Development of Epirubicin-Loaded Biocompatible Polymer PLA–PEG–PLA Nanoparticles: Synthesis, Characterization, Stability, and In Vitro Anticancerous Assessment
Abstract
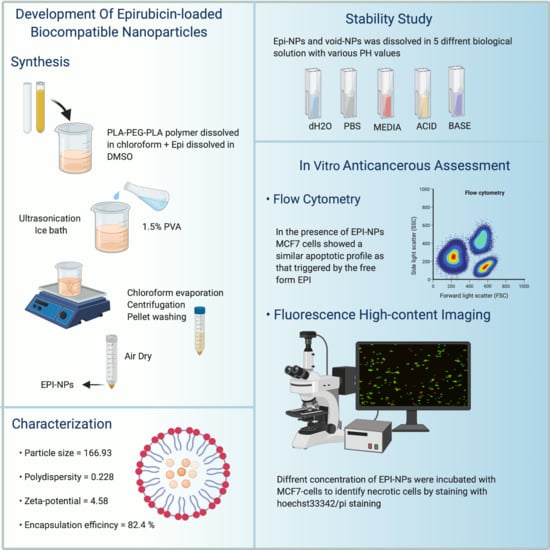
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of EPI Polymeric NPs
2.3. Characterization of the EPI-Loaded NPs Formulations
2.3.1. Particle Size and Polydispersity Index Analysis
2.3.2. Measurement of Zeta-Potential
2.3.3. Transmission Electron Microscopy (TEM)
2.3.4. Measurement of Drug Entrapment Efficiency (%EE) and Drug Release Study
2.4. Stability Study
2.5. Differential Scanning Calorimetry
2.6. Anticancer Activity of EPI-NPs
2.6.1. Assessment of Anticancer Activity Using Flow Cytometry
2.6.2. Fluorescence High-Content Imaging
2.6.3. Statistical Analysis
3. Results and Discussion
3.1. Dynamic Light Scattering
3.2. Transmission Electron Microscopy
3.3. Entrapment Efficiency
3.4. Stability Study
3.5. Differential Scanning Calorimetry
3.6. The Effect of EPI-NPs on Estrogen Positive Cancer Cells MCF-7
3.7. Fluorescence Imaging of MCF-7 Cells Treated with EPI-NPS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- BC Cancer Agency Epirubicin. BC Cancer Agency Cancer Drug Manual; BC Cancer Agency Epirubicin: Vancouver, BC, Canada, 2015. [Google Scholar]
- Plosker, G.L.; Faulds, D. Epirubicin. Drugs 1993, 45, 788–856. [Google Scholar] [CrossRef]
- Petrioli, R.; Roviello, G.; Zanotti, L.; Roviello, F.; Polom, K.; Bottini, A.; Marano, L.; Francini, E.; Marrelli, D.; Generali, D. Epirubicin-Based Compared with Docetaxel-Based Chemotherapy for Advanced Gastric Carcinoma: A Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2016, 102, 82–88. [Google Scholar] [CrossRef]
- Khasraw, M.; Bell, R.; Dang, C. Epirubicin: Is It like Doxorubicin in Breast Cancer? A Clinical Review. Breast 2012, 21, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Tang, W.; Fu, M.; Gong, X.-Q.; Kong, L.; Yao, X.-M.; Jing, M.; Cai, F.-Y.; Li, X.-T.; Ju, R.-J. Development of R8 Modified Epirubicin-Dihydroartemisinin Liposomes for Treatment of Non-Small-Cell Lung Cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1947–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukagoshi, S. Epirubicin 4′-epi-adriamycin. Gan Kagaku Ryoho 1990, 17, 151–159. [Google Scholar]
- Nielsen, D.; Dombernowsky, P.; Larsen, S.K.; Hansen, O.P.; Skovsgaard, T. Epirubicin or Epirubicin and Cisplatin as First-Line Therapy in Advanced Breast Cancer. A Phase III Study. Cancer Chemother. Pharmacol. 2000, 46, 459–466. [Google Scholar] [CrossRef]
