New Hybrid Copper Nanoparticles/Conjugated Polyelectrolyte Composite with Antibacterial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurements
2.3. Synthesis of Conjugated Polymer (CP) and Polyelectrolyte (CPE)
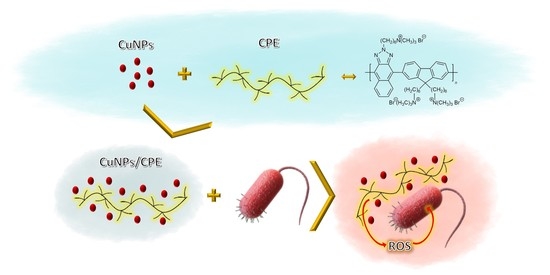
2.4. Preparation of CuNP/CPE Composite
2.5. Computational Details
2.6. Preparation of Microorganism–CuNP/CPE Suspensions
3. Results and Discussion
3.1. Synthesis and Characterization of CuNP/CPE Composite
3.2. Theoretical Calculations
3.3. Microbiological Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, S.J.; Wong, E.H.H.; Boyer, C.; Qiao, G.G. Antimicrobial polymeric nanoparticles. Prog. Polym. Sci. 2018, 76, 40–64. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomedicine 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Niu, L.N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Zhai, L.; Zhang, Z.; Zhao, Y.; Tang, Y. Efficient Antibacterial Performance and Effect of Structure on Property Based on Cationic Conjugated Polymers. Macromolecules 2018, 51, 7239–7247. [Google Scholar] [CrossRef]
- Xu, Q.; He, P.; Wang, J.; Chen, H.; Lv, F.; Liu, L.; Wang, S.; Yoon, J. Antimicrobial activity of a conjugated polymer with cationic backbone. Dyes. Pigm. 2019, 160, 519–523. [Google Scholar] [CrossRef]
- Gupta, A.; Landis, R.F.; Li, C.H.; Schnurr, M.; Das, R.; Lee, Y.W.; Yazdani, M.; Liu, Y.; Kozlova, A.; Rotello, V.M. Engineered Polymer Nanoparticles with Unprecedented Antimicrobial Efficacy and Therapeutic Indices against Multidrug-Resistant Bacteria and Biofilms. J. Am. Chem. Soc. 2018, 140, 12137–12143. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Hafeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnology 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, L.; Azocar, M.; Kogan, M.; Riveros, A.; Paez, M. Copper-polymer nanocomposites: An excellent and cost-effective biocide for use on antibacterial surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 1391–1409. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.D.; Yang, S.S.; Wilson, B.K.; McManus, S.A.; Chen, C.V.H.H.; Prud’homme, R.K. Nanoparticle targeting of Gram-positive and Gram-negative bacteria for magnetic-based separations of bacterial pathogens. Appl. Nanosci. 2017, 7, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Tamilvanan, A.; Balamurugan, K.; Ponappa, K.; Kumar, B.M. Copper Nanoparticles: Synthetic Strategies, Properties and Multifunctional Application. Int. J. Nanosci 2014, 13. [Google Scholar] [CrossRef]
- Parveen, F.; Sannakki, B.; Mandke, M.V.; Pathan, H.M. Copper nanoparticles: Synthesis methods and its light harvesting performance. Sol. Energy Mater. Sol. Cells 2016, 144, 371–382. [Google Scholar] [CrossRef]
- Pham, L.Q.; Sohn, J.H.; Kim, C.W.; Park, J.H.; Kang, H.S.; Lee, B.C.; Kang, Y.S. Copper nanoparticles incorporated with conducting polymer: Effects of copper concentration and surfactants on the stability and conductivity. J. Colloid Interface Sci. 2012, 365, 103–109. [Google Scholar] [CrossRef]
- Wu, S.H.; Chen, D.H. Synthesis of high-concentration Cu nanoparticles in aqueous CTAB solutions. J. Colloid Interface Sci. 2004, 273, 165–169. [Google Scholar] [CrossRef]
