Photophysical Properties of Linked Zinc Phthalocyanine to Acryloyl Chloride:N-vinylpyrrolidone Copolymer
Abstract
:1. Introduction
2. Preparation and Characterization of Photosensitizer
2.1. Materials
2.1.1. N-VP:ClAC Synthesis
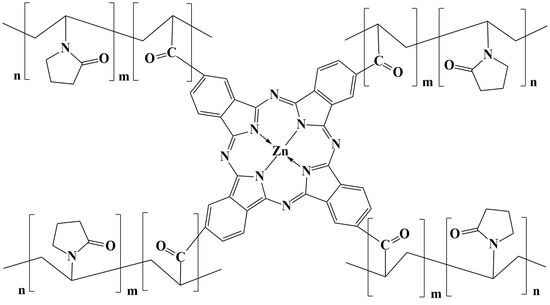
2.1.2. ZnPc: N-VP:ClAC Synthesis
2.1.3. Physical Characterization Methods
2.2. Preparation of Cell Culture
2.2.1. Cell Culture
2.2.2. Dark Cytotoxicity
3. Results and Discussion
3.1. Absorbance and Fluorescence Spectra
3.2. Cytotoxicity Analysis
3.3. Structural Determination
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Na, K. Self-Assembled Chlorin e6 Conjugated Chondroitin Sulfate Nanodrug for Photodynamic Therapy. Biomacromolecules 2011, 12, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, W.; Na, K. Doxorubicin loaded singlet-oxygen producible polymeric micelle based on chlorine e6 conjugated pluronic F127 for overcoming drug resistance in cancer. Biomaterials 2014, 35, 7963–7969. [Google Scholar] [CrossRef]
- Park, W.; Park, S.-J.; Na, K. The controlled photoactivity of nanoparticles derived from ionic interactions between a water soluble polymeric photosensitizer and polysaccharide quencher. Biomaterials 2011, 32, 8261–8270. [Google Scholar] [CrossRef]
- Li, F.; Bae, B.-C.; Na, K. Acetylated Hyaluronic Acid/Photosensitizer Conjugate for the Preparation of Nanogels with Controllable Phototoxicity: Synthesis, Characterization, Autophotoquenching Properties, andin vitroPhototoxicity against HeLa Cells. Bioconjugate Chem. 2010, 21, 1312–1320. [Google Scholar] [CrossRef]
- Liu, Q.; Pang, M.; Tan, S.; Wang, J.; Chen, Q.; Wang, K.; Wu, W.; Hong, Z. Potent peptide-conjugated silicon phthalocyanines for tumor photodynamic therapy. J. Cancer 2018, 9, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Makhseed, S.; Machacek, M.; Alfadly, W.; Tuhl, A.; Vinodh, M.; Simunek, T.; Novakova, V.; Kubat, P.; Rudolf, E.; Zimcik, P. Water-soluble non-aggregating zinc phthalocyanine and in vitro studies for photodynamic therapy. Chem. Commun. 2013, 49, 11149–11151. [Google Scholar] [CrossRef]
- Çakır, D.; Göksel, M.; Çakır, V.; Durmuş, M.; Biyiklioglu, Z.; Kantekin, H. Amphiphilic zinc phthalocyanine photosensitizers: Synthesis, photophysicochemical properties and in vitro studies for photodynamic therapy. Dalton Trans. 2015, 44, 9646–9658. [Google Scholar] [CrossRef]
- Dube, E.; Oluwole, D.O.; Prinsloo, E.; Nyokong, T. A gold–chitosan composite with low symmetry zinc phthalocyanine for enhanced singlet oxygen generation and improved photodynamic therapy activity. New J. Chem. 2018, 42, 10214–10225. [Google Scholar] [CrossRef]
- Kuzyniak, W.; Schmidt, J.; Glac, W.; Berkholz, J.; Steinemann, G.; Hoffmann, B.; Ermilov, E.A.; Gürek, A.G.; Ahsen, V.; Nitzsche, B.; et al. Novel zinc phthalocyanine as a promising photosensitizer for photodynamic treatment of esophageal cancer. Int. J. Oncol. 2017, 50, 953–963. [Google Scholar] [CrossRef] [Green Version]
