Silanization of Chitosan and Hydrogel Preparation for Skeletal Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Si-HPMC and Si-Chito Synthesis
2.3. Hydrogel Preparation
2.4. Viscosity Measurements
2.5. Syringeability
2.6. Gel Point Measurements
2.7. Viscoelastic Moduli and Breaking Strength
2.8. Adhesion
2.9. Cell Investigations
2.9.1. Cell Viability in 2D
2.9.2. Cell Viability in 3D/Cell Adhesion
2.10. In Vivo Experiment
2.10.1. Implants Preparation
2.10.2. Subcutaneous Implantation
2.10.3. Histological Experiments
2.11. Statistics
3. Results
3.1. Chitosan Functionalization
3.2. Rheology
3.3. Viscoelastic Properties
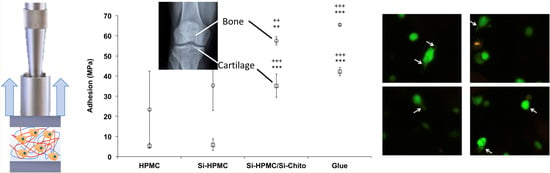
3.4. Tissue Adhesion
3.5. Cell Investigations
3.6. In Vivo Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Flégeau, K.; Pace, R.; Gautier, H.; Rethore, G.; Guicheux, J.; Le Visage, C.; Weiss, P. Toward the development of biomimetic injectable and macroporous biohydrogels for regenerative medicine. Adv. Colloid Interface Sci. 2017, 247, 589–609. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Park, K.; Kim, C.; Khang, G. Novel Biomaterials for Regenerative Medicine; Springer: Singapore, 2018; p. 1077. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badylak, S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 2007, 28, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- McCall, J.D.; Luoma, J.E.; Anseth, K.S. Covalently tethered transforming growth factor beta in PEG hydrogels promotes chondrogenic differentiation of encapsulated human mesenchymal stem cells. Drug Deliv. Transl. Res. 2012, 2, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Edgar, L.; McNamara, K.; Wong, T.; Tamburrini, R.; Katari, R.; Orlando, G. Heterogeneity of Scaffold Biomaterials in Tissue Engineering. Materials 2016, 9, 332. [Google Scholar] [CrossRef] [Green Version]
- Dornish, M.; Kaplan, D.; Skaugrud, O. Bioartificial Organs III: Tissue Sourcing, Immunoisolation, and Clinical Trials. Ann. N. Y. Acad. Sci. 2001, 944, 62–73. [Google Scholar]
- Guan, X.; Avci-Adali, M.; Alarçin, E.; Cheng, H.; Kashaf, S.S.; Li, Y.; Chawla, A.; Jang, H.L.; Khademhosseini, A. Development of hydrogels for regenerative engineering. Biotechnol. J. 2017, 12, 1600394. [Google Scholar] [CrossRef]
- Boyer, C.; Figueiredo, L.; Pace, R.; Lesoeur, J.; Rouillon, T.; Le Visage, C.; Tassin, J.-F.; Weiss, P.; Guicheux, J.; Rethore, G. Laponite nanoparticle-associated silated hydroxypropylmethyl cellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering. Acta Biomater. 2018, 65, 112–122. [Google Scholar] [CrossRef]
- Boyer, C.; Réthoré, G.; Weiss, P.; D’Arros, C.; Lesoeur, J.; Vinatier, C.; Halgand, B.; Geffroy, O.; Fusellier, M.; Vaillant, G.; et al. A Self-Setting Hydrogel of Silylated Chitosan and Cellulose for the Repair of Osteochondral Defects: From in vitro Characterization to Preclinical Evaluation in Dogs. Front. Bioeng. Biotechnol. 2020, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, L.; Pace, R.; D’Arros, C.; Réthoré, G.; Guicheux, J.; Le Visage, C.; Weiss, P. Assessing glucose and oxygen diffusion in hydrogels for the rational design of 3D stem cell scaffolds in regenerative medicine. J. Tissue Eng. Regen. Med. 2018, 12, 1238–1246. [Google Scholar] [CrossRef]
