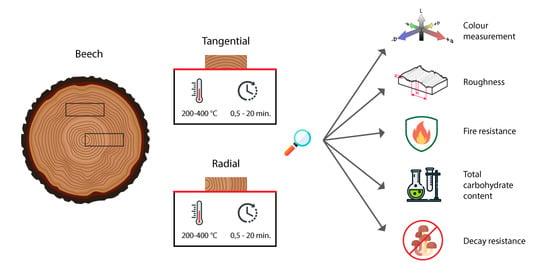
Surface Characteristics of One-Sided Charred Beech Wood
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Microscopic Measurements—Colour and Roughness
2.3. Fire Resistance Test
2.4. Total Carbohydrate Content
2.5. Decay Test
3. Results and Discussion
3.1. Colour Measurements
3.2. Results of Surface Roughness
3.3. Fire Resistance Test Results
3.4. Total Carbohydrate Content
3.5. Decay Resistance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Thieme, H. Lower palaeolithic hunting spears from Germany. Nat. Cell Biol. 1997, 385, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Aranguren, B.; Revedin, A.; Amico, N.; Cavulli, F.; Giachi, G.; Grimaldi, S.; Macchioni, N.; Santaniello, F. Wooden tools and fire technology in the early Neanderthal site of Poggetti Vecchi (Italy). Proc. Natl. Acad. Sci. USA 2018, 115, 2054–2059. [Google Scholar] [CrossRef] [Green Version]
- Čechák, P.; Adámek, F.; Pacák, M.; Kolert, V.M. Paleolitická štípaná industrie a její použitelnost k lovu. Živá Archeol. 2014, 16, 67–76. [Google Scholar]
- Steele, J. Contemporary Japanese Architecture: Tracing the Next Generation; Taylor & Francis: New York, NY, USA, 2017; ISBN 978-1-138-94124-3. [Google Scholar]
- Shou Sugi Ban. The Japanese Art of Charred Cedar. Available online: https://shousugiban.com/ (accessed on 11 January 2021).
- Graham, R.D.; Miller, D.J. Service Life of Treated and Untreated Fence Posts; Forest Research Laboratory, Oregon State University: Corvallis, OR, USA, 1957; Volume 11. [Google Scholar]
- Ebner, D.; Stelzer, R.; Barbu, M.C. Study of wooden surface carbonization using the traditional Japanese Yakisugi technique. Pro Ligno 2019, 15, 278–283. [Google Scholar]
- Kymäläinen, M.; Hautamäki, S.; Lillqvist, K.; Segerholm, K.; Rautkari, L. Surface modification of solid wood by charring. J. Mater. Sci. 2017, 52, 6111–6119. [Google Scholar] [CrossRef]
- Kymäläinen, M.; Turunen, H.; Čermák, P.; Hautamäki, S.; Rautkari, L. Sorption-related characteristics of surface charred spruce wood. Materials 2018, 11, 2083. [Google Scholar] [CrossRef] [Green Version]
- Čermák, P.; Dejmal, A.; Paschová, Z.; Kymäläinen, M.; Dömény, J.; Brabec, M.; Hess, D.; Rautkari, L. One-sided surface charring of beech wood. J. Mater. Sci. 2019, 54, 9497–9506. [Google Scholar] [CrossRef]
- Bartlett, A.I.; Hadden, R.M.; Bisby, L.A. A Review of factors affecting the burning behaviour of wood for application to tall timber construction. Fire Technol. 2019, 55, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Sehlstedt-Persson, M. Properties of Solid Wood Responses to Drying and Heat Treatment; Luleå University of Technology: Skellefteå, Sweden, 2005. [Google Scholar]
- Oltean, L.; Teischinger, A.; Hansmann, C. Influence of temperature on cracking and mechanical properties of wood during wood drying—A review. BioResources 2007, 2, 789–811. [Google Scholar]
- White, R.; Dietenberger, M. Wood products: Thermal degradation and fire. Encycl. Mater. Sci. Technol. 2001, 9712–9716. [Google Scholar] [CrossRef]
- Hill, C.; Altgen, M.; Rautkari, L. Thermal modification of wood—A review: Chemical changes and hygroscopicity. J. Mater. Sci. 2021, 56, 6581–6614. [Google Scholar] [CrossRef]
- Hill, C.A.S. Wood Modification: Chemical, Thermal and Other Processes; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 0470021721. [Google Scholar]
- Byrne, C.E.; Nagle, D.C. Carbonization of wood for advanced materials applications. Carbon 1997, 35, 259–266. [Google Scholar] [CrossRef]