- Olad, A.; Alipour, M.; Nosrati, R. The use of biodegradable polymers for the stabilization of copper nanoparticles synthesized by chemical reduction method. Bull. Mater. Sci. 2017, 40, 1013–1020. [Google Scholar] [CrossRef]
- Athawale, A.A.; Katre, P.P.; Kumar, M.; Majumdar, M.B. Synthesis of CTAB–IPA reduced copper nanoparticles. Mater. Chem. Phys. 2005, 91, 507–512. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, B.; Lv, F.; Liu, L.; Wang, S. Conjugated-polymer-based energy-transfer systems for antimicrobial and anticancer applications. Adv. Mater. 2014, 26, 6978–6982. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, J.; Liu, B. Conjugated-polyelectrolyte-based polyprodrug: Targeted and image-guided photodynamic and chemotherapy with on-demand drug release upon irradiation with a single light source. Angew. Chem. Int. Ed. Engl. 2014, 53, 7163–7168. [Google Scholar] [CrossRef]
- Wu, W.; Mao, D.; Xu, S.; Kenry; Hu, F.; Li, X.; Kong, D.; Liu, B. Polymerization-Enhanced Photosensitization. Chem 2018, 4, 1937–1951. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, H.; Wang, Z.; Feng, L. Photothermal Conjugated Polymers and Their Biological Applications in Imaging and Therapy. ACS Appl. Polym. Mater. 2020, 2, 4222–4240. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, L.; Wang, S. Conjugated Polymer Nanoparticles for Imaging, Cell Activity Regulation, and Therapy. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Meng, Z.; Hou, W.; Zhou, H.; Zhou, L.; Chen, H.; Wu, C. Therapeutic Considerations and Conjugated Polymer-Based Photosensitizers for Photodynamic Therapy. Macromol. Rapid Commun. 2018, 39. [Google Scholar] [CrossRef]
- Wu, W.; Bazan, G.C.; Liu, B. Conjugated-Polymer-Amplified Sensing, Imaging, and Therapy. Chem 2017, 2, 760–790. [Google Scholar] [CrossRef] [Green Version]
- Repenko, T.; Rix, A.; Ludwanowski, S.; Go, D.; Kiessling, F.; Lederle, W.; Kuehne, A.J.C. Bio-degradable highly fluorescent conjugated polymer nanoparticles for bio-medical imaging applications. Nat. Commun. 2017, 8, 470. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, P.; Jiang, J.; Wu, J.; Chen, Y.; Tan, Y.; Tan, C.; Jiang, Y. Conjugated Polyelectrolyte Nanoparticles for Apoptotic Cell Imaging. ACS Appl. Mater. Interfaces 2016, 8, 21984–21989. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, X.; Yang, Y.; Bai, H.; Cui, Q.; Sun, H.; Li, L.; Wang, S. Conjugated Polymer with Aggregation-Directed Intramolecular Förster Resonance Energy Transfer Enabling Efficient Discrimination and Killing of Microbial Pathogens. Chem. Mater. 2018, 30, 3244–3253. [Google Scholar] [CrossRef]
- Wang, Y.; Canady, T.D.; Zhou, Z.; Tang, Y.; Price, D.N.; Bear, D.G.; Chi, E.Y.; Schanze, K.S.; Whitten, D.G. Cationic phenylene ethynylene polymers and oligomers exhibit efficient antiviral activity. ACS Appl. Mater. Interfaces 2011, 3, 2209–2214. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Liu, L.; Feng, L. Photothermal-Responsive Conjugated Polymer Nanoparticles for Rapid and Effective Killing of Bacteria. ACS Appl. Biol. Mater. 2018, 1, 27–32. [Google Scholar] [CrossRef]
