Multiresponsive Cellulose Nanocrystal Cross-Linked Copolymer Hydrogels for the Controlled Release of Dyes and Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Cellulose Nanocrystals (CNCs)
2.3. Desulfation Procedure of the Cellulose Nanocrystals (CNCs)
2.4. Preparation of the PAAc/PAAm/CNC Composite Hydrogels
2.5. Shrinking/Swelling Measurement of the Hydrogels
2.6. Dye/Drug Loading and Release
2.7. Characterizations
3. Results and Discussion
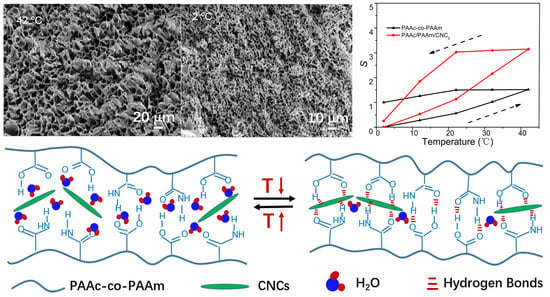
3.1. Preparation and Characterization of the PAAc/PAAm/CNC Hydrogels
3.2. Shrinkage and Swelling Properties of the PAAc/PAAm/CNC Hydrogels
3.2.1. Temperature Effects
3.2.2. pH Effects
3.3. Rheological Properties of PAAc/PAAm/CNC Hydrogels
3.4. Adsorption and Controlled Release of Dyes or Drugs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weng, G.; Thanneeru, S.; He, J. Dynamic coordination of Eu-iminodiacetate to control fluorochromic response of polymer hydrogels to multistimuli. Adv. Mater. 2018, 30, 1706526. [Google Scholar] [CrossRef] [PubMed]
- Morelle, X.P.; Illeperuma, W.R.; Tian, K.; Bai, R.; Suo, Z.; Vlassak, J.J. Highly stretchable and tough hydrogels below water freezing temperature. Adv. Mater. 2018, 30, 1801541. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Meng, X.; Xu, W.; Zhang, S.; Ouyang, J.; Zhang, Z.; Liu, Y.; Niu, Y.; Ma, S.; Xue, Z.; et al. Single network double cross-linker (SNDCL) hydrogels with excellent stretchability, self-recovery, adhesion strength, and conductivity for human motion monitoring. Soft Matter 2020, 16, 7323–7331. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Chen, L.; Yin, Y.; Zhou, J.; Liu, M. Anti-freezing, conductive self-healing organohydrogels with stable strain-sensitivity at subzero temperatures. Angew. Chem. Int. Ed. 2017, 56, 14347–14351. [Google Scholar] [CrossRef]
- Huang, S.; Kong, X.; Xiong, Y.; Zhang, X.; Chen, H.; Jiang, W.; Niu, Y.; Xu, W.; Ren, C. An overview of dynamic covalent bonds in polymer material and their applications. Eur. Polym. J. 2020, 141, 110094. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Hu, J.; Hu, J.; Zhang, S.; Yang, Z.; Li, Y.; Cheng, Y. Smart hydrogels with antibacterial properties built from all natural building blocks. Chem. Mater. 2019, 31, 7678–7685. [Google Scholar] [CrossRef]
- Veleva, V.R.; Cue, B.W.; Todorova, S. Benchmarking green chemistry adoption by the global pharmaceutical supply chain. ACS Sustain. Chem. Eng. 2018, 6, 2–14. [Google Scholar] [CrossRef]
- Hua, L.; Xie, M.; Jian, Y.; Wu, B.; Chen, C.; Zhao, C. Multiple-responsive and amphibious hydrogel actuator based on asymmetric UCST-type volume phase transition. ACS Appl. Mater. Interfaces 2019, 11, 43641–43648. [Google Scholar] [CrossRef]
- Downs, F.G.; Lunn, D.J.; Booth, M.J.; Sauer, J.B.; Ramsay, W.J.; Klemperer, R.G.; Hawker, C.J.; Bayley, H. Multi-responsive hydrogel structures from patterned droplet networks. Nat. Chem. 2020, 12, 363–371. [Google Scholar] [CrossRef]
- Zarzar, L.D.; Kim, P.; Aizenberg, J. Bio-inspired design of submerged hydrogel-actuated polymer microstructures operating in response to pH. Adv. Mater. 2011, 23, 1442–1446. [Google Scholar] [CrossRef]
- Deng, Z.; Guo, Y.; Zhao, X.; Ma, P.X.; Guo, B. Multifunctional stimuli-responsive hydrogels with self-healing, high conductivity, and rapid recovery through Host–Guest interactions. Chem. Mater. 2018, 30, 1729–1742. [Google Scholar] [CrossRef]