- Beall, F.C.; Eickner, H.W. Thermal Degradation of Wood Components: A Review of the Literature; U.S. Forest Products Laboratory: Madison, WI, USA, 1970. [Google Scholar]
- Ryszard, K.; Małgorzata, M. Smart environmentally friendly composite coatings for wood protection. In Smart Composite Coatings and Membranes: Transport, Structural, Environmental and Energy Applications; Montemor, M.F., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 293–325. ISBN 9781782422839. [Google Scholar]
- Kawamoto, H. Lignin pyrolysis reactions. J. Wood Sci. 2017, 63, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Burhenne, L.; Messmer, J.; Aicher, T.; Laborie, M.-P. The effect of the biomass components lignin, cellulose and hemicellulose on TGA and fixed bed pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 177–184. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Martinka, J.; Chrebet, T.; Balog, K. Impact of oxygen concentration on ignition time of birchwood. In Proceedings of the 22nd International DAAAM Symposium, Vienna, Austria, 23–26 November 2011; pp. 1231–1232. [Google Scholar]
- Marková, I.; Ladomerský, J.; Hroncová, E.; Mračková, E. Thermal parameters of beech wood dust. BioResources 2018, 13, 3098–3109. [Google Scholar] [CrossRef]
- Leach, R.K. Surface topography measurement instrumentation. In Fundamental Principles of Engineering Nanometrology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 133–204. [Google Scholar]
- European Standard EN 113—Wood Preservatives—Test Method for Determining the Protective Effectiveness against Wood Destroying Basidiomycetes—Determination of the Toxic Values; European Committee for Standardization: Brussels, Belgium, 1996.
- Aydemir, D.; Gunduz, G.; Ozden, S. The influence of thermal treatment on color response of wood materials. Color Res. Appl. 2010, 37, 148–153. [Google Scholar] [CrossRef]
- González-Peña, M.M.; Hale, M.D. Colour in thermally modified wood of beech, Norway spruce and Scots pine. Part 1: Colour evolution and colour changes. Holzforschung 2009, 63, 385–393. [Google Scholar] [CrossRef]
- Bourgois, J.; Bartholin, M.-C.; Guyonnet, R. Thermal treatment of wood: Analysis of the obtained product. Wood Sci. Technol. 1989, 23, 303–310. [Google Scholar] [CrossRef]
- Huang, X.; Kocaefe, D.; Kocaefe, Y.; Boluk, Y.; Pichette, A. A spectrocolorimetric and chemical study on color modification of heat-treated wood during artificial weathering. Appl. Surf. Sci. 2012, 258, 5360–5369. [Google Scholar] [CrossRef]
- Korkut, D.S.; Korkut, S.; Bekar, I.; Budakçı, M.; Dilik, T.; Çakıcıer, N. The effects of heat treatment on the physical properties and surface roughness of Turkish hazel (Corylus colurna L.) wood. Int. J. Mol. Sci. 2008, 9, 1772–1783. [Google Scholar] [CrossRef] [Green Version]
- Ayrilmis, N.; Winandy, J.E.; Laufenberg, T.L. Effects of post thermal-treatment on wettability, surface roughness, and adhesive bonding performance of exterior medium density fiberboard. Polym. Degrad. Stabil 2008, 9, 1772–1783. [Google Scholar]
- Korkut, S.; Akgül, M.; Dündar, T. The effects of heat treatment on some technological properties of Scots pine (Pinus sylvestris L.) wood. Bioresour. Technol. 2008, 99, 1861–1868. [Google Scholar] [CrossRef]
- Dornyak, O.R. Modeling of the rheological behavior of wood in compression processes. J. Eng. Phys. Thermophys. 2003, 76, 648–654. [Google Scholar] [CrossRef]
- Lavery, D.J.; McLarnon, D.; Taylor, J.M.; Moloney, S.; Atanackovic, A. Parameters affecting the surface finish of planed sitka spruce. For. Prod. J. 1995, 45, 45. [Google Scholar]