- Cersosimo, R.J.; Hong, W.K. Epirubicin: A Review of the Pharmacology, Clinical Activity, and Adverse Effects of an Adriamycin Analogue. JCO 1986, 4, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D. A Critical Evaluation of the Mechanisms of Action Proposed for the Antitumor Effects of the Anthracycline Antibiotics Adriamycin and Daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Mattu, C.; Pabari, R.M.; Boffito, M.; Sartori, S.; Ciardelli, G.; Ramtoola, Z. Comparative Evaluation of Novel Biodegradable Nanoparticles for the Drug Targeting to Breast Cancer Cells. Eur. J. Pharm. Biopharm. 2013, 85, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, C. Nanomedicine Transforms Drug Delivery. Drug Discov. Today 2005, 10. [Google Scholar] [CrossRef]
- Stylios, G.K.; Giannoudis, P.V.; Wan, T. Applications of Nanotechnologies in Medical Practice. Injury 2005, 36. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M. Drug Targeting with Nano-Sized Carrier Systems. J. Artif. Organs 2005, 8. [Google Scholar] [CrossRef]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-Based Drug Delivery Systems. J. Occup. Med. Toxicol. 2007, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- De Jong, W.H.; Borm, P.J. Drug Delivery and Nanoparticles: Applications and Hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Badran, M.M.; Alomrani, A.H.; Harisa, G.I.; Ashour, A.E.; Kumar, A.; Yassin, A.E. Novel Docetaxel Chitosan-Coated PLGA/PCL Nanoparticles with Magnified Cytotoxicity and Bioavailability. Biomed. Pharmacother. 2018, 106, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, K.B.; Amin, M.L. Nanotechnology in Cancer Drug Delivery and Selective Targeting. ISRN Nanotechnol. 2014, 2014, 12. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Lai, S.K.; Yu, T.; Wang, Y.-Y.; Happe, C.; Zhong, W.; Zhang, M.; Anonuevo, A.; Fridley, C.; Hung, A.; et al. Nanoparticle Penetration of Human Cervicovaginal Mucus: The Effect of Polyvinyl Alcohol. J. Control. Release 2014, 192, 202–208. [Google Scholar] [CrossRef] [Green Version]
- Massadeh, S.; Alaamery, M.; Al-Qatanani, S.; Alarifi, S.; Bawazeer, S.; Alyafee, Y. Synthesis of Protein-Coated Biocompatible Methotrexate-Loaded PLA-PEG-PLA Nanoparticles for Breast Cancer Treatment. Nano Rev. Exp. 2016. [Google Scholar] [CrossRef] [Green Version]
- Massadeh, S.; Omer, M.E.; Alterawi, A.; Ali, R.; Alanazi, F.H.; Almutairi, F.; Almotairi, W.; Alobaidi, F.F.; Alhelal, K.; Almutairi, M.S.; et al. Optimized Polyethylene Glycolylated Polymer–Lipid Hybrid Nanoparticles as a Potential Breast Cancer Treatment. Pharmaceutics 2020, 12, 666. [Google Scholar] [CrossRef]
- Iqbal, S.; Rashid, M.H.; Arbab, A.S.; Khan, M. Encapsulation of Anticancer Drugs (5-Fluorouracil and Paclitaxel) into Polycaprolactone (PCL) Nanofibers and In Vitro Testing for Sustained and Targeted Therapy. J. Biomed. Nanotechnol. 2017, 13, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Hulderman, T.; Salmen, R.; Chapman, R.; Leonard, S.S.; Young, S.H. Cardiovascular Effects of Pulmonary Exposure to Single-Wall Carbon Nanotubes. Environ. Health Perspect. 2007, 115. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Gendelman, H.E. Nanomedicine in the Diagnosis and Therapy of Neurodegenerative Disorders. Prog. Polym. Sci. 2007, 32, 1054–1082. [Google Scholar] [CrossRef] [Green Version]