- Moussa, L.; Demarquay, C.; Réthoré, G.; Benadjaoud, M.A.; Siñeriz, F.; Pattappa, G.; Guicheux, J.; Weiss, P.; Barritault, D.; Mathieu, N. Heparan Sulfate Mimetics: A New Way to Optimize Therapeutic Effects of Hydrogel-Embedded Mesenchymal Stromal Cells in Colonic Radiation-Induced Damage. Sci. Rep. 2019, 9, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, F.; Boyer, C.; Gaborit, V.; Rouillon, T.; Guicheux, J.; Tassin, J.-F.; Geoffroy, V.; Rethore, G.; Weiss, P. A Cellulose/Laponite Interpenetrated Polymer Network (IPN) Hydrogel: Controllable Double-Network Structure with High Modulus. Polymers 2018, 10, 634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fellah, B.H.; Weiss, P.; Gauthier, O.; Rouillon, T.; Pilet, P.; Daculsi, G.; Layrolle, P. Bone repair using a new injectable self-crosslinkable bone substitute. J. Orthop. Res. 2006, 24, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Rederstorff, E.; Weiss, P.; Sourice, S.; Pilet, P.; Xie, F.; Sinquin, C.; Colliec-Jouault, S.; Guicheux, J.; Laïb, S. An in vitro study of two GAG-like marine polysaccharides incorporated into injectable hydrogels for bone and cartilage tissue engineering. Acta Biomater. 2011, 7, 2119–2130. [Google Scholar] [CrossRef] [Green Version]
- Vinatier, C.; Magne, D.; Moreau, A.; Gauthier, O.; Malard, O.; Vignes-Colombeix, C.; Daculsi, G.; Weiss, P.; Guicheux, J. Engineering cartilage with human nasal chondrocytes and a silanized hydroxypropyl methylcellulose hydrogel. J. Biomed. Mater. Res. Part A 2007, 80A, 66–74. [Google Scholar] [CrossRef]
- Vinatier, C.; Gauthier, O.; Fatimi, A.; Merceron, C.; Masson, M.; Moreau, A.; Moreau, F.; Fellah, B.; Weiss, P.; Guicheux, J. An injectable cellulose-based hydrogel for the transfer of autologous nasal chondrocytes in articular cartilage defects. Biotechnol. Bioeng. 2009, 102, 1259–1267. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Durkin, C.A.; Mock, T.; Armbrust, E.V. Chitin in Diatoms and Its Association with the Cell Wall. Eukaryot. Cell 2009, 8, 1038–1050. [Google Scholar] [CrossRef] [Green Version]
- Agboh, O.C.; Qin, Y. Chitin and chitosan fibers. Polym. Adv. Technol. 1997, 8, 355–365. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, S.J.; Kim, S.I. Swelling behavior of interpenetrating polymer network hydrogels composed of poly(vinyl alcohol) and chitosan. React. Funct. Polym. 2003, 55, 53–59. [Google Scholar] [CrossRef]
- Ong, S.-Y.; Wu, J.; Moochhala, S.M.; Tan, M.-H.; Lu, J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials 2008, 29, 4323–4332. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Khor, E.; Lim, L.Y. Implantable applications of chitin and chitosan. Biomaterials 2003, 24, 2339–2349. [Google Scholar] [CrossRef]
- Shahidi, F.; Abuzaytoun, R. Chitin, Chitosan, and Co-Products: Chemistry, Production, Applications, and Health Effects; Elsevier: Amsterdam, The Netherlands, 2005; Volume 49. [Google Scholar]
- Ahmadi, F.; Oveisi, Z.; Mohammadi-Samani, S.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1–16. [Google Scholar] [PubMed]
- Bourges, X.; Weiss, P.; Daculsi, G.; Legeay, G. Synthesis and general properties of silated-hydroxypropyl methylcellulose in prospect of biomedical use. Adv. Colloid Interface Sci. 2002, 99, 215–228. [Google Scholar] [CrossRef] [Green Version]