- Dundar, T.; As, N.; Korkut, S.; Unsal, O. The effect of boiling time on the surface roughness of rotary-cut veneers from oriental beech (Fagus orientalis L.). J. Mater. Process. Technol. 2008, 199, 119–123. [Google Scholar] [CrossRef]
- Gardner, D.J.; Generalla, N.C.; Gunnells, D.W.; Wolcott, M.P. Dynamic wettability of wood. Langmuir 1991, 7, 2498–2502. [Google Scholar] [CrossRef]
- Gunnells, D.W.; Gardner, D.J.; Wolcott, M.P. Temperature dependence of wood surface energy. Wood Fiber Sci. 1994, 26, 447–455. [Google Scholar]
- Ye, H.; Asante, B.; Schmidt, G.; Krause, A.; Zhang, Y.; Yu, Z. Interfacial bonding properties of the eco-friendly geopoly-mer-wood composites: Influences of embedded wood depth, wood surface roughness, and moisture conditions. J. Mater. Sci. 2021, 56, 7420–7433. [Google Scholar] [CrossRef]
- Akbulut, T.; Ayrilmis, N. Effect of compression wood on surface roughness and surface absorption of medium density fiber-board. Silva Fenn. 2006, 40, 161. [Google Scholar] [CrossRef] [Green Version]
- Thybring, E.E. The decay resistance of modified wood influenced by moisture exclusion and swelling reduction. Int. Biodeterior. Biodegrad. 2013, 82, 87–95. [Google Scholar] [CrossRef]
- Michalec, J.; Niklasova, S. Water Uptake of Hardwoods. Master’s Thesis, Linnaeus University, Växjö, Sweden, 2006. [Google Scholar]
- Sweet, M.S. Fire Performance of Wood: Test Methods and Fire Retardant Treatments; Business Communications Co. Inc.: Ridgeland, MS, USA, 1993. [Google Scholar]
- Gan, W.; Chen, C.; Wang, Z.; Song, J.; Kuang, Y.; He, S.; Mi, R.; Sunderland, P.B.; Hu, L. Dense, self-formed char layer enables a fire-retardant wood structural material. Adv. Funct. Mater. 2019, 29, 1807444. [Google Scholar] [CrossRef]
- Buchanan, A.H. Fire performance of timber construction. Prog. Struct. Eng. Mater. 2000, 2, 278–289. [Google Scholar] [CrossRef]
- Frangi, A.; Fontana, M. Charring rates and temperature profiles of wood sections. Fire Mater. 2003, 27, 91–102. [Google Scholar] [CrossRef]
- White, M. Charred Timber Cladding: Performance Investigation. Master’s Thesis, Conventry University, Conventry, UK, 2017. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Brummer, Y.; Cui, W.S. Understanding carbohydrate analysis. In Food Carbohydrates: Chemistry, Physical Properties, and Applications; Cui, W.S., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 67–105. ISBN 9780429095641. [Google Scholar]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1989. [Google Scholar]
- Sjöström, E.; Alén, R. Analytical Methods in Wood Chemistry, Pulping, and Papermaking; Springer: Berlin, Germany, 1999. [Google Scholar]
- Nielsen, S.S. Total carbohydrate by phenol-sulfuric acid method. In Food Analysis Laboratory Manual; Springer: Cham, Switzerland, 2017; pp. 137–141. [Google Scholar]
- Wang, Y.; Zhang, Z.; Fan, H.; Wang, J. Wood carbonization as a protective treatment on resistance to wood destroying fungi. Int. Biodeterior. Biodegrad. 2018, 129, 42–49. [Google Scholar] [CrossRef]
- Bari, E.; Ohno, K.; Yilgor, N.; Singh, A.; Morrell, J.; Pizzi, A.; Ghanbary, M.T.; Ribera, J. Characterizing fungal decay of beech wood: Potential for biotechnological applications. Microorganisms 2021, 9, 247. [Google Scholar] [CrossRef]
- Hakkou, M.; Pétrissans, M.; Gérardin, P.; Zoulalian, A. Investigations of the reasons for fungal durability of heat-treated beech wood. Polym. Degrad. Stab. 2006, 91, 393–397. [Google Scholar] [CrossRef]
