films. J. Appl. Polym. Sci. 2002, 84, 2554–2560. [Google Scholar] [CrossRef]
- Harmayani, E.; Aprilia, V.; Marsono, Y. Characterization of glucomannan from Amorphophallus oncophyllus and its prebiotic activity in vivo. Carbohydr. Polym. 2014, 112, 475–479. [Google Scholar] [CrossRef]
- Yanuriati, A.; Marseno, D.W.; Rochmadi Harmayani, E. Characteristics of glucomannan isolated from fresh tuber of Porang (Amorphophallus muelleri Blume). Carbohydr. Polym. 2017, 156, 56–63. [Google Scholar] [CrossRef]
- Septiawan, A.R.; Darma, G.C.; Aryani, R. Preparation and Characterization of Glucomannan from Porang Bulbs (Amorphophallus muelleri Blume.) as a tablet binder. Pros. Farm. 2021, 7, 508–515. [Google Scholar] [CrossRef]
- Cui, T.; Liu, R.; Wu, T.; Sui, W.; Zhang, M. Influence of konjac glucomannan and frozen storage on rheological and tensile properties of frozen dough. Polymers 2019, 11, 794. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Li, J.; Chen, J.; Li, B. Effect of degree of deacetylation on physicochemical and gelation properties of konjac glucomannan. Food Res. Int. 2012, 46, 270–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Lindström, M.E.; Stepan, A.; Gatenholm, P. Spruce glucomannan: Preparation, structural characteristics and basic film forming ability. Nord. Pulp Pap. Res. J. 2013, 28, 323–330. [Google Scholar] [CrossRef]
- Wang, K.; Fan, J.; Liu, Y.; He, Z. Konjac glucomannan and xanthan gum as compression coat for colonic drug delivery: Experimental and theoretical evaluations. Front. Chem. Eng. China 2010, 4, 102–108. [Google Scholar] [CrossRef]
- Long, X.Y.; Luo, X.G.; Zou, N.W.; Ma, Y.H. Preparation and in vitro evaluation of Carboxymethyl konjac glucomannan coated 5-aminosalicylic acid tablets for colonic delivery. Adv. Mater. Res. 2011, 152–153, 1712–1715. [Google Scholar] [CrossRef]
- Wu, K.; Zhu, Q.; Qian, H.; Xiao, M.; Corke, H.; Nishinari, K.; Jiang, F. Controllable hydrophilicity-hydrophobicity and related properties of konjac glucomannan and ethyl cellulose composite films. Food Hydrocoll. 2018, 79, 301–309. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, M.; Li, R.; Cai, Z.; Zhang, H. Thermostable physically crosslinked cryogel from carboxymethylated konjac glucomannan fabricated by freeze-thawing. Food Hydrocoll. 2022, 122, 107103. [Google Scholar] [CrossRef]
- Ai, T.; Shang, L.; He, C.; Teng, Y.; Ren, C.; Zhou, P.; Wang, L.; Li, J.; Li, B. Development of multi-layered gastric floating tablets based on konjac glucomannan: A modified calcium supplement with enhanced bioavailability. Food Funct. 2019, 10, 6429–6437. [Google Scholar] [CrossRef]
- Zhu, G.-Q.; Zhang, Y.; Liu, J.-H. Studies on drug release from aminophylline konjac glucomannan matrix tablet. China J. Chin. Mater. Med. 2007, 32, 2236–2239. [Google Scholar]
- Liu, J.; Zhang, L.; Wang, C.; Yuan, P.; Xin, Y. Study on novel colon position pulsatile capsule and its release in vitro. China J. Chin. Mater. Med. 2010, 35, 3127–3130. [Google Scholar]
- Cuña, M.; Alonso-Sande, M.; Remunãn-López, C.; Pivel, J.P.; Alonso-Lebrero, J.L.; Alonso, M.J. Development of phosphorylated glucomannan-coated Chitosan nanoparticles as nanocarriers for protein delivery. J. Nanosci. Nanotechnol. 2006, 6, 2887–2895. [Google Scholar] [CrossRef]
- Deshpande, G.G.; Borate, H.P.; Wangikar, S.S. Fabrication and Characterization of Composite Material Connecting Rod. Techno-Societal 2021, 2, 87–95. [Google Scholar]
- Patria, D.G.; Sutrisno, A.; Sukamto, S.; Lin, J. Process optimization in the development of porang glucomannan (Amorphophallus mulleri B.) incorporated into the restructured rice using a pasta extruder: Physicochemical properties, cooking characteristics, and an estimated glycemic index. Food Sci. Technol. 2021, 42, 1–9. [Google Scholar] [CrossRef]