- Machacek, M.; Cidlina, A.; Novakova, V.; Svec, J.; Rudolf, E.; Miletin, M.; Kučera, R.; Simunek, T.; Zimcik, P. Far-Red-Absorbing Cationic Phthalocyanine Photosensitizers: Synthesis and Evaluation of the Photodynamic Anticancer Activity and the Mode of Cell Death Induction. J. Med. Chem. 2015, 58, 1736–1749. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, Q.; Liang, Z.; Wang, J.; Pang, M.; Huang, W.; Wu, W.; Hong, Z. Synthesis and biological evaluation of peptide-conjugated phthalocyanine photosensitizers with highly hydrophilic modifications. Org. Biomol. Chem. 2016, 14, 3409–3422. [Google Scholar] [CrossRef] [PubMed]
- Setaro, F.; Wennink, J.W.H.; Mäkinen, P.I.; Holappa, L.; Trohopoulos, P.N.; Ylä-Herttuala, S.; van Nostrum, C.F.; de la Escosura, A.; Torres, T. Amphiphilic phthalocyanines in polymeric micelles: A supramolecular approach toward efficient third-generation photosensitizers. J. Mater. Chem. B 2019, 8, 282–289. [Google Scholar] [CrossRef]
- Feuser, P.E.; Gaspar, P.C.; Jacques, A.V.; Tedesco, A.C.; Santos Silva, M.C.D.; Ricci-Júnior, E.; Sayer, C.; de Araújo, P.H.H. Synthesis of ZnPc loaded poly(methyl methacrylate) nanoparticles via miniemulsion polymerization for photodynamic therapy in leukemic cells. Mater. Sci. Eng. C 2016, 60, 458–466. [Google Scholar] [CrossRef]
- Obata, M.; Tanaka, S.; Mizukoshi, H.; Ishihara, E.; Takahashi, M.; Hirohara, S. RAFT synthesis of polystyrene-block-poly(polyethylene glycol monomethyl ether acrylate) for zinc phthalocyanine-loaded polymeric micelles as photodynamic therapy photosensitizers. J. Polym. Sci. Part A Polym. Chem. 2017, 56, 560–570. [Google Scholar] [CrossRef]
- Obata, M.; Masuda, S.; Takahashi, M.; Yazaki, K.; Hirohara, S. Effect of the hydrophobic segment of an amphiphilic block copolymer on micelle formation, zinc phthalocyanine loading, and photodynamic activity. Eur. Polym. J. 2021, 147, 110325. [Google Scholar] [CrossRef]
- Shen, A.; Chen, D.; Kaur, M.; Bartels, P.; Xu, B.; Shi, Q.; Martinez, J.M.; Man, K.M.; Cintron, M.; Hell, Y.; et al. β-blockers augment L-type Ca2+channelactivity by targeting spatially restrictedb2AR signaling in neurons. ELife 2019, 8, e49464. [Google Scholar] [CrossRef] [PubMed]
- Motloung, M.; Babu, B.; Prinsloo, B.; Nyokong, E. The photophysicochemical properties and photodynamic therapy activity of In and Zn phthalocyanines when incorporated into individual or mixed Pluronic® micelles. Polyhedron 2020, 188, 114683. [Google Scholar] [CrossRef]
- Chiarante, N.; Vior, M.C.G.; Awruch, J.; Marino, J.; Roguin, L.P. Phototoxic action of a zinc(II) phthalocyanine encapsulated into poloxamine polymeric micelles in 2D and 3D colon carcinoma cell cultures. J. Photochem. Photobiol. B Biol. 2017, 170, 140–151. [Google Scholar] [CrossRef]
- Lamch, A.; Kulbacka, J.; Pietkiewicz, J.; Rossowska, J.; Dubińska-Magiera, M.; Choromańska, A.; Wilk, K.A. Preparation and characterization of new zinc(II) phthalocyanine—Containing poly(l-lactide)-b-poly(ethylene glycol) copolymer micelles for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2016, 160, 185–197. [Google Scholar] [CrossRef]
- Li, L.; Yang, Q.; Shi, L.; Zheng, N.; Li, Z.; Li, K.; Qiao, S.; Jia, T.; Sun, T.; Wang, Y. Novel phthalocyanine-based polymeric micelles with high near-infrared photothermal conversion efficiency under 808 nm laser irradiation for in vivo cancer therapy. J. Mater. Chem. B 2019, 7, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, W.; Qu, Z.; Shi, L.; Tan, S.; Ha, E.; Jia, T.; Sun, T. Novel phthalocyanine-based micelles/PNIPAM composite hydrogels: Spatially/temporally controlled drug release triggered by NIR laser irradiation. New J. Chem. 2020, 44, 8705–8709. [Google Scholar] [CrossRef]
