Effect of Fillers on the Recovery of Rubber Foam: From Theory to Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Rubber Foams
2.3. Characterization
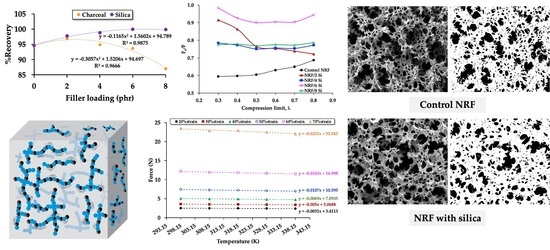
3. Results and Discussion
3.1. Physical and Chemical Properties
3.2. Mechanical and Morphological Properties
3.3. Thermodynamic Aspects
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chollakup, R.; Suwanruji, P.; Tantatherdtam, R.; Smitthipong, W. New approach on structure-property relationships of stabilized natural rubbers. J. Polym. Res. 2019, 26, 37. [Google Scholar] [CrossRef]
- Oliveira-Salmazo, L.; Lopez-Gil, A.; Silva-Bellucci, F.; Job, A.E.; Rodriguez-Perez, M.A. Natural rubber foams with anisotropic cellular structures: Mechanical properties and modeling. Ind. Crop. Prod. 2016, 80, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.Y.; Park, J.W.; Lee, D.Y.; Seo, K.H. Correlation between the Crosslink Characteristics and Mechanical Properties of Natural Rubber Compound via Accelerators and Reinforcement. Polymers 2020, 12, 2020. [Google Scholar] [CrossRef]
- Nawamawat, K.; Sakdapipanich, J.T.; Ho, C.C.; Ma, Y.; Song, J.; Vancso, J.G. Surface nanostructure of Hevea brasiliensis natural rubber latex particles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 390, 157–166. [Google Scholar] [CrossRef]
- Suethao, S.; Shah, D.U.; Smitthipong, W. Recent Progress in Processing Functionally Graded Polymer Foams. Materials 2020, 13, 4060. [Google Scholar] [CrossRef]
- Phomrak, S.; Nimpaiboon, A.; Newby, B.-m.Z.; Phisalaphong, M. Natural Rubber Latex Foam Reinforced with Micro- and Nanofibrillated Cellulose via Dunlop Method. Polymers 2020, 12, 1959. [Google Scholar] [CrossRef]
- Suksup, R.; Sun, Y.; Sukatta, U.; Smitthipong, W. Foam rubber from centrifuged and creamed latex. J. Polym. Eng. 2019, 39. [Google Scholar] [CrossRef]
- Blackley, D.C. Polymer Latices: Science and Technology Volume 2: Types of Latices, 2nd ed.; Springer: Dordrecht, The Netherlands, 1997. [Google Scholar]
- Kamila, S. Introduction, Classification and Applications of Smart Materials: An Overview. Am. J. Appl. Sci. 2013, 10, 876–880. [Google Scholar] [CrossRef]
- Cavicchi, K.A. Shape Memory Polymers from Blends of Elastomers and Small Molecule Additives. Macromol. Symp. 2015, 358, 194–201. [Google Scholar] [CrossRef]
- Gunes, I.S.; Cao, F.; Jana, C.S. Evaluation of Nanoparticulate Fillers for Shape Memory Polyurethane Nanocomposites. Polymer 2008, 49, 2223–2234. [Google Scholar] [CrossRef]
- Bashir, A.S.M.; Manusamy, Y.; Chew, T.L.; Ismail, H.; Ramasamy, S. Mechanical, thermal, and morphological properties of (eggshell powder)-filled natural rubber latex foam. J. Vinyl Addit. Technol. 2017, 23, 3–12. [Google Scholar] [CrossRef]
- Ramasamy, S.; Ismail, H.; Munusamy, Y. Effect of Rice Husk Powder on Compression Behavior and Thermal Stability of Natural Rubber Latex Foam. BioResources 2013, 8, 4258–4269. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Smitthipong, W.; Nardin, M.; Schultz, J.; Suchiva, K. Adhesion and self-adhesion of rubbers, crosslinked by electron beam irradiation. Int. J. Adhes. Adhes. 2007, 27, 352–357. [Google Scholar] [CrossRef]
