Preparation and Characterization of Highly Porous Polyacrylonitrile Electrospun Nanofibers Using Lignin as Soft Template via Selective Chemical Dissolution Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
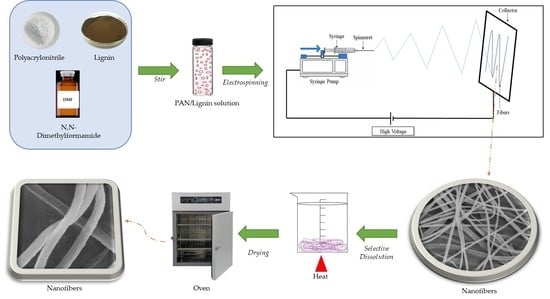
2.2. Preparation of Electrospun Nanofibers
2.3. Selective Chemical Dissolution Technique
2.4. Characterizations
3. Results and Discussion
3.1. Fourier Transform Infrared Spectra (FTIR) Analysis
3.2. Field Emission Scanning Electron Microscope (FESEM) Analysis
3.3. Thermal Analysis
3.4. X-ray Diffractometer (XRD) Analysis
3.5. BET Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philip, P.; Jose, E.T.; Chacko, J.K.; Philip, K.; Thomas, P. Preparation and characterisation of surface roughened PMMA electrospun nanofibers from PEO-PMMA polymer blend nanofibers. Polym. Test. 2019, 74, 257–265. [Google Scholar] [CrossRef]
- Yin, J.; Roso, M.; Boaretti, C.; Lorenzetti, A.; Martucci, A.; Modesti, M. PVDF-TiO2 core-shell fibrous membranes by microwave-hydrothermal method: Preparation, characterization, and photocatalytic activity. J. Environ. Chem. Eng. 2021, 9, 106250. [Google Scholar] [CrossRef]
- Moon, S.; Choi, J.; Farris, R.J. Highly porous polyacrylonitrile/polystyrene nanofibers by electrospinning. Fibers Polym. 2008, 9, 276–280. [Google Scholar] [CrossRef]
- Deeney, C.; McKiernan, E.P.; Belhout, S.A.; Rodriguez, B.J.; Redmond, G.; Quinn, S.J. Template-Assisted Synthesis of Luminescent Carbon Nanofibers from Beverage-Related Precursors by Microwave Heating. Molecules 2019, 24, 1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manafi, S.A.; Badiee, S.H. Production of Carbon Nanofibers Using a CVD Method with Lithium Fluoride as a Supported Cobalt Catalyst. Res. Lett. Mater. Sci. 2008, 2008, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Yalcinkaya, F. Preparation of various nanofiber layers using wire electrospinning system. Arab. J. Chem. 2019, 12, 5162–5172. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Ruiz-Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Controlling the Composition, Morphology, Porosity, and Surface Chemistry of Lignin-Based Electrospun Carbon Materials. Front. Mater. 2019, 6, 114. [Google Scholar] [CrossRef] [Green Version]
- Hoque, M.E.; Nuge, T.; Yeow, T.K.; Nordin, N. Chapter 5—Electrospun Matrices from Natural Polymers for Skin Regeneration. In Nanostructured Polymer Composites for Biomedical Applications; Swain, S.K., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–104. [Google Scholar]
- Kong, L.; Ziegler, G. Fabrication of pure starch fibers by electrospinning. Food Hydrocoll. 2014, 36, 20–25. [Google Scholar] [CrossRef]
- Chew, S.Y.; Wen, Y.; Dzenis, Y.; Leong, K. The Role of Electrospinning in the Emerging Field of Nanomedicine. Curr. Pharm. Des. 2006, 12, 4751–4770. [Google Scholar] [CrossRef] [Green Version]
- Haghi, A.K.; Zaikov, G.E. Electrospinning Process and Nanofiber Research; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 1–196. [Google Scholar]
- Kanafi, N.M.; Rahman, N.A.; Rosdi, N.H.; Bahruji, H.; Maarof, H. Hydrogel Nanofibers from Carboxymethyl Sago Pulp and Its Controlled Release Studies as a Methylene Blue Drug Carrier. Fibers 2019, 7, 56. [Google Scholar] [CrossRef] [Green Version]
- Kanafi, N.M.; Rahman, N.A.; Rosdi, N.H. Citric acid cross-linking of highly porous carboxymethyl cellulose/poly(ethylene oxide) composite hydrogel films for controlled release applications. Mater. Today Proc. 2019, 7, 721–731. [Google Scholar] [CrossRef]
- Nordin, N.A.; Rahman, N.A.; Abdullah, A.H. Effective Removal of Pb(II) Ions by Electrospun PAN/Sago Lignin-based Activated Carbon Nanofibers. Molecules 2020, 25, 3081. [Google Scholar] [CrossRef]