- Freire, N.F.; Feuser, P.; Abel, J.; Machado-de-Ávila, R.; Fialho, R.L.; Albuquerque, E.C.; Sayer, C.; Hermes de Araújo, P.H. Zinc phthalocyanine encapsulation via thiolene miniemulsion polymerization and in vitro photoxicity studies. Int. J. Polym. Mater. Polym. Biomaterials 2020, 2, 1–11. [Google Scholar] [CrossRef]
- Durmuş, M.; Ahsen, V. Water-soluble cationic gallium(III) and indium(III) phthalocyanines for photodynamic therapy. J. Inorg. Biochem. 2010, 104, 297–309. [Google Scholar] [CrossRef]
- Tiuleanu, P.; Robu, Ș.; Prisakari, V.; Furtuna, V.; Rusnac, R.; Potlog, T. Synthesis of New Zinc Phthalocyanine with Block Copolymers in Nanomedicine Applications. In Proceedings of the 4th International Conference on Nanotechnologies and Biomedical Engineering, Chisinau, Moldova, 18–21 September 2019; Tighineanu, I., Sontea, V., Railean, S., Eds.; Springer: Cham, Switzerland, 2020; pp. 727–730. [Google Scholar]
- Włodarczyk, J.; Kierdaszuk, B. Interpretation of Fluorescence Decays using a Power-like Model. Biophys. J. 2003, 1, 589–598. [Google Scholar] [CrossRef] [Green Version]
- ISO 10993-5:2009. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Szuwarzyński, M.; Wolski, K.; Pomorska, A.; Uchacz, T.; Gut, A.; Łapok, Ł.; Zapotoczny, S. Photoactive Surface-Grafted Polymer Brushes with Phthalocyanine Bridging Groups as an Advanced Architecture for Light-Harvesting. Chem. A Eur. J. 2017, 23, 11239–11243. [Google Scholar] [CrossRef]
- Qian, T.; Chen, F.; Chen, Y.; Wang, Y.-X.; Hu, W. Photolysis of polymeric self-assembly controlled by donor–acceptor interaction. Chem. Commun. 2017, 53, 11822–11825. [Google Scholar] [CrossRef]
- Zhu, X.; Su, Q.; Feng, W.; Li, F. Anti-Stokes shift luminescent materials for bio-applications. Chem. Soc. Rev. 2016, 46, 1025–1039. [Google Scholar] [CrossRef]
- Vilsinski, B.H.; Witt, M.A.; Barbosa, P.M.; Montanha, M.C.; Nunes, C.S.; Bellettini, I.C.; de Castro, L.V.; Sato, F.; Baesso, M.L.; Muniz, E.C.; et al. Formulation of chloroaluminum phthalocyanine incorporated into PS-b-PAA diblock copolymer nanomicelles. J. Mol. Liq. 2018, 271, 949–958. [Google Scholar] [CrossRef]
- Demazeau, M.; Gibot, L.; Mingotaud, A.-F.; Vicendo, P.; Roux, C.; Lonetti, B. Rational design of block copolymer self-assemblies in photodynamic therapy. Beilstein J. Nanotechnol. 2020, 11, 180–212. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potlog, T.; Lungu, I.; Tiuleanu, P.; Robu, S. Photophysical Properties of Linked Zinc Phthalocyanine to Acryloyl Chloride:N-vinylpyrrolidone Copolymer. Polymers 2021, 13, 4428. https://doi.org/10.3390/polym13244428
Potlog T, Lungu I, Tiuleanu P, Robu S. Photophysical Properties of Linked Zinc Phthalocyanine to Acryloyl Chloride:N-vinylpyrrolidone Copolymer. Polymers. 2021; 13(24):4428. https://doi.org/10.3390/polym13244428
Chicago/Turabian StylePotlog, Tamara, Ion Lungu, Pavel Tiuleanu, and Stefan Robu. 2021. "Photophysical Properties of Linked Zinc Phthalocyanine to Acryloyl Chloride:N-vinylpyrrolidone Copolymer" Polymers 13, no. 24: 4428. https://doi.org/10.3390/polym13244428