- Cattel, L.; Ceruti, M.; Dosio, F. From Conventional to Stealth Liposomes: A New Frontier in Cancer Chemotherapy. Tumori 2003, 89, 237–249. [Google Scholar] [CrossRef]
- Wong, C.Y.; Luna, G.; Martinez, J.; Al-Salami, H.; Dass, C.R. Bio-Nanotechnological Advancement of Orally Administered Insulin Nanoparticles: Comprehensive Review of Experimental Design for Physicochemical Characterization. Int. J. Pharm. 2019, 572, 118720. [Google Scholar] [CrossRef]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.; Bantz, K.C.; Haynes, C.L. Stabilization of Silver and Gold Nanoparticles: Preservation and Improvement of Plasmonic Functionalities. Chem. Rev. 2019, 119, 664–699. [Google Scholar] [CrossRef] [PubMed]
- Curtis, C.; Toghani, D.; Wong, B.; Nance, E. Colloidal Stability as a Determinant of Nanoparticle Behavior in the Brain. Colloids Surf. B Biointerfaces 2018, 170, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Ball, R.L.; Bajaj, P.; Whitehead, K.A. Achieving Long-Term Stability of Lipid Nanoparticles: Examining the Effect of PH, Temperature, and Lyophilization. Int. J. Nanomed. 2017, 12, 305–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, J.; McClements, D.J. Standardization of Nanoparticle Characterization: Methods for Testing Properties, Stability, and Functionality of Edible Nanoparticles. Crit. Rev. Food Sci. Nutr. 2016, 56, 1334–1362. [Google Scholar] [CrossRef]
- Lima, F.V.; Mendes, C.; Zanetti-Ramos, B.G.; Nandi, J.K.; Cardoso, S.G.; Bernardon, J.K.; Silva, M.A.S. Carbamide Peroxide Nanoparticles for Dental Whitening Application: Characterization, Stability and in Vivo/in Situ Evaluation. Colloids Surf. B Biointerfaces 2019, 179, 326–333. [Google Scholar] [CrossRef]
- Gou, J.; Feng, S.; Liang, Y.; Fang, G.; Zhang, H.; Yin, T.; Zhang, Y.; He, H.; Wang, Y.; Tang, X. Polyester-Solid Lipid Mixed Nanoparticles with Improved Stability in Gastro-Intestinal Tract Facilitated Oral Delivery of Larotaxel. Mol. Pharm. 2017, 14, 3750–3761. [Google Scholar] [CrossRef]
- Bakhaidar, R.; Green, J.; Alfahad, K.; Samanani, S.; Moollan, N.; O’Neill, S.; Ramtoola, Z. Effect of Size and Concentration of PLGA-PEG Nanoparticles on Activation and Aggregation of Washed Human Platelets. Pharmaceutics 2019, 11, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuo, X.; Lei, T.; Miao, L.; Chu, W.; Li, X.; Luo, L.; Gou, J.; Zhang, Y.; Yin, T.; He, H.; et al. Disulfiram-Loaded Mixed Nanoparticles with High Drug-Loading and Plasma Stability by Reducing the Core Crystallinity for Intravenous Delivery. J. Colloid Interface Sci. 2018, 529, 34–43. [Google Scholar] [CrossRef]
- Drug Nanoparticle Stability Assessment Using Isothermal and Nonisothermal Approaches. Available online: https://www.hindawi.com/journals/jnm/2018/3047178/ (accessed on 9 November 2020).