- Nimpaiboon, A.; Sakdapipanich, J. Properties of peroxide cured highly purified natural rubber. Kautsch. Gummi Kunstst. 2012, 65, 55–59. [Google Scholar]
- Sadeghi Ghari, H.; Jalali-Arani, A. Nanocomposites based on natural rubber, organoclay and nano-calcium carbonate: Study on the structure, cure behavior, static and dynamic-mechanical properties. Appl. Clay Sci. 2016, 119, 348–357. [Google Scholar] [CrossRef]
- Kudori, S.N.I.; Ismail, H. The effects of filler contents and particle sizes on properties of green kenaf-filled natural rubber latex foam. Cell. Polym. 2019, 39, 57–68. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, X.; Chen, W.; Zhang, H.; Han, D. Modification of Rigid Polyurethane Foams with the Addition of Nano-SiO2 or Lignocellulosic Biomass. Polymers 2020, 12, 107. [Google Scholar] [CrossRef] [Green Version]
- Rodrigue, D.; Souici, S.; Twite-Kabamba, E. Effect of wood powder on polymer foam nucleation. J. Vinyl Addit. Technol. 2006, 12, 19–24. [Google Scholar] [CrossRef]
- Aussawasathien, D.; Jariyakun, K.; Pomrawan, T.; Hrimchum, K.; Yeetsorn, R.; Prissanaroon-Ouajai, W. Preparation and properties of low density polyethylene-activated carbon composite foams. AIP Conf. Proc. 2017, 1914, 060003. [Google Scholar] [CrossRef] [Green Version]
- Thongsang, S.; Sombatsompop, N. Effect of filler surface treatment on properties of fly ash/NR blends. In ANTEC 2005—Volume 8, Proceedings of the Annual Technical Conference—ANTEC, Boston, MA, USA, 1–5 May 2005; Society of Plastics Engineers: Brookfield, WI, USA, 2005; pp. 3278–3282. [Google Scholar]
- Promhuad, K.; Smitthipong, W. Effect of Stabilizer States (Solid vs. Liquid) on Properties of Stabilized Natural Rubbers. Polymers 2020, 12, 741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wang, H.; Chen, C.; Li, X.; Deng, Q.; Gong, M.; Li, D. Size effect of charcoal particles on the properties of bamboo charcoal/ultra-high molecular weight polyethylene composites. J. Appl. Polym. Sci. 2017, 134, 45530. [Google Scholar] [CrossRef]
- Ain, Z.N.; Azura, A.R. Effect of different types of filler and filler loadings on the properties of carboxylated acrylonitrile–butadiene rubber latex films. J. Appl. Polym. Sci. 2011, 119, 2815–2823. [Google Scholar] [CrossRef]
- Forest, C.; Chaumont, P.; Cassagnau, P.; Swoboda, B.; Sonntag, P. Polymer nano-foams for insulating applications prepared from CO2 foaming. Prog. Polym. Sci. 2015, 41, 122–145. [Google Scholar] [CrossRef]
- Treloar, L.R.G. The Physics of Rubber Elasticity; Oxford University Press: New York, NY, USA, 1975. [Google Scholar]
- Rubinstein, M.; Colby, R.H. Polymer Physics; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Hiemenz, P.C.; Lodge, T.P. Polymer Chemistry; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Roe, R.J.; Krigbaum, W.R. The contribution of internal energy to the elastic force of natural rubber. J. Polym. Sci. 1962, 61, 167–183. [Google Scholar] [CrossRef]
- Pakornpadungsit, P.; Smitthipong, W.; Chworos, A. Self-assembly nucleic acid