- Bai, H.; Yuan, H.; Nie, C.; Wang, B.; Lv, F.; Liu, L.; Wang, S. A Supramolecular Antibiotic Switch for Antibacterial Regulation. Angew. Chem. Int. Ed. Engl. 2015, 54, 13208–13213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Corbitt, T.S.; Jett, S.D.; Tang, Y.; Schanze, K.S.; Chi, E.Y.; Whitten, D.G. Direct visualization of bactericidal action of cationic conjugated polyelectrolytes and oligomers. Langmuir 2012, 28, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Visurraga, J.; Daza, C.; Pozo, C.; Becerra, A.; von Plessing, C.; Garcia, A. Study on antibacterial alginate-stabilized copper nanoparticles by FT-IR and 2D-IR correlation spectroscopy. Int. J. Nanomedicine 2012, 7, 3597–3612. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Lu, F.; Hu, W.; Zhao, H.; Tang, Y.; Li, B.; Hu, X.; Li, X.; Lu, X.; Fan, Q.; et al. Tandem activated photodynamic and chemotherapy: Using pH-Sensitive nanosystems to realize different tumour distributions of photosensitizer/prodrug for amplified combination therapy. Biomaterials 2019, 219, 119393. [Google Scholar] [CrossRef]
- Zhu, X.; Xiao, Y.; Jiang, X.; Li, J.; Qin, H.; Huang, H.; Zhang, Y.; He, X.; Wang, K. A ratiometric nanosensor based on conjugated polyelectrolyte-stabilized AgNPs for ultrasensitive fluorescent and colorimetric sensing of melamine. Talanta 2016, 151, 68–74. [Google Scholar] [CrossRef]
- Sun, M.; Sun, B.; Liu, Y.; Shen, Q.D.; Jiang, S. Dual-Color Fluorescence Imaging of Magnetic Nanoparticles in Live Cancer Cells Using Conjugated Polymer Probes. Sci. Rep. 2016, 6, 22368. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Ding, D.; Li, K.; Liu, J.; Liu, B. Tumor-responsive fluorescent light-up probe based on a gold nanoparticle/conjugated polyelectrolyte hybrid. Small 2014, 10, 1967–1975. [Google Scholar] [CrossRef]
- Kazim, S.; Pfleger, J.; Prochazka, M.; Bondarev, D.; Vohlidal, J. Colloidal systems of silver nanoparticles and high-regioregular cationic polythiophene with ionic-liquid-like pendant groups: Optical properties and SERS. J. Colloid Interface Sci. 2011, 354, 611–619. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ishida, S.; Ihara, K.; Yasuda, Y.; Morita, T.; Yamada, S. Synthesis of metallic copper nanoparticles coated with polypyrrole. Colloid Polym. Sci. 2009, 287, 877–880. [Google Scholar] [CrossRef]
- Kahveci, Z.; Vazquez-Guillo, R.; Martinez-Tome, M.J.; Mallavia, R.; Mateo, C.R. New Red-Emitting Conjugated Polyelectrolyte: Stabilization by Interaction with Biomolecules and Potential Use as Drug Carriers and Bioimaging Probes. ACS Appl. Mater. Interfaces 2016, 8, 1958–1969. [Google Scholar] [CrossRef]
- Lee, S.H.; Komurlu, S.; Zhao, X.; Jiang, H.; Moriena, G.; Kleiman, V.D.; Schanze, K.S. Water-Soluble Conjugated Polyelectrolytes with Branched Polyionic Side Chains. Macromolecules 2011, 44, 4742–4751. [Google Scholar] [CrossRef]
- Yen, Y.S.; Ni, J.S.; Hung, W.I.; Hsu, C.Y.; Chou, H.H.; Lin, J.T. Naphtho[2,3-c][1,2,5]thiadiazole and 2H-Naphtho[2,3-d][1,2,3]triazole-Containing D-A-pi-A Conjugated Organic Dyes for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 6117–6126. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Bazan, G.C. Synthesis of cationic conjugated polymers for use in label-free DNA microarrays. Nat. Protoc. 2006, 1, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens Matter. 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 2006, 19, 553–566. [Google Scholar] [CrossRef]