- Chen, X.; Han, W.; Zhao, X.; Tang, W.; Wang, F. Epirubicin-Loaded Marine Carrageenan Oligosaccharide Capped Gold Nanoparticle System for PH-Triggered Anticancer Drug Release. Sci. Rep. 2019, 9, 6754. [Google Scholar] [CrossRef] [PubMed]
- Takemae, K.; Okamoto, J.; Horise, Y.; Masamune, K.; Muragaki, Y. Function of Epirubicin-Conjugated Polymeric Micelles in Sonodynamic Therapy. Front. Pharmacol. 2019, 10, 546. [Google Scholar] [CrossRef]
- Evangelatov, A.; Skrobanska, R.; Mladenov, N.; Petkova, M.; Yordanov, G.; Pankov, R. Epirubicin Loading in Poly(Butyl Cyanoacrylate) Nanoparticles Manifests via Altered Intracellular Localization and Cellular Response in Cervical Carcinoma (HeLa) Cells. NULL 2016, 23, 2235–2244. [Google Scholar] [CrossRef]
- Rao, Y.; Chen, W.; Liang, X.; Huang, Y.; Miao, J.; Liu, L.; Lou, Y.; Zhang, X.; Wang, B.; Tang, R.; et al. Epirubicin-Loaded Superparamagnetic Iron-Oxide Nanoparticles for Transdermal Delivery: Cancer Therapy by Circumventing the Skin Barrier. Small 2015, 11, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Kunjiappan, S.; Panneerselvam, T.; Somasundaram, B.; Arunachalam, S.; Sankaranarayanan, M.; Parasuraman, P. Preparation of Liposomes Encapsulated Epirubicin-Gold Nanoparticles for Tumor Specific Delivery and Release. Biomed. Phys. Eng. Express 2018, 4, 045027. [Google Scholar] [CrossRef]
- Ding, J.; Chen, G.; Chen, G.; Guo, M. One-Pot Synthesis of Epirubicin-Capped Silver Nanoparticles and Their Anticancer Activity against Hep G2 Cells. Pharmaceutics 2019, 11, 123. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, R.; Abdollahi, M.; Emamgholi Zadeh, E.; Khodabakhshi, K.; Badeli, A.; Bagheri, H.; Hosseinkhani, S. MPEG-PLA and PLA-PEG-PLA Nanoparticles as New Carriers for Delivery of Recombinant Human Growth Hormone (RhGH). Sci. Rep. 2018, 8, 9854. [Google Scholar] [CrossRef]
- Danafar, H.; Rostamizadeh, K.; Davaran, S.; Hamidi, M. Drug-Conjugated PLA–PEG–PLA Copolymers: A Novel Approach for Controlled Delivery of Hydrophilic Drugs by Micelle Formation. Pharm. Dev. Technol. 2017, 22, 947–957. [Google Scholar] [CrossRef]
- Danafar, H.; Rostamizadeh, K.; Hamidi, M. Polylactide/Poly(Ethylene Glycol)/Polylactide Triblock Copolymer Micelles as Carrier for Delivery of Hydrophilic and Hydrophobic Drugs: A Comparison Study. J. Pharm. Investig. 2018, 48, 381–391. [Google Scholar] [CrossRef]
- Singh, N.A.; Mandal, A.K.A.; Khan, Z.A. Fabrication of PLA-PEG Nanoparticles as Delivery Systems for Improved Stability and Controlled Release of Catechin. J. Nanomater. 2017, 2017, e6907149. [Google Scholar] [CrossRef] [Green Version]
- Shalgunov, V.; Zaytseva-Zotova, D.; Zintchenko, A.; Levada, T.; Shilov, Y.; Andreyev, D.; Dzhumashev, D.; Metelkin, E.; Urusova, A.; Demin, O.; et al. Comprehensive Study of the Drug Delivery Properties of Poly(l-Lactide)-Poly(Ethylene Glycol) Nanoparticles in Rats and Tumor-Bearing Mice. J. Control. Release 2017, 261, 31–42. [Google Scholar] [CrossRef]