- Fatimi, A.; Tassin, J.F.; Quillard, S.; Axelos, M.A.; Weiss, P. The rheological properties of silated hydroxypropylmethylcellulose tissue engineering matrices. Biomaterials 2008, 29, 533–543. [Google Scholar] [CrossRef] [Green Version]
- Fatimi, A.; Tassin, J.-F.; Turczyn, R.; Axelos, M.A.; Weiss, P. Gelation studies of a cellulose-based biohydrogel: The influence of pH, temperature and sterilization. Acta Biomater. 2009, 5, 3423–3432. [Google Scholar] [CrossRef] [Green Version]
- Cross, M.M. Rheology of non-Newtonian fluids: A new flow equation for pseudoplastic systems. J. Colloid Sci. 1965, 20, 417–437. [Google Scholar] [CrossRef]
- Vo, A.; Doumit, M.; Rockwell, G. The Biomechanics and Optimization of the Needle-Syringe System for Injecting Triamcinolone Acetonide into Keloids. J. Med. Eng. 2016, 2016, 5162394. [Google Scholar] [CrossRef] [Green Version]
- Dash, B.C.; Réthoré, G.; Monaghan, M.; Fitzgerald, K.; Gallagher, W.; Pandit, A. The influence of size and charge of chitosan/polyglutamic acid hollow spheres on cellular internalization, viability and blood compatibility. Biomaterials 2010, 31, 8188–8197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rethore, G.; Mathew, A.; Naik, H.; Pandit, A. Preparation of Chitosan/Polyglutamic Acid Spheres Based on the Use of Polystyrene Template as a Nonviral Gene Carrier. Tissue Eng. Part C Methods 2009, 15, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Debnath, T.; Ghosh, S.; Potlapuvu, U.S.; Kona, L.; Kamaraju, S.R.; Sarkar, S.; Gaddam, S.; Chelluri, L.K. Proliferation and Differentiation Potential of Human Adipose-Derived Stem Cells Grown on Chitosan Hydrogel. PLoS ONE 2015, 10, e0120803. [Google Scholar] [CrossRef]
- Montheil, T.; Echalier, C.; Martinez, J.; Subra, G.; Mehdi, A. Inorganic polymerization: An attractive route to biocompatible hybrid hydrogels. J. Mater. Chem. B 2018, 6, 3434–3448. [Google Scholar] [CrossRef] [Green Version]
- Trojani, C.; Boukhechba, F.; Scimeca, J.-C.; Vandenbos, F.; Michiels, J.-F.; Daculsi, G.; Boileau, P.; Weiss, P.; Carle, G.F.; Rochet, N. Ectopic bone formation using an injectable biphasic calcium phosphate/Si-HPMC hydrogel composite loaded with undifferentiated bone marrow stromal cells. Biomaterials 2006, 27, 3256–3264. [Google Scholar] [CrossRef]
- Chichiricco, P.M.; Riva, R.; Thomassin, J.-M.; Lesoeur, J.; Struillou, X.; Le Visage, C.; Jérôme, C.; Weiss, P. In situ photochemical crosslinking of hydrogel membrane for Guided Tissue Regeneration. Dent. Mater. 2018, 34, 1769–1782. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Gauthier, O.; Sourice, S.; Pilet, P.; Rethore, G.; Khairoun, K.; Bouler, J.-M.; Tancret, F.; Weiss, P. A simple and effective approach to prepare injectable macroporous calcium phosphate cement for bone repair: Syringe-foaming using a viscous hydrophilic polymeric solution. Acta Biomater. 2016, 31, 326–338. [Google Scholar] [CrossRef]
- Duggan, E.; Waghorne, W.E. Effect of addition of chitosan on rheological properties of acidified milk gels. In Trends in Colloid and Interface Science XV; Springer: Berlin/Heidelberg, Germany, 2001; Volume 118, pp. 196–201. [Google Scholar] [CrossRef]