- Xie, W.; Du, Y.; Yuan, S.; Pang, J. Dihydromyricetin incorporated active films based on konjac glucomannan and gellan gum. Int. J. Biol. Macromol. 2021, 180, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhu, P.; Chen, N.; Ye, X.; Wang, Y.; Xiao, S. Preparation and sustainable release of modified konjac glucomannan/chitosan nanospheres. Int. J. Biol. Macromol. 2016, 91, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ye, T.; Zhou, B.; Li, J.; He, L.; Li, Y.; Liu, S.; Chen, Y.; Li, B. Fabrication of gastric floating controlled release tablet based on konjac glucomannan. Food Res. Int. 2015, 72, 47–53. [Google Scholar] [CrossRef]
- Chéret, R.; Chapleau, N.; Delbarre-Ladrat, C.; Verrez-Bagnis, V.; Lamballerie, M.D. Effects of High Pressure on Texture and Microstructure of Sea Bass (Dicentrarchus labrax L.) Fillets. Science 2005, 70, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Xu, X.; Gong, J.; Mu, R.; Li, Y.; Wu, C.; Pang, J. Fabrication of chitosan-coated konjac glucomannan/sodium alginate/graphene oxide microspheres with enhanced colon-targeted delivery. Int. J. Biol. Macromol. 2019, 131, 209–217. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Zheng, B.; Guo, Z. Effects of high pressure processing on gelation properties and molecular forces of myosin containing deacetylated konjac glucomannan. Food Chem. 2019, 291, 117–125. [Google Scholar] [CrossRef]
- Li, B.; Li, J.; Xia, J.; Kennedy, J.F.; Yie, X.; Liu, T.G. Effect of gamma irradiation on the condensed state structure and mechanical properties of konjac glucomannan/chitosan blend films. Carbohydr. Polym. 2011, 83, 44–51. [Google Scholar] [CrossRef]
- Fan, L.; Yang, J.; Wu, H.; Hu, Z.; Yi, J.; Tong, J.; Zhu, X. Preparation and characterization of quaternary ammonium chitosan hydrogel with significant antibacterial activity. Int. J. Biol. Macromol. 2015, 79, 830–836. [Google Scholar] [CrossRef]
- Al-Ghazzewi, F.H.; Tester, R.F. Efficacy of cellulase and mannanase hydrolysates of konjac glucomannan to promote the growth of lactic acid bacteria. J. Sci. Food Agric. 2012, 92, 2394–2396. [Google Scholar] [CrossRef]
- Mikkelson, A.; Maaheimo, H.; Hakala, T.K. Hydrolysis of konjac glucomannan by Trichoderma reesei mannanase and endoglucanases Cel7B and Cel5A for the production of glucomannooligosaccharides. Carbohydr. Res. 2013, 372, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Ogaji, I.J.; Nep, E.I.; Audu-Peter, J.D. Advances in Natural Polymers as Pharmaceutical Excipients. Pharm. Anal. Acta 2012, 3, 146. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Graft copolymers from cellulose: Synthesis, characterization and evaluation. Carbohydr. Polym. 2013, 97, 18–25. [Google Scholar] [CrossRef]
- Ciric, J.; Loos, K. Synthesis of branched polysaccharides with tunable degree of branching. Carbohydr. Polym. 2013, 93, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Chaudhury, A.; Maity, S.K.; Ghosh, R. Efficient routes toward the synthesis of the D-rhamno-trisaccharide related to the A-band polysaccharide of Pseudomonas aeruginosa. Beilstein J. Org. Chem. 2014, 10, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, V.; Mukhopadhyay, B. Chemical synthesis of the tetrasaccharide repeating unit of the O-polysaccharide isolated from Azospirillum brasilense SR80. Carbohydr. Res. 2015, 406, 65–70. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kashiwa, K.; Shimada, J.; Kawasaki, T.; Shoda, S. Enzymatic polymerization: The first in Vitro Sínthesis of Cellulose Via Nonbiosynthetic Path Catalyzed by Cellulase. Makromol. Chem. Macromol. Symp. 1992, 54–55, 509–518. [Google Scholar] [CrossRef]
- Uryu, T.; Yamanaka, M.; Date, M.; Ogawa, M.; Hatanaka, K. Selective synthesis of polysaccharide macromers by ring-opening polymerization of anhydro sugar. Macromolecules 1988, 21, 1916–1920. [Google Scholar] [CrossRef]