- Woo, S.J.; Park, S.; Jeong, J.E.; Hong, Y.; Ku, M.; Kim, B.Y.; Jang, I.H.; Heo, S.C.; Wang, T.; Kim, K.H.; et al. Synthesis and Characterization of Water-Soluble Conjugated Oligoelectrolytes for Near-Infrared Fluorescence Biological Imaging. ACS Appl. Mater. Interfaces 2016, 8, 15937–15947. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Abd El-Hack, M.E.; Taha, A.E.; Fouda, M.M.G.; Ajarem, J.S.; Maodaa, S.N.; Allam, A.A.; Elshaer, N. Ecofriendly Synthesis and Insecticidal Application of Copper Nanoparticles against the Storage Pest Tribolium castaneum. Nanomaterials 2020, 10, 587. [Google Scholar] [CrossRef] [Green Version]
- Din, M.I.; Arshad, F.; Hussain, Z.; Mukhtar, M. Green Adeptness in the Synthesis and Stabilization of Copper Nanoparticles: Catalytic, Antibacterial, Cytotoxicity, and Antioxidant Activities. Nanoscale Res. Lett. 2017, 12, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, V.D.; Tran, N.Q.; Nguyen, T.P.P. Synergistic effect of citrate dispersant and capping polymers on controlling size growth of ultrafine copper nanoparticles. J. Exp. Nanosci. 2013, 10, 576–587. [Google Scholar] [CrossRef] [Green Version]
- Park, B.K.; Jeong, S.; Kim, D.; Moon, J.; Lim, S.; Kim, J.S. Synthesis and size control of monodisperse copper nanoparticles by polyol method. J. Colloid Interface Sci. 2007, 311, 417–424. [Google Scholar] [CrossRef]
- Dang, T.M.D.; Le, T.T.T.; Fribourg-Blanc, E.; Dang, M.C. Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 015009. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Gellini, C.; Giorgetti, E. Surface-Enhanced Raman Scattering from Copper Nanoparticles Obtained by Laser Ablation. J. Chem. Phys. C 2011, 115, 5021–5027. [Google Scholar] [CrossRef]
- Jessop, I.A.; Bustos, M.; Hidalgo, D.; Terraza, C.A.; Tundidor-Camba, A.; Pardo, M.A.; Fuentealba, D.; Hssein, M.; Bernede, J.C. Synthesis of 2H-benzotriazole based donor-acceptor polymers bearing carbazole derivative as pendant groups: Optical, electronical and photovoltaic properties. Int. J. Electrochem. Sci. 2016, 11, 9822–9838. [Google Scholar] [CrossRef]
- Jessop, I.A.; Diaz, F.R.; Terraza, C.A.; Tundidor-Camba, A.; Leiva, A.; Cattin, L.; Bernede, J.C. PANI Branches onto Donor-Acceptor Copolymers: Synthesis, Characterization and Electroluminescent Properties of New 2D-Materials. Polymers 2018, 10, 553. [Google Scholar] [CrossRef] [Green Version]
- Jessop, I.A.; Chong, A.; Graffo, L.; Camarada, M.B.; Espinoza, C.; Angel, F.A.; Saldias, C.; Tundidor-Camba, A.; Terraza, C.A. Synthesis and Characterization of a 2,3-Dialkoxynaphthalene-Based Conjugated Copolymer via Direct Arylation Polymerization (DAP) for Organic Electronics. Polymers 2020, 12, 1377. [Google Scholar] [CrossRef]
- Kowenje, C.O.; Doetschman, D.C.; Schulte, J.; Kanyi, C.W.; DeCoste, J.; Yang, S.-W.; Jones, B.R. Effects of Copper Exchange Levels on Complexation of Ammonia in Cu (II)-exchanged X Zeolite. S. Afr J. Chem. 2010, 63, 6–10. [Google Scholar]
- Gladstone, J.H. On the Chemical Action of Water on Soluble Salts. Proc. R Soc. 1857, 9, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yang, Z.; Wang, J. Fenton-like degradation of sulfamethoxazole in Cu0/Zn0-air system over abroad pH range: Performance, kinetics and mechanism. Chem. Eng. J. 2021, 403, 126320. [Google Scholar] [CrossRef]