- Alyafee, Y.A.; Alaamery, M.; Bawazeer, S.; Almutairi, M.S.; Alghamdi, B.; Alomran, N.; Sheereen, A.; Daghestani, M.; Massadeh, S. Preparation of Anastrozole Loaded PEG-PLA Nanoparticles: Evaluation of Apoptotic Response of Breast Cancer Cell Lines. Int. J. Nanomed. 2017, 13, 199–208. [Google Scholar] [CrossRef] [Green Version]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. Methods Mol. Biol. 2011, 697, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.Z.; Zeng, Z.W.; Zhou, G.L.; Wang, J.J.; Li, F.Z.; Wang, A.M. Recent Advances in PEG–PLA Block Copolymer Nanoparticles. Int. J. Nanomed. 2010, 5, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.-C.; Wu, S.-C.; Tsai, J.-W.; Yu, T.-J.; Tsai, T.-R. Optimization of Epirubicin Nanoparticles Using Experimental Design for Enhanced Intravesical Drug Delivery. Int. J. Pharm. 2009, 376, 195–203. [Google Scholar] [CrossRef]
- Esim, O.; Bakirhan, N.K.; Sarper, M.; Savaser, A.; Ozkan, S.A.; Ozkan, Y. Influence of Emulsifiers on the Formation and in Vitro Anticancer Activity of Epirubicin Loaded PLGA Nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 60, 102027. [Google Scholar] [CrossRef]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. PH-Responsive High Stability Polymeric Nanoparticles for Targeted Delivery of Anticancer Therapeutics. Commun. Biol. 2020, 3. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, S.; Moscatelli, D.; Codari, F.; Salmona, M.; Morbidelli, M.; Diomede, L. Colloidal Stability of Polymeric Nanoparticles in Biological Fluids. J. Nanoparticle Res. 2012, 14. [Google Scholar] [CrossRef] [Green Version]
- Reference Standards Catalogue—British Pharmacopoeia. Available online: https://www.pharmacopoeia.com/Catalogue/ProductDetails?productid=1000015033 (accessed on 14 December 2020).
- Msds.Pdf. Available online: https://www.alfa.com/en/msds/?language=EN&subformat=CLP1&sku=J60411 (accessed on 13 December 2020).
- Physical, Thermal, and Mechanical Properties of Polymers. In Biosurfaces; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 329–344. ISBN 978-1-118-95062-3.
- Bijarimi, M.; Ahmad, S.; Rasid, R.; Khushairi, M.A.; Zakir, M. Poly(Lactic Acid)/Poly(Ethylene Glycol) Blends: Mechanical, Thermal and Morphological Properties. AIP Conf. Proc. 2016, 1727, 020002. [Google Scholar] [CrossRef] [Green Version]
- Lavor, E.P.; Navarro, M.V.M.; Freire, F.D.; Aragão, C.F.S.; Raffin, F.N.; Barbosa, E.G.; de Lima e Moura, T.F.A. Application of Thermal Analysis to the Study of Antituberculosis Drugs–Excipient Compatibility. J. Therm. Anal. Calorim. 2014, 115, 2303–2309. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Estimating the Concentration of DNA by Fluorometry Using Hoechst 33258. Cold Spring Harb. Protoc. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, A.; Canning, S.L.; Kato, I. Flow Cytometric Detection of G0 in Live Cells by Hoechst 33342 and Pyronin Y Staining. Methods Mol. Biol. 2018, 1686, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Crowley, L.C.; Marfell, B.J.; Waterhouse, N.J. Analyzing Cell Death by Nuclear Staining with Hoechst 33342. Cold Spring Harb. Protoc. 2016, 2016. [Google Scholar] [CrossRef]