- Nthumbi, R.; Ngila, J.; Kindness, A.; Moodley, B.; Petrik, L. Method Development for Flow Adsorption and Removal of Lead and Copper in Contaminated Water Using Electrospun Nanofibers of Chitosan Blend. Anal. Lett. 2011, 44, 1937–1955. [Google Scholar] [CrossRef]
- Park, J.-A.; Kang, J.; Lee, S.-C.; Kim, S.-B. Electrospun poly(acrylic acid)/poly(vinyl alcohol) nanofibrous adsorbents for Cu(II) removal from industrial plating wastewater. RSC Adv. 2017, 7, 18075–18084. [Google Scholar] [CrossRef] [Green Version]
- Bates, I.I.C.; Loranger, É; Chabot, B. Chitosan-PEO nanofiber mats for copper removal in aqueous solution using a new versatile electrospinning collector. SN Appl. Sci. 2020, 2, 1–14. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Bhattacharya, A. Drinking water contamination and treatment techniques. Appl. Water Sci. 2016, 7, 1043–1067. [Google Scholar] [CrossRef] [Green Version]
- Awad, R.; Mamaghani, A.H.; Boluk, Y.; Hashisho, Z. Synthesis and characterization of electrospun PAN-based activated carbon nanofibers reinforced with cellulose nanocrystals for adsorption of VOCs. Chem. Eng. J. 2021, 410, 128412. [Google Scholar] [CrossRef]
- Mokhtari-Shourijeh, Z.; Montazerghaem, L.; Olya, M.E. Preparation of Porous Nanofibers from Electrospun Polyacrylonitrile/Polyvinylidene Fluoride Composite Nanofibers by Inexpensive Salt Using for Dye Adsorption. J. Polym. Environ. 2018, 26, 3550–3563. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Lee, H.I.; Lee, J.W.; Park, M.; Rhee, K.Y.; Park, S.-J. Optimization of the pore structure of PAN-based carbon fibers for enhanced supercapacitor performances via electrospinning. Compos. Part B Eng. 2019, 161, 10–17. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, Y.; Luo, X.; Sun, C.; Chen, N. Polarization Effects of a Rayon and Polyacrylonitrile Based Graphite Felt for Iron-Chromium Redox Flow Batteries. ChemElectroChem 2019, 6, 3175–3188. [Google Scholar] [CrossRef]
- Qiu, L.; Zheng, X.; Zhu, J.; Su, G.; Tang, D. The effect of grain size on the lattice thermal conductivity of an individual polyacrylonitrile-based carbon fiber. Carbon 2013, 51, 265–273. [Google Scholar] [CrossRef]
- Ramasubramanian, G. Influence of Lignin Modification on PAN-Lignin Copolymers as Potential Carbon Fiber Precursors. Master’s Thesis, Iowa State University, Ames, IA, USA, 2013. [Google Scholar]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y.; Chu, F. Preparation and Formation Mechanism of Renewable Lignin Hollow Nanospheres with a Single Hole by Self-Assembly. ACS Sustain. Chem. Eng. 2017, 5, 2273–2281. [Google Scholar] [CrossRef]
- Branco, R.H.R.; Serafim, L.S.; Xavier, A.M.R.B. Second Generation Bioethanol Production: On the Use of Pulp and Paper Industry Wastes as Feedstock. Fermentation 2018, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Sabetzadeh, N.; Gharehaghaji, A.A. How Porous Nanofibers Have Enhanced Engineering of Advanced Materials: A Review. Mat. Sci. 2017, 5, 3–21. [Google Scholar]
- Chen, X.; Huang, J.; Ling, X.; Lm, H. Preparation of porous ultrafine SF fibers via selective dissolution of electrospun SF/PLA blend fibers. IOP Conf. Ser. Mater. Sci. Eng. 2018, 423, 012153. [Google Scholar] [CrossRef]
- Kim, M.N.; Koh, J.; Lee, Y.; Kim, H. Preparation of PVA/PAN bicomponent nanofiber via electrospinning and selective dissolution. J. Appl. Polym. Sci. 2009, 113, 274–282. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Iqbal, A.; Shen, C.C.; Bhawani, S.A.; Adam, F. Synthesis of lignin based composites of TiO2 for potential application as radical scavengers in sunscreen formulation. BMC Chem. 2019, 13, 17. [Google Scholar] [CrossRef] [Green Version]