- Yagura, T.; Ikegami, W.; Kamitakahara, H.; Takano, T. Synthesis of an enantiomer of cellulose via cationic ring-opening polymerization. Cellulose 2020, 27, 9755–9766. [Google Scholar] [CrossRef]
- Shi, X.D.; Yin, J.Y.; Zhang, L.J.; Huang, X.J.; Nie, S.P. Studies on O-acetyl-glucomannans from Amorphophallus species: Comparison of physicochemical properties and primary structures. Food Hydrocoll. 2019, 89, 503–511. [Google Scholar] [CrossRef]
- Wu, W.T.; Cheng, H.C.; Chen, H.L. Ameliorative effects of konjac glucomannan on human faecal -glucuronidase activity, secondary bile acid levels and faecal water toxicity towards Caco-2 cells. Br. J. Nutr. 2011, 105, 593–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, J.; Wu, K.; Li, C.; Liu, J.; Ni, X.; Xiao, M.; Xu, Y.; Kuang, Y.; Jiang, F. pH-Sensitive drug delivery system based on hydrophobic modified konjac glucomannan. Carbohydr. Polym. 2017, 171, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, M.; Lv, W.; Qiu, P.; Gong, Y.; Li, D. Study on Rheological Behavior of Konjac Glucomannan. Phys. Procedia 2012, 33, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Tatirat, O.; Charoenrein, S. Physicochemical properties of konjac glucomannan extracted from konjac flour by a simple centrifugation process. LWT Food Sci. Technol. 2011, 44, 2059–2063. [Google Scholar] [CrossRef]
- Koroskenyi, B.; McCarthy, S.P. Synthesis of acetylated konjac glucomannan and effect of degree of acetylation on water absorbency. Biomacromolecules 2001, 2, 824–826. [Google Scholar] [CrossRef]
- Guo, L.; Yokoyama, W.; Chen, L.; Liu, F.; Chen, M.; Zhong, F. Characterization and physicochemical properties analysis of konjac glucomannan: Implications for structure-properties relationships. Food Hydrocoll. 2021, 120, 106818. [Google Scholar] [CrossRef]
- Alves, A.; Miguel, S.P.; Araujo, A.R.T.S.; Jes, D. Xanthan Gum–Konjac Glucomannan Blend Hydrogel for Wound Dressings. Polymers 2020, 12, 99. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Tian, J.; Zou, J.; Yuan, X.; Li, J.; Liang, H.; Zhan, F.; Li, B. Partial removal of acetyl groups in konjac glucomannan significantly improved the rheological properties and texture of konjac glucomannan and κ-carrageenan blends. Int. J. Biol. Macromol. 2019, 123, 1165–1171. [Google Scholar] [CrossRef]
- Gong, J.; Wang, L.; Wu, J.; Yuan, Y.; Mu, R.J.; Du, Y.; Wu, C.; Pang, J. The rheological and physicochemical properties of a novel thermosensitive hydrogel based on konjac glucomannan/gum tragacanth. LWT 2019, 100, 271–277. [Google Scholar] [CrossRef]
- Kurt, A.; Kahyaoglu, T. Characterization of a new biodegradable edible film made from salep glucomannan. Carbohydr. Polym. 2014, 104, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Blumentritt, M.; Gardner, D.J.; Cole, B.J.W.; Shaler, S.M. Influence of hot-water extraction on ultrastructure and distribution of glucomannans and xylans in poplar xylem as detected by gold immunolabeling. Holzforschung 2016, 70, 243–252. [Google Scholar] [CrossRef]
- Wardhani, D.H.; Nugroho, F.; Muslihudin, M.; Aryanti, N. Application of response surface method on purification of glucomannan from amorphophallus oncophyllus by using 2-propanol. Sci Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2016, 17, 63–74. [Google Scholar]
- Xu, W.; Wang, S.; Ye, T.; Jin, W.; Liu, J.; Lei, J.; Li, B.; Wang, C. A simple and feasible approach to purify konjac glucomannan from konjac flour–Temperature effect. Food Chem. 2014, 158, 171–176. [Google Scholar] [CrossRef]
- Nurlela Andriani, D.; Arizal, R. Extraction of Glucomannan from porang (Amorphophallus muelleri Blume) flour using Ethanol. J. Sains. Dan Terap. Kim. 2020, 14, 88–98. [Google Scholar] [CrossRef]
- Widjanarko, S.B.; Sutrisno, A.; Faridah, A. Effect of hydrogen peroxide on physicochemical properties of Common Konjac (Amorphophallus oncophyllus) flour by maceration and ultrasonic methods. J. Teknol. Pertan. 2011, 12, 143–152. [Google Scholar]