- Bazhulina, N.P.; Nikitin, A.M.; Rodin, S.A.; Surovaya, A.N.; Kravatsky, Y.V.; Pismensky, V.F.; Archipova, V.S.; Martin, R.; Gursky, G.V. Binding of Hoechst 33258 and Its Derivatives to DNA. J. Biomol. Struct. Dyn. 2009, 26, 701–718. [Google Scholar] [CrossRef]










| Mean Zeta Potential (ζ)/ mV for Void NPs | ||
|---|---|---|
| Day 0 | Day 31 | |
| H2O (pH 7.02) | −0.18 ± 0.5 | −0.11 ± 0.2 |
| PBS (pH 7.15) | 0.15 ± 0.4 | −0.50 ± 1.5 |
| DMEM Media (pH 7.10) | −0.37 ± 0.04 | −0.16 ± 0.1 |
| KOH (pH 14.05) | 0.45 ± 0.4 | −0.97 ± 1.1 |
| HCL (pH 3.26) | −0.26 ± 0.4 | −0.09 ± 0.2 |
| Mean Zeta Potential (ζ)/mV for EPI-NPs | ||
|---|---|---|
| Day 0 | Day 31 | |
| H2O (pH 7.02) | −0.21 ± 0.9 | 0.03 ± 0.1 |
| PBS (pH 7.15) | −2.56 ± 11.4 | −0.26 ± 0.3 |
| DMEM Media (pH 7.10) | −0.08 ± 0.3 | 0.17 ± 0.5 |
| KOH (pH 14.05) | −1.05 ± 0.4 | 0.33 ± 0.5 |
| HCL (pH 3.26) | 0.16 ± 0.1 | 0.21 ± 0.7 |
| Solution | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | PBS | Media | KOH | HCL | |||||||
| Day 0 | Day 31 | Day 0 | Day 31 | Day 0 | Day 31 | Day 0 | Day 31 | Day 0 | Day 31 | ||
| Particle size | EPI-NPs | 105.2 ± 12 | 170.8 ± 9.4 | 304.8 ± 72.8 | 243.4 ± 2.8 | 165.4 ± 23.3 | 495.8 ± 15.5 | 159.5 ± 7.11 | 160.5 ± 4.5 | 203.9 ± 19.7 | 315.8 ± 16 |
| Void NPs | 87.7 ± 1.2 | 244.9 ± 7.9 | 113.8 ± 2.5 | 128.1 ± 3 | 79.4 ± 9.6 | 656.7 ± 41.5 | 316.5 ± 21.2 | 245.5 ± 7.3 | 146 ± 8 | 432.1 ± 17.2 | |
| Solution | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Water | PBS | Media | KOH | HCL | |||||||
| Day 0 | Day 31 | Day 0 | Day 31 | Day 0 | Day 31 | Day 0 | Day 31 | Day 0 | Day 31 | ||
| Polydispersity | EPI-NPs | 0.41 ± 0.02 | 0.33 ± 0.02 | 4.63 ± 2.8 | 0.24 ± 0.02 | 0.35 ± 0.03 | ± 0.34 ± 0.08 | 0.29 ± 0.1 | 0.22 ± 0.04 | 0.56 ± 0.65 | 0.76 ± 0.14 |
| Void NPs | 0.24 ± 0.02 | 0.32 ± 0.02 | 1.18 ± 0.9 | 0.16 ± 0.04 | 0.60 ± 0.03 | 0.36 ± 0.02 | 0.17 ± 0.03 | 0.39 ± 0.03 | 0.46 ± 0.01 | 0.94 ± 1.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massadeh, S.; Almohammed, I.; Barhoush, E.; Omer, M.; Aldhawi, N.; Almalik, A.; Alaamery, M. Development of Epirubicin-Loaded Biocompatible Polymer PLA–PEG–PLA Nanoparticles: Synthesis, Characterization, Stability, and In Vitro Anticancerous Assessment. Polymers 2021, 13, 1212. https://doi.org/10.3390/polym13081212
Massadeh S, Almohammed I, Barhoush E, Omer M, Aldhawi N, Almalik A, Alaamery M. Development of Epirubicin-Loaded Biocompatible Polymer PLA–PEG–PLA Nanoparticles: Synthesis, Characterization, Stability, and In Vitro Anticancerous Assessment. Polymers. 2021; 13(8):1212. https://doi.org/10.3390/polym13081212
Chicago/Turabian StyleMassadeh, Salam, Iman Almohammed, Eman Barhoush, Mustafa Omer, Nouf Aldhawi, Abdulaziz Almalik, and Manal Alaamery. 2021. "Development of Epirubicin-Loaded Biocompatible Polymer PLA–PEG–PLA Nanoparticles: Synthesis, Characterization, Stability, and In Vitro Anticancerous Assessment" Polymers 13, no. 8: 1212. https://doi.org/10.3390/polym13081212