- Fernandes, R.; Gracias, D.H. Self-folding polymeric containers for encapsulation and delivery of drugs. Adv. Drug Deliv. Rev. 2012, 64, 1579–1589. [Google Scholar] [CrossRef] [Green Version]
- Rus, D.; Tolley, M.T. Design, fabrication and control of soft robots. Nature 2015, 521, 467–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Cho, Y.; Kim, S.; Lee, J. 3D cocontinuous composites of hydrophilic and hydrophobic soft materials: High modulus and fast actuation time. ACS Macro Lett. 2017, 6, 1119–1123. [Google Scholar] [CrossRef]
- Yang, M.; Liu, C.; Li, Z.; Gao, G.; Liu, F. Temperature-responsive properties of poly(acrylic acid-co-acrylamide) hydrophobic association hydrogels with high mechanical strength. Macromolecules 2010, 43, 10645–10651. [Google Scholar] [CrossRef]
- Xu, Y.; Ghag, O.; Reimann, M.; Sitterle, P.; Chatterjee, P.; Nofen, E.; Yu, H.; Jiang, H.; Dai, L.L. Development of visible-light responsive and mechanically enhanced “Smart” UCST interpenetrating network hydrogels. Soft Matter 2018, 14, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Tuan, H.N.A.; Nhu, V.T.T. Synthesis and properties of pH-thermo dual responsive semi-iPN hydrogels based on N, N’-diethylacrylamide and itaconamic acid. Polymers 2020, 12, 1139. [Google Scholar] [CrossRef]
- Jana, S.; Biswas, Y.; Anas, M.; Saha, A.; Mandal, T.K. Poly [oligo(2-ethyl-2-oxazoline)acrylate]-based poly(ionic liquid) random copolymers with coexistent and tunable lower critical solution temperature- and upper critical solution temperature-type phase transitions. Langmuir 2018, 34, 12653–12663. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yan, Y.; Peng, Q.; Wang, B.; Xing, Y.; Hua, Z.; Wang, Z. Multiple stimuli-responsive cellulose hydrogels with tunable LCST and UCST as smart windows. ACS Appl. Polym. Mater. 2020, 2, 3259–3266. [Google Scholar] [CrossRef]
- Zheng, J.; Xiao, P.; Le, X.; Lu, W.; Théato, P.; Ma, C.; Du, B.; Zhang, J.; Huang, Y.; Chen, T. Mimosa inspired bilayer hydrogel actuator functioning in multi-environments. J. Mater. Chem. C 2018, 6, 1320–1327. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, S.; Ionov, L.; Agarwal, S. Thermophilic films and fibers from photo cross-linkable UCST-type polymers. Polym. Chem. 2015, 6, 2769–2776. [Google Scholar] [CrossRef] [Green Version]
- Isogai, A. Emerging nanocellulose technologies: Recent developments. Adv. Mater. 2020, 2020, 2000630. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, X.; Han, J.; Yu, L.; Chen, J.; Wu, Q.; Jiang, J. Effects of nanocellulose on sodium alginate/polyacrylamide hydrogel: Mechanical properties and adsorption-desorption capacities. Carbohydr. Polym. 2019, 206, 289–301. [Google Scholar] [CrossRef]
- Zhang, T.; Zuo, T.; Hu, D.; Chang, C. Dual physically cross-linked nanocomposite hydrogels reinforced by tunicate cellulose nanocrystals with high toughness and good self-recoverability. ACS Appl. Mater. Interfaces 2017, 9, 24230–24237. [Google Scholar] [CrossRef]
- Li, B.; Han, Y.; Zhang, Y.; Cao, X.; Luo, Z. Dual physically crosslinked nanocomposite hydrogels reinforced by poly (N-vinylpyrrolidone) grafted cellulose nanocrystal with high strength, toughness, and rapid self-recovery. Cellulose 2020, 27, 9913–9925. [Google Scholar] [CrossRef]
- Kloser, E.; Gray, D. Surface grafting of cellulose nanocrystals with poly (ethylene oxide) in aqueous media. Langmuir 2010, 26, 13450. [Google Scholar] [CrossRef]