- Weiland, J.J.; Guyonnet, R. Study of chemical modifications and fungi degradation of thermally modified wood using DRIFT spectroscopy. Holz Roh Werkst. 2003, 61, 216–220. [Google Scholar] [CrossRef]
- Schwarze, F.W.M.R.; Engels, J.; Mattheck, C. Fungal Strategies of Wood Decay in Trees; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Bari, E.; Daniel, G.; Yilgor, N.; Kim, J.S.; Tajick-Ghanbary, M.A.; Singh, A.P.; Ribera, J. Comparison of the decay behavior of two white-rot fungi in relation to wood type and exposure conditions. Microorganisms 2020, 8, 1931. [Google Scholar] [CrossRef] [PubMed]
- Borůvka, V.; Novák, D.; Šedivka, P. Comparison and analysis of radial and tangential bending of softwood and hardwood at static and dynamic loading. Forests 2020, 11, 896. [Google Scholar] [CrossRef]
- Vay, O.; De Borst, K.; Hansmann, C.; Teischinger, A.; Müller, U. Thermal conductivity of wood at angles to the principal anatomical directions. Wood Sci. Technol. 2015, 49, 577–589. [Google Scholar] [CrossRef]
- Sonderegger, W.; Hering, S.; Niemz, P. Thermal behaviour of Norway spruce and European beech in and between the principal anatomical directions. Holzforschung 2011, 65, 369–375. [Google Scholar] [CrossRef]





| Group | Direction | Temperature [°C] | Time [min] | Coding | Group | Direction | Temperature [°C] | Time [min] | Coding |
|---|---|---|---|---|---|---|---|---|---|
| REF | R | - | - | REFR | Charred specimens | R | 300 | 4 | 300/4R |
| T | REFT | T | 300/4T | ||||||
| Charred specimens | R | 200 | 20 | 200/20R | R | 350 | 1 | 350/1R | |
| T | 200/20T | T | 350/1T | ||||||
| R | 250 | 4 | 250/4R | R | 350 | 2 | 350/2R | ||
| T | 250/4T | T | 350/2T | ||||||
| R | 250 | 6 | 250/6R | R | 400 | 0.5 | 400/0.5R | ||
| T | 250/6T | T | 400/0.5T | ||||||
| R | 300 | 2 | 300/2R | R | 400 | 1 | 400/1R | ||
| T | 300/2T | T | 400/1T |
| Specimen | Radial | Tangential | ||
|---|---|---|---|---|
| Sa [mm] | Sz [mm] | Sa [mm] | Sz [mm] | |
| 200/20 | 0.201 (0.01) | 1.179 (0.08) | 0.142 (0.01) | 1.260 (0.16) |
| 250/4 | 0.146 (0.06) | 1.410 (0.63) | 0.122 (0.04) | 1.349 (0.83) |
| 250/6 | 0.137 (0.03) | 1.370 (0.26) | 0.156 (0.02) | 1.454 (0.23) |
| 300/2 | 0.118 (0.02) | 1.168 (0.21) | 0.132 (0.02) | 1.424 (0.34) |
| 300/4 | 0.136 (0.02) | 1.365 (0.27) | 0.171 (0.05) | 1.479 (0.34) |
| 350/1 | 0.118 (0.01) | 1.103 (0.15) | 0.128 (0.01) | 1.394 (0.27) |
| 350/2 | 0.110 (0.01) | 1.112 (0.19) | 0.130 (0.01) | 1.306 (0.30) |
| 400/0.5 | 0.135 (0.02) | 1.262 (0.27) | 0.116 (0.03) | 1.204 (0.20) |
| 400/1 | 0.133 (0.00) | 1.189 (0.10) | 0.117 (0.02) | 1.135 (0.16) |
| REF | 0.151 (0.07) | 1.438 (0.03) | 0.123 (0.00) | 1.283 (0.24) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machová, D.; Oberle, A.; Zárybnická, L.; Dohnal, J.; Šeda, V.; Dömény, J.; Vacenovská, V.; Kloiber, M.; Pěnčík, J.; Tippner, J.; et al. Surface Characteristics of One-Sided Charred Beech Wood. Polymers 2021, 13, 1551. https://doi.org/10.3390/polym13101551
Machová D, Oberle A, Zárybnická L, Dohnal J, Šeda V, Dömény J, Vacenovská V, Kloiber M, Pěnčík J, Tippner J, et al. Surface Characteristics of One-Sided Charred Beech Wood. Polymers. 2021; 13(10):1551. https://doi.org/10.3390/polym13101551
Chicago/Turabian StyleMachová, Dita, Anna Oberle, Lucie Zárybnická, Jakub Dohnal, Vít Šeda, Jakub Dömény, Veronika Vacenovská, Michal Kloiber, Jan Pěnčík, Jan Tippner, and et al. 2021. "Surface Characteristics of One-Sided Charred Beech Wood" Polymers 13, no. 10: 1551. https://doi.org/10.3390/polym13101551