- Faridah, A.; Widjanarko, S.B. Optimization of Multilevel Ethanol Leaching Process of Porang Flour (Amorphophallus muelleri) Using Response Surface Methodology. Int. J. Adv. Sci. Eng. Inf. Technol. 2013, 3, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Ariesta, N.; Santosa, E.; Muhandri, T. Effect of harvest timing and length of storage time on glucomannan content in porang tubers. IOP Conf. Ser. Earth Environ. Sci. 2019, 299, 012012. [Google Scholar]
- Campestrini, L.H.; Silveira, J.L.M.; Duarte, M.E.R.; Koop, H.S.; Noseda, M.D. NMR and rheological study of Aloe barbadensis partially acetylated glucomannan. Carbohydr. Polym. 2013, 94, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Chua, M.; Chan, K.; Hocking, T.J.; Williams, P.A.; Perry, C.J.; Baldwin, T.C. Methodologies for the extraction and analysis of konjac glucomannan from corms of Amorphophallus konjac K. Koch. Carbohydr. Polym. 2012, 87, 2202–2210. [Google Scholar] [CrossRef]
- Chairul, C.; Chairul, S.M. Isolasi Glukomanan dari Dua Jenis Araceae: Talas {Colocasia esculenta (L.) Schott} dan Iles-iles (Amorphophallus campanulatus Blumei) [Isolation of Glucomannan from Two Species of Araceae: Talas {Colocasia esculenta (L.) Schott} and Iles-iles. Ber. Biol. 2006, 8, 171–178. [Google Scholar]
- Anindita, F.; Bahri, S.; Hardi, J. Extraction and Characterization of Glucomannan From Salak (Salacca Edulis Reinw.) Seed Flours. KOVALEN J. Ris. Kim. 2016, 2, 1–10. [Google Scholar]
- Darmawati, D.; Bahri, S.; Sosidi, H. Analysis of glucomannan content from durian seeds (Durio zeibethinus Murr) by spectrophotometric method at various times and hydrolysis temperatures. KOVALEN J. Ris. Kim. 2020, 6, 158–164. [Google Scholar] [CrossRef]
- Sareu, P.L.; Ridhay, A.; Mirzan, M. Extraction of Glucomannan from Gembili Bulbs (Dioscorea esculenta L.). KOVALEN J. Ris. Kim. 2021, 7, 51–58. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Chen, L.; Mi, Z.; Xu, Y.; Zhao, G.; Liu, S.; Lei, H.; Wang, Z.; Niu, J. Extraction, purification, and determination of the gastroprotective activity of glucomannan from Bletilla striata. Carbohydr. Polym. 2020, 246, 116620. [Google Scholar] [CrossRef] [PubMed]
- Fatmawati, S.; Nurgraheni, B.; Setyani, D.K. Ekstraksi Berbantu Ultrasonik dan Penetapan Kadar Glukomanan Dalam Umbi Porang (Amorphophallus oncophyllus). Media Farm. 2013, 11, 1075–1083. [Google Scholar]
- Huang, D.; Zhang, M.; Yi, P.; Yan, C. Structural characterization and osteoprotective effects of a novel oligo-glucomannan obtained from the rhizome of Cibotium barometz by alkali extraction. Ind. Crops Prod. 2018, 113, 202–209. [Google Scholar] [CrossRef]
- Jin, W.; Mei, T.; Wang, Y.; Xu, W.; Li, J.; Zhou, B.; Li, B. Synergistic degradation of konjac glucomannan by alkaline and thermal method. Carbohydr. Polym. 2014, 99, 270–277. [Google Scholar] [CrossRef]
- Su, L.; Ji, W.K.; Lan, W.Z.; Dong, X.Q. Chemical modification of xanthan gum to increase dissolution rate. Carbohydr. Polym. 2003, 53, 497–499. [Google Scholar] [CrossRef]
- Yu, H.; Xiao, C. Synthesis and properties of novel hydrogels from oxidized konjac glucomannan crosslinked gelatin for in vitro drug delivery. Carbohydr. Polym. 2008, 72, 479–489. [Google Scholar] [CrossRef]
- Lu, M.; Li, Z.; Liang, H.; Shi, M.; Zhao, L.; Li, W.; Chen, Y.; Wu, J.; Wang, S.; Chen, X.; et al. Controlled release of anthocyanins from oxidized konjac glucomannan microspheres stabilized by chitosan oligosaccharides. Food Hydrocoll. 2015, 51, 476–485. [Google Scholar] [CrossRef]
- Hongbo, T.; Lan, W.; Yanping, L.; Siqing, D. Effect of acidolysis and oxidation on structure and properties of konjac glucomannan. Int. J. Biol. Macromol. 2019, 130, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wu, Q.; Luo, X.; Liu, F.; Luo, X.; He, P. Effect of degree of acetylation on thermoplastic and melt rheological properties of acetylated konjac glucomannan. Carbohydr. Polym. 2010, 82, 167–172. [Google Scholar] [CrossRef]