- Deng, D.; Hao, Y.; Xue, J.; Liu, X.; Xu, X.; Liu, L. A Colorimetric Enzyme-Linked Immunosorbent Assay with CuO Nanoparticles as Signal Labels Based on the Growth of Gold Nanoparticles In Situ. Nanomaterials 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.S.; Kim, S.H.; Man, M.T.; Lee, H.S. Synthesis and Characterization of Water Soluble Fluorescent Copper Nanoparticles. Appl. Sci. Converg. Technol. 2018, 27, 75–77. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Z.; Zhu, J.; Tang, Y.; Canady, T.D.; Chi, E.Y.; Schanze, K.S.; Whitten, D.G. Dark Antimicrobial Mechanisms of Cationic Phenylene Ethynylene Polymers and Oligomers against Escherichia coli. Polymers 2011, 3, 1199–1214. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.; Chen, H.; Hu, R.; Li, M.; Lv, F.; Liu, L.; Wang, S. Supramolecular Conjugated Polymer Materials for in Situ Pathogen Detection. ACS Appl. Mater. Interfaces 2016, 8, 31550–31557. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Schanze, K.S.; Chi, E.Y.; Whitten, D.G. When worlds collide: Interactions at the interface between biological systems and synthetic cationic conjugated polyelectrolytes and oligomers. Langmuir 2013, 29, 10635–10647. [Google Scholar] [CrossRef]
- Alzahrani, E. Synthesis of Copper Nanoparticles with Various Sizes and Shapes: Application as a Superior Non-Enzymatic Sensor and Antibacterial Agent. Int. J. Electrochem. Sci. 2016, 4712–4723. [Google Scholar] [CrossRef]







| Complex | Anchor Bond | dX–Cu a | qx b | qCu b | Δqcluster b | −Eint c |
|---|---|---|---|---|---|---|
| 1 | Cu–N | 2.074 | −0.353 | 0.305 | −0.234 | 17.231 (29.958) |
| 2 | Cu–Br | 2.407 | −0.727 | 0.376 | −0.399 | 37.278 (49.935) |
| 3 | Cu–C | 2.394 | −0.257 | 0.317 | −0.056 | 18.303 (24.716) |
| 4 | Cu–Br | 2.456 | −0.744 | 0.33 | −0.572 | 45.984 (60.315) |
| 5 | Cu–Br | 2.449 | −0.726 | 0.322 | ||
| Cu–Br | 2.417 | −0.717 | 0.306 | −0.624 | 42.495 (56.552) | |
| Cu–Br | 2.455 | −0.651 | −0.012 |
| Bacterial Strain | CPE | CuNP/CPE | CPE | CuNP/CPE | CPE | CuNP/CPE |
|---|---|---|---|---|---|---|
| 1 × 10−1 mg·mL−1 | 1 × 10−2 mg·mL−1 | 1 × 10−3 mg·mL−1 | ||||
| E. coli | + | - | + | - | + | + |
| S. enteriditis | + | - | + | - | + | + |
| S. aureus | + | - | + | + | + | + |
| E. faecalis | + | - | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jessop, I.A.; Pérez, Y.P.; Jachura, A.; Nuñez, H.; Saldías, C.; Isaacs, M.; Tundidor-Camba, A.; Terraza, C.A.; Araya-Durán, I.; Camarada, M.B.; et al. New Hybrid Copper Nanoparticles/Conjugated Polyelectrolyte Composite with Antibacterial Activity. Polymers 2021, 13, 401. https://doi.org/10.3390/polym13030401
Jessop IA, Pérez YP, Jachura A, Nuñez H, Saldías C, Isaacs M, Tundidor-Camba A, Terraza CA, Araya-Durán I, Camarada MB, et al. New Hybrid Copper Nanoparticles/Conjugated Polyelectrolyte Composite with Antibacterial Activity. Polymers. 2021; 13(3):401. https://doi.org/10.3390/polym13030401
Chicago/Turabian StyleJessop, Ignacio A., Yasmín P. Pérez, Andrea Jachura, Hipólito Nuñez, Cesar Saldías, Mauricio Isaacs, Alain Tundidor-Camba, Claudio A. Terraza, Ingrid Araya-Durán, María B. Camarada, and et al. 2021. "New Hybrid Copper Nanoparticles/Conjugated Polyelectrolyte Composite with Antibacterial Activity" Polymers 13, no. 3: 401. https://doi.org/10.3390/polym13030401