- Abitbol, T.; Kloser, E.; Gray, D. Estimation of the surface sulfur content of cellulose nanocrystals prepared by sulfuric acid hydrolysis. Cellulose 2013, 20, 785. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q.; Yue, Y.; Zhang, Q. Application of rod-shaped cellulose nanocrystals in polyacrylamide hydrogels. J. Colloid Interface Sci. 2011, 353, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Wang, M.; Meng, L.; Chang, H.; Wang, B.; Xu, F.; Yang, J.; Wan, P. Mussel-inspired cellulose nanocomposite tough hydrogels with synergistic self-healing, adhesive, and strain-sensitive properties. Chem. Mater. 2018, 30, 3110–3121. [Google Scholar] [CrossRef]
- Dai, H.; Chen, Q.; Qin, H.; Guan, Y.; Shen, D.; Hua, Y.; Tang, Y.; Xu, J. A temperature-responsive copolymer hydrogel in controlled drug delivery. Macromolecules 2006, 39, 6584–6589. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, C.; Kim, Y.; Jung, S. Dual crosslinked carboxymethyl cellulose/polyacrylamide interpenetrating hydrogels with highly enhanced mechanical strength and superabsorbent properties. Eur. Polym. J. 2020, 127, 109586. [Google Scholar] [CrossRef]
- Ryu, J.H.; Han, N.K.; Lee, J.S.; Jeong, Y.G. Microstructure, thermal and mechanical properties of composite films based on carboxymethylated nanocellulose and polyacrylamide. Carbohydr. Polym. 2019, 211, 84–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Z.; Fu, Y.; Wang, Z.; Qin, M.; Yuan, Z. Responsive and patterned cellulose nanocrystal films modified by N-methylmorpholine-N-oxide. Carbohydr. Polym. 2020, 228, 115387. [Google Scholar] [CrossRef]
- Yang, N.; Ji, X.; Sun, J.; Zhang, Y.; Xu, Q.; Fu, Y.; Li, H.; Qin, M.; Yuan, Z. Photonic actuators with predefined shapes. Nanoscale 2019, 11, 10088–10096. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ji, X.; Li, G.; Zhang, Y.; Liu, N.; Li, H.; Qin, M.; Yuan, Z. Chiral nematic latex-GO composite films with synchronous response of color and actuation. J. Mater. Chem. C 2019, 7, 104–110. [Google Scholar] [CrossRef]
- Gómez Ribelles, J.L.; Pradas, M.M.; Dueñas, J.M.M.; Cabanilles, C.T. Glass transition in homogeneous and heterogeneous interpenetrating polymer networks and its relation to concentration fluctuations. J. Non-Cryst. Solids. 2002, 307–310, 731–737. [Google Scholar] [CrossRef]
- Ilmain, F.; Tanaka, T.; Kokufuta, E. Volume transition in a gel driven by hydrogen bonding. Nature 1991, 349, 400–401. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Chen, L.W.; Zhong, M.; Shi, F.K.; Liu, X.Y.; Xie, X.M. Phase transition temperature controllable poly(acrylamide-co-acrylic acid) nanocomposite physical hydrogels with high strength. Chin. J. Polym Sci. 2016, 34, 1261–1269. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, S.; Fan, W.; Zhao, Y. Ultrasensitive pH-induced water solubility switch using UCST polymers. Macromolecules 2016, 49, 1424–1433. [Google Scholar] [CrossRef]
- Yue, Y.F.; Haque, M.A.; Kurokawa, T.; Nakajima, T.; Gong, J.P. Lamellar hydrogels with high toughness and ternary tunable photonic stop-band. Adv. Mater. 2013, 25, 3106–3110. [Google Scholar] [CrossRef]
- Zhang, Y.; Furyk, S.; Bergbreiter, D.E.; Cremer, P.S. Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J. Am. Chem. Soc. 2005, 127, 14505–14510. [Google Scholar] [CrossRef]