- Wang, M.; He, W.; Wang, S.; Song, X. Carboxymethylated glucomannan as paper strengthening agent. Carbohydr. Polym. 2015, 125, 334–339. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tsujihata, S.; Hibi, N.; Tsukamoto, Y. Preparation and rheological characterization of carboxymethyl konjac glucomannan. Food Hydrocoll. 2002, 16, 289–294. [Google Scholar] [CrossRef]
- Xiao, M.; Dai, S.; Wang, L.; Ni, X.; Yan, W.; Fang, Y.; Corke, H.; Jiang, F. Carboxymethyl modification of konjac glucomannan affects water binding properties. Carbohydr. Polym. 2015, 130, 1–8. [Google Scholar] [CrossRef]
- Xie, Y.; Yi, Z.X.; Wang, J.X.; Hou, T.G.; Jiang, Q. Carboxymethyl konjac glucomannan-crosslinked chitosan sponges for wound dressing. Int. J. Biol. Macromol. 2018, 112, 1225–1233. [Google Scholar] [CrossRef]
- Fadilah Distantina, S.; Kaavessina, M.; Wijayanti, S.T.; Andayani, R. Study on the carboxymethylation of glucomannan from porang. AIP Conf. Proc. 2018, 1931, 030005. [Google Scholar]
- Wang, L.; Xiao, M.; Dai, S.; Song, J.; Ni, X.; Fang, Y.; Corke, H.; Jiang, F. Interactions between carboxymethyl konjac glucomannan and soy protein isolate in blended films. Carbohydr. Polym. 2014, 101, 136–145. [Google Scholar] [CrossRef]
- Xiao, J.X.; Wang, L.H.; Xu, T.C.; Huang, G.Q. Complex coacervation of carboxymethyl konjac glucomannan and chitosan and coacervate characterization. Int. J. Biol. Macromol. 2019, 123, 436–445. [Google Scholar] [CrossRef]
- Wang, C.; Li, B.; Chen, T.; Mei, N.; Wang, X.; Tang, S. Preparation and bioactivity of acetylated konjac glucomannan fibrous membrane and its application for wound dressing. Carbohydr. Polym. 2020, 229, 115404. [Google Scholar] [CrossRef] [PubMed]
- Korkiatithaweechai, S.; Umsarika, P.; Praphairaksit, N.; Muangsin, N. Controlled release of diclofenac from matrix polymer of chitosan and oxidized konjac glucomannan. Mar. Drugs 2011, 9, 1649–1663. [Google Scholar] [CrossRef]
- Liu, C.; Li, J.; Li, K.; Xie, C.; Liu, J. Oxidized konjac glucomannan-cassava starch and sucrose esters as novel excipients for sustained-release matrix tablets. Int. J. Biol. Macromol. 2020, 156, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Pelton, R.; Leduc, M. Mechanical properties of polyelectrolyte complex films based on polyvinylamine and carboxymethyl cellulose. Ind. Eng. Chem. Res. 2006, 45, 6665–6671. [Google Scholar] [CrossRef]
- Rashid, S.; Dutta, H. Physicochemical characterization of carboxymethyl cellulose from differently sized rice husks and application as cake additive. LWT 2022, 154, 112630. [Google Scholar] [CrossRef]
- Ohya, Y.; Ihara, K.; Murata, J.; Sugitou, T.; Ouchi, T. Preparation and biological properties of dicarboxy-glucomannan: Enzymatic degradation and stimulating activity against cultured macrophages. Carbohydr. Polym. 1994, 25, 123–130. [Google Scholar] [CrossRef]
- Sa, B.; Mukherjee, S.; Roy, S.K. Effect of polymer concentration and solution pH on viscosity affecting integrity of a polysaccharide coat of compression coated tablets. Int. J. Biol. Macromol. 2019, 125, 922–930. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, B.Z.; Wang, Q.L.; Jin, X.; Guo, X.D. Fabrication of coated polymer microneedles for transdermal drug delivery. J. Control. Release 2017, 265, 14–21. [Google Scholar] [CrossRef]
- Fowler, P.D.; Ruscher, C.; McGraw, J.D.; Forrest, J.A.; Dalnoki-Veress, K. Controlling Marangoni-induced instabilities in spin-cast polymer films: How to prepare uniform films. Eur. Phys. J. E 2016, 39, 90. [Google Scholar] [CrossRef]
- Nair, S.B.; Jyothi, A.N.; Sajeev, M.S.; Misra, R. Rheological, mechanical and moisture sorption characteristics of cassava starch-konjac glucomannan blend films. Starch-Staerke 2011, 63, 728–739. [Google Scholar] [CrossRef]