- Kubo, S.; Kadla, J.F. Lignin-based Carbon Fibers: Effect of Synthetic Polymer Blending on Fiber Properties. J. Polym. Environ. 2005, 13, 97–105. [Google Scholar] [CrossRef]
- Seydibeyoğlu, M.Ö. A Novel Partially Biobased PAN-Lignin Blend as a Potential Carbon Fiber Precursor. J. Biomed. Biotechnol. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Yue, Z.; Benak, K.R.; Wang, J.; Mangun, C.L.; Economy, J. Elucidating the porous and chemical structures of ZnCl2-activated polyacrylonitrile on a fiberglass substrate. J. Mater. Chem. 2005, 15, 3142–3148. [Google Scholar] [CrossRef]
- Wangxi, Z.; Jie, L.; Gang, W. Evolution of structure and properties of PAN precursors during their conversion to carbon fibers. Carbon 2003, 41, 2805–2812. [Google Scholar] [CrossRef]
- Jenab, A.; Roghanian, R.; Ghorbani, N.; Ghaedi, K.; Emtiazi, G. The Efficacy of Electrospun PAN/Kefiran Nanofiber and Kefir in Mammalian Cell Culture: Promotion of PC12 Cell Growth, Anti-MCF7 Breast Cancer Cells Activities, and Cytokine Production of PBMC. Int. J. Nanomed. 2020, 15, 717–728. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.I.; Lee, J.-N.; Song, J.; Kang, P.-H.; Park, J.-K.; Lee, Y.M. Fabrication of polyacrylonitrile/lignin-based carbon nanofibers for high-power lithium ion battery anodes. J. Solid State Electrochem. 2013, 17, 2471–2475. [Google Scholar] [CrossRef]
- Pashaloo, F.; Bazgir, S.; Tamizifar, M.; Faghihisani, M.; Zakerifar, S. Preparation and Characterization of Carbon Nanofibers via Electrospun PAN Nanofibers. Text. Sci. Technol. J. 2009, 3, 2. [Google Scholar]
- Dang, W.; Liu, J.; Wang, X.; Yan, K.; Zhang, A.; Yang, J.; Chen, L.; Liang, J. Structural Transformation of Polyacrylonitrile (PAN) Fibers during Rapid Thermal Pretreatment in Nitrogen Atmosphere. Polymers 2020, 12, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafov, S.D.; Mohanty, A.K.; Misra, M.; Seydibeyoğlu, M.Ö. Fabrication of conductive Lignin/PAN carbon nanofibers with enhanced graphene for the modified electrodes. Carbon 2019, 147, 262–275. [Google Scholar] [CrossRef]
- Goudarzi, A.; Lin, L.-T.; Ko, F.K. X-ray Diffraction Analysis of Kraft Lignins and Lignin-Derived Carbon Nanofibers. J. Nanotechnol. Eng. Med. 2014, 5, 021006. [Google Scholar] [CrossRef]
- Ji, L.; Saquing, C.; Khan, S.A.; Zhang, X. Preparation and characterization of silica nanoparticulate-polyacrylonitrile composite and porous nanofibers. Nanotechnology 2008, 19, 085605. [Google Scholar] [CrossRef] [PubMed]









| PAN | 9:1 | 8:2 | 7:3 | 6:4 | |
|---|---|---|---|---|---|
| Specific surface area, SBET/m2g−1 | 16.17 | 20.94 | 32.42 | 29.13 | 28.15 |
| Pore volume, Vtotal/m3g−1 | 0.2360 | 0.2371 | 0.1974 | 0.1800 | 0.2970 |
| Average pore diameter/nm | 5.16 | 5.13 | 5.02 | 5.34 | 5.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.A.T.; Abdul Rahman, N. Preparation and Characterization of Highly Porous Polyacrylonitrile Electrospun Nanofibers Using Lignin as Soft Template via Selective Chemical Dissolution Technique. Polymers 2021, 13, 3938. https://doi.org/10.3390/polym13223938
Ahmad MAT, Abdul Rahman N. Preparation and Characterization of Highly Porous Polyacrylonitrile Electrospun Nanofibers Using Lignin as Soft Template via Selective Chemical Dissolution Technique. Polymers. 2021; 13(22):3938. https://doi.org/10.3390/polym13223938
Chicago/Turabian StyleAhmad, Mohd Adib Tajuddin, and Norizah Abdul Rahman. 2021. "Preparation and Characterization of Highly Porous Polyacrylonitrile Electrospun Nanofibers Using Lignin as Soft Template via Selective Chemical Dissolution Technique" Polymers 13, no. 22: 3938. https://doi.org/10.3390/polym13223938