- Salis, A.; Ninham, B.W. Models and mechanisms of Hofmeister effects in electrolyte solutions, and colloid and protein systems revisited. Chem. Soc. Rev. 2014, 43, 7358–7377. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Liu, Y.; Jin, L.; Chen, T.; Yin, B. Coordination-induced gelation of an L-glutamic acid Schiff base derivative: The anion effect and cyanide-specific selectivity. Chem. Commun. 2016, 52, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, P.; Mao, G.; Yin, T.; Zhong, D.; Yiming, B.; Hu, X.; Jia, Z.; Nian, G.; Qu, S.; et al. Dual pH-responsive hydrogel actuator for lipophilic drug delivery. ACS Appl. Mater. Interfaces 2020, 12, 12010–12017. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Ma, M.; Xu, J.; Si, C.; Wang, X.; Liu, Z.; Ni, Y. All-lignin-based hydrogel with fast pH-stimuli responsiveness for mechanical switching and actuation. Chem. Mater. 2020, 32, 4324–4330. [Google Scholar] [CrossRef]
- Li, G.; Liu, M.; Song, C.; Yuan, Z. Printable and conductive supramolecular hydrogels facilitated by peptides and group 1B metal ions. Appl. Surf. Sci. 2019, 493, 94–104. [Google Scholar] [CrossRef]
- Wang, H.; Xu, W.; Song, S.; Feng, L.; Song, A.; Hao, J. Hydrogels facilitated by monovalent cations and their use as efficient dye adsorbents. J. Phys. Chem. B 2014, 118, 4693. [Google Scholar] [CrossRef] [PubMed]
- Rohani Rad, E.; Vahabi, H.; Formela, K.; Saeb, M.R.; Thomas, S. Injectable poloxamer/graphene oxide hydrogels with well-controlled mechanical and rheological properties. Polym. Advan. Technol. 2019, 30, 2250–2260. [Google Scholar] [CrossRef]
- Yang, K.; Li, X.; Cui, J.; Zhang, M.; Wang, Y.; Lou, Z.; Shan, W.; Xiong, Y. Facile synthesis of novel porous graphene-like carbon hydrogel for highly efficient recovery of precious metal and removal of organic dye. Appl. Surf. Sci. 2020, 528, 146928. [Google Scholar] [CrossRef]
- Adhikari, B.; Palui, G.; Banerjee, A. Self-assembling tripeptide based hydrogels and their use in removal of dyes from waste-water. Soft Matter 2009, 5, 3452–3460. [Google Scholar] [CrossRef]
- Basak, S.; Nandi, N.; Paul, S.; Hamley, I.W.; Banerjee, A. A tripeptide-based self-shrinking hydrogel for waste-water treatment: Removal of toxic organic dyes and lead (Pb2+) ions. Chem. Commun. 2017, 53, 5910–5913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Hui, P.C.; Kan, C.; Wang, W. Dual-responsive (pH/temperature) Pluronic F-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 2019, 9, 11658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasparakis, G.; Tsitsilianis, C. LCST polymers: Thermoresponsive nanostructured assemblies towards bioapplications. Polymer 2020, 211, 123146. [Google Scholar] [CrossRef]
- Xu, W.; Hong, Y.; Song, A.; Hao, J. Peptide-assembled hydrogels for pH-controllable drug release. Colloids Surf. B 2020, 185, 110567. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Li, G.; Yang, C.; Kong, F.; Yuan, Z. Multiresponsive Cellulose Nanocrystal Cross-Linked Copolymer Hydrogels for the Controlled Release of Dyes and Drugs. Polymers 2021, 13, 1219. https://doi.org/10.3390/polym13081219
Jiang Y, Li G, Yang C, Kong F, Yuan Z. Multiresponsive Cellulose Nanocrystal Cross-Linked Copolymer Hydrogels for the Controlled Release of Dyes and Drugs. Polymers. 2021; 13(8):1219. https://doi.org/10.3390/polym13081219
Chicago/Turabian StyleJiang, Yuchen, Guihua Li, Chenyu Yang, Fangong Kong, and Zaiwu Yuan. 2021. "Multiresponsive Cellulose Nanocrystal Cross-Linked Copolymer Hydrogels for the Controlled Release of Dyes and Drugs" Polymers 13, no. 8: 1219. https://doi.org/10.3390/polym13081219