- Xu, M.; Li, D.S.; Li, B.; Wang, C.; Zhu, Y.P.; Lv, W.P.; Xie, B.J. Comparative study on molecular weight of konjac glucomannan by gel permeation chromatography-laser light scattering-refractive index and laser light-scattering methods. J. Spectrosc. 2013, 2013, 685698. [Google Scholar] [CrossRef]
- Jacon, S.A.; Rao, M.A.; Cooley, H.J.; Walter, R.H. The isolation and characterization of a water extract of konjac flour gum. Carbohydr. Polym. 1993, 20, 35–41. [Google Scholar] [CrossRef]
- He, Y.; Wang, S.; Li, J.; Liang, H.; Wei, X.; Peng, D.; Jiang, Z.; Li, B. Interaction between konjac glucomannan and tannic acid: Effect of molecular weight, pH and temperature. Food Hydrocoll. 2019, 94, 451–458. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, H.; Liu, X.; Li, Z.; Liu, B.; Wu, J.; Shi, M.; Norde, W.; Li, Y. TEMPO-oxidized Konjac glucomannan as appliance for the preparation of hard capsules. Carbohydr. Polym. 2016, 143, 262–269. [Google Scholar] [CrossRef]
- Bin, L.K.; Mohammed Helaluddin, A.B.; Islam Sarker, M.Z.; Mandal, U.K.; Gaurav, A. Effect of processing methods on xylitol-starch base co-processed adjuvant for orally disintegrating tablet application. Pak. J. Pharm. Sci. 2020, 33, 551–559. [Google Scholar]
- Narang, A.S.; Boddu, S.H. Excipient applications in formulation design and drug delivery. In Excipient Applications in Formulation Design and Drug Delivery; Springer: Cham, Switzerland, 2015; pp. 1–681. [Google Scholar]
- Hardikar, S.; Bhosale, A. Formulation and evaluation of gastro retentive tablets of clarithromycin prepared by using novel polymer blend. Bull. Fac. Pharm. Cairo Univ. 2018, 56, 147–157. [Google Scholar] [CrossRef]
- Ermawati, D.E.; Andini, B.P.; Prihapsara, F.; Farida, Y.; Rohmani, S.; Kundarto, W.; Nugraheni, E.R. Optimization of Suweg starch (Amorphophallus paeoniifolius (Dennst.) Nicolson) and lactose as co-processed excipient of Ibuprofen-PEG 6000 solid dispersion of effervescent tablet. AIP Conf. Proc. 2020, 2237, 020061. [Google Scholar]
- Shevkar, B.; Ahirrao, S.; Bhavsar, G.; Patel, A.; Rajurkar, V.; Amale, P. Konjac Glucomannan Matrix Tablet for Extended Release of Diclofenac Sodium. Int. J. Adv. Pharm. Sci. 2014, 5, 2098–2108. [Google Scholar]
- Wang, S.; Zhou, B.; Wang, Y.; Li, B. Preparation and characterization of konjac glucomannan microcrystals through acid hydrolysis. Food Res. Int. 2015, 67, 111–116. [Google Scholar] [CrossRef]
- Pawar Harshal, A.; Jadhav Surekha, M.; Jadhav Pravin, T.; Rachel, G. Development and Evaluation of Mucoadhesive Patch Using a Natural Polysaccharide Isolated from Cordia dichotoma Fruit. J. Mol Pharm. Org. Process Res. 2014, 2, 91–98. [Google Scholar]
- Bisht, S.S.; Pandey, K.K.; Joshi, G.; Naithani, S. New route for carboxymethylation of cellulose: Synthesis, structural analysis and properties. Cellul. Chem. Technol. 2017, 51, 609–619. [Google Scholar]
- Mansouri, S.; Khiari, R.; Bettaieb, F.; El-Gendy, A.A.; Mhenni, F. Synthesis and Characterization of Carboxymethyl Cellulose from Tunisian Vine Stem: Study of Water Absorption and Retention Capacities. J. Polym. Environ. 2015, 23, 190–198. [Google Scholar] [CrossRef]
- Yahoum, M.M.; Moulai-Mostefa, N.; Le Cerf, D. Synthesis, physicochemical, structural and rheological characterizations of carboxymethyl xanthan derivatives. Carbohydr. Polym. 2016, 154, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lei, Y.; Zhang, X.; Liu, Y.; Sui, H.; Feng, J.; Wang, W. Konjac Glucomannan and Its Modifications as Tablet Disintegrants. Acta Pol. Pharm. Drug Res. 2017, 74, 1841–1849. [Google Scholar]
- Wang, L.; Lin, L.; Pang, J. A novel glucomannan incorporated functionalized carbon nanotube films: Synthesis, characterization and antimicrobial activity. Carbohydr. Polym. 2020, 245, 116619. [Google Scholar] [CrossRef]