- Moore, G.K.; Roberts, G.A. Chitosan gels: 1. Study of reaction variables. Int. J. Biol. Macromol. 1980, 2, 73–77. [Google Scholar] [CrossRef]
- Ramanathan, S.; Block, L.H. The use of chitosan gels as matrices for electrically-modulated drug delivery. J. Control. Release 2001, 70, 109–123. [Google Scholar] [CrossRef]
- Buchtová, N.; Réthoré, G.; Boyer, C.; Guicheux, J.; Rambaud, F.; Vallé, K.; Belleville, P.; Sanchez, C.; Chauvet, O.; Weiss, P.; et al. Nanocomposite hydrogels for cartilage tissue engineering: Mesoporous silica nanofibers interlinked with siloxane derived polysaccharide. J. Mater. Sci. Mater. Med. 2013, 24, 1875–1884. [Google Scholar] [CrossRef] [PubMed]





| Relative Quantity Chitosan/ICPTS | 1/1 | 1/1.5 | ||||||||||
| Reaction time (H) | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | ||
| Uncontroled pH | grafting efficiency | + | + | ++ | ++ | ++ | + | + | ++ | ++ | ++ | |
| Solubility | - | - | + | + | + | - | - | + | + | + | ||
| Hydrogel formation | alone | - | - | + (syneresis) | + (syneresis) | + (syneresis) | - | - | + (syneresis) | + (syneresis) | + (syneresis) | |
| mixt with Si-HPMC | - | - | + | + | + | - | - | + | + | + (syneresis) | ||
| Controled pH | grafting efficiency | - | - | - | - | - | - | - | - | - | - | |
| Solubility | - | - | - | - | - | - | - | - | - | - | ||
| Hydrogel formation | alone | - | - | - | - | - | - | - | - | - | - | |
| mixt with Si-HPMC | - | - | - | - | - | - | - | - | - | - | ||
| Relative Quantity Chitosan/ICPTS | 1/2 | 1/2.5 | ||||||||||
| Reaction time (H) | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | ||
| Uncontroled pH | grafting efficiency | + | + | ++ | ++ | ++ | + | + | ++ | ++ | ++ | |
| Solubility | - | - | + | + | + | - | - | + | + | + | ||
| Hydrogel formation | alone | - | - | + (syneresis) | + (syneresis) | + (syneresis) | - | - | + (syneresis) | + (syneresis) | + (syneresis) | |
| mixt with Si-HPMC | - | - | + | + (syneresis) | + (syneresis) | - | - | + (syneresis) | + (syneresis) | + (syneresis) | ||
| Controled pH | grafting efficiency | - | - | - | - | - | - | - | - | - | - | |
| Solubility | - | - | - | - | - | - | - | - | - | - | ||
| Hydrogel formation | alone | - | - | - | - | - | - | - | - | - | - | |
| mixt with Si-HPMC | - | - | - | - | - | - | - | - | - | - | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Réthoré, G.; Boyer, C.; Kouadio, K.; Toure, A.; Lesoeur, J.; Halgand, B.; Jordana, F.; Guicheux, J.; Weiss, P. Silanization of Chitosan and Hydrogel Preparation for Skeletal Tissue Engineering. Polymers 2020, 12, 2823. https://doi.org/10.3390/polym12122823
Réthoré G, Boyer C, Kouadio K, Toure A, Lesoeur J, Halgand B, Jordana F, Guicheux J, Weiss P. Silanization of Chitosan and Hydrogel Preparation for Skeletal Tissue Engineering. Polymers. 2020; 12(12):2823. https://doi.org/10.3390/polym12122823
Chicago/Turabian StyleRéthoré, Gildas, Cécile Boyer, Kouakou Kouadio, Amadou Toure, Julie Lesoeur, Boris Halgand, Fabienne Jordana, Jérôme Guicheux, and Pierre Weiss. 2020. "Silanization of Chitosan and Hydrogel Preparation for Skeletal Tissue Engineering" Polymers 12, no. 12: 2823. https://doi.org/10.3390/polym12122823