- Sow, L.C.; Toh, N.Z.Y.; Wong, C.W.; Yang, H. Combination of sodium alginate with tilapia fish gelatin for improved texture properties and nanostructure modification. Food Hydrocoll. 2019, 94, 459–467. [Google Scholar] [CrossRef]
- Liu, R.; Saunders, J.M.; Freemont, T.J.; Saunders, B.R. Doubly crosslinked microgel–polyelectrolyte complexes: Three simple methods to tune and improve gel mechanical properties. Soft Matter. 2012, 8, 10932–10940. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, Y.; Chen, H.; Huang, Y.; Yuan, P.; Zhang, L. Preparation of a colon-specific sustained-release capsule with curcumin-loaded SMEDDS alginate beads. RSC Adv. 2017, 7, 22280–22285. [Google Scholar] [CrossRef] [Green Version]
- Yui, T.; Ogawa, K.; Sarko, A. Molecular and crystal structure of konjac glucomannan in the mannan II polymorphic form. Carbohydr. Res. 1992, 229, 41–55. [Google Scholar] [CrossRef]
- Ogawa, K.; Yui, T.; Mizuno, T. X-ray diffraction study of glucomannans and their acetates. Agric. Biol. Chem. 1991, 55, 2105–2111. [Google Scholar]
- Ainurofiq, A.; Choiri, S. Model and release pattern of water soluble drug from natural-polymer based sustained release tablet dosage form. Int. J. Pharm. Pharm. Sci. 2014, 6, 179–182. [Google Scholar]
- Ainurofiq, A.; Choiri, S. Drug release model and kinetics of naturl polymers-based sustained release tablet. Lat. Am. J. Pharm. 2015, 34, 1328–1337. [Google Scholar]








| Plant Sources | Part | Extraction Method | Principle | Extraction Solvent | Molecular Weight | % Yield | Ref |
|---|---|---|---|---|---|---|---|
| Aloe barbadensis M. | Leaves | Cold method (maceration for 24 h) | Maceration at room temperature with frequent agitation intended to soften and break the plant’s cell wall to release glucomannan | Ethanol precipitation | 1.2 MDa | 23.4% | [59] |
| Amorphophallus muelleri B. | Tubers | Cold method (maceration for 3 h) | Multilevel concentration of ethanol (40, 60, and 80%) | NA | 62.2% | [55] | |
| Amorphophallus konjac | Tubers | Cold method for 90 min | 50% ethanol | 9.5 × 105 g/mol | 91.4% | [60] | |
| Colocasia esculenta L. | Tubers | Cold method with centrifugal rotational | Separation of starch and glucomannan is done by adding electrolyte salts such as NaCl to break the bond between starch and glucomannan Maceration at room temperature with frequent agitation intended to soften and break the plant’s cell wall to release the soluble glucomannan. Centrifugal rotational promotes the starch precipitate faster. | Isopropyl alcohol precipitation. Crude extract was extracted with water for 2 h | NA | 4.08% | [61] |
| Amorphophallus campanulatus B.) | Tubers | Cold method with centrifugal rotational | Isopropyl alcohol precipitation. Crude extract was extracted with water for 2 h | NA | 5.64% | [61] | |
| Salacca edulis R. | Seeds | Hot water extraction (T = 95 °C for 2 h) | Glucomannan has greater solubility in hot water and is stable enough for minimum destruction with hot water extraction. | 95% isopropyl alcohol solvent in a ratio (1:17) | 2.057 × 104 g/mol | 40.19% | [62] |
| Durio zeibethinus M. | Seeds | Hot method (T = 95 °C for 2 h) | Isopropyl alcohol precipitation. Crude extract was washed with ethanol 95% | NA | 39.60% | [63] | |
| Dioscorea esculenta | Tubers | Hot method (T = 105 °C for 90 min) | Hot water extraction of the precipitate with isopropyl alcohol | 1.865 × 104 g/mol | 53.09% | [64] | |
| Bletilla striata | Tubers | Hot water extraction (T = 80 °C for 4 h) | 95% ethanol precipitation. Crude extract was purified with DEAE-52 cellulose column | 1.7 × 105 Da | 27.21% | [65] | |
| Amorphophallus oncophyllus | Tubers | Hot water extraction (T = 55 °C for 1.5 h) | Purified with 95% ethanol | NA | 93.84% | [6] | |
| Amorphophallus oncophyllus | Tubers | Ultrasonic | Ultrasonic breaking of plant cell wall significantly improves glucomannan extraction efficiency | 60% isopropanol | NA | 59.36% | [66] |
| Cibotium barometz | Rhizomes | Alkali extraction | Glucomannan, a higher molecular weight polysaccharide, has greater solubility in dilute alkaline solutions than in hot water. Generally, extraction of the polysaccharides is first carried out in hot water and thereafter a dilute alkaline solution is employed for the extraction of residual polysaccharides. | Sodium hydroxide ([NaOH] 0.3 mol/L) | 1445 Da | 8.25% | [67] |
| Combination of Excipients | Co-Processed | Application | Mechanism | Ref. |
|---|---|---|---|---|
| GM and HPMC K 100 LV | Microwave on level 5 (350 W) for 30 min | Matrix for gastro-retentive tablets forming a porous channel that allows the polymer mixture to absorb more water and expand, followed by prolonged drug release | Hydrogen bonds in single polymers have low energy, but the simultaneous formation of interlinked hydrogen bonds between polymer components provides significant interaction strength, resulting in a matrix that floats quickly and maintains the integrity of the polymer mixture under acidic conditions. | [97] |
| GM and lactose | Wet granulation | Filler–binder for direct compression of effervescent tablets | GM has a high viscosity and strong adhesive properties, thus providing good tablet binding effectiveness. GM has poor solubility in water, so it is combined with lactose as a water-soluble ingredient and to improve the poor flowability of lactose. | [98] |
| GM, sodium alginate (SA), and graphene oxide (GO) | Freeze dried | Microsphere-making polymers that enhance targeted delivery of drugs or nutrients to the colon | GM interacts with SA via hydrogen bonding and physical entanglement, and GO enhances these interactions in the microspheres. In addition, GO can greatly improve the loading efficiency of ciprofloxacin (CPFX) of microspheres, and achieve the sustained release effect of CPFX. | [26] |
| Oxidized GM, cassava starch, and sucrose esters | Dry heated | The OGM–CS combination exhibits low solubility and swellability, which makes it a possible excipient for the formulation of sustained-release drugs. However, the addition of SE significantly decreased porosity and swelling of the tablets, which inhibited immediate drug release. | Heating OGM and CS to high temperatures causes structural damage that limits the solubility and swelling ability of the polymer. The addition of SE with HLB 5 decreased porosity and slowed drug release because the more closed structure inhibited free movement of the drug out of the matrix. In addition, more hydroxyl groups in SE form hydrogen bonds, increasing intergranular bonding. | [84] |
| CMGM and 2-hydroxypropyl trimethyl ammonium chloride chitosan (HACC) | Complex coacervation and freeze dried | The coaservation complex formed can encapsulate and control the release of the molecular model for the vaccine, namely ovalbumin (OVA). | The anionic carboxyl group of CMGM and the cationic quaternary amine group of HACC cause intramolecular electrostatic attraction that causes the HACC and CMGM macromolecular chains to aggress and coil, forming the CMGM/HACC composite nanosphere. | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aanisah, N.; Wardhana, Y.W.; Chaerunisaa, A.Y.; Budiman, A. Review on Modification of Glucomannan as an Excipient in Solid Dosage Forms. Polymers 2022, 14, 2550. https://doi.org/10.3390/polym14132550
Aanisah N, Wardhana YW, Chaerunisaa AY, Budiman A. Review on Modification of Glucomannan as an Excipient in Solid Dosage Forms. Polymers. 2022; 14(13):2550. https://doi.org/10.3390/polym14132550
Chicago/Turabian StyleAanisah, Nuur, Yoga W. Wardhana, Anis Y. Chaerunisaa, and Arif Budiman. 2022. "Review on Modification of Glucomannan as an Excipient in Solid Dosage Forms" Polymers 14, no. 13: 2550. https://doi.org/10.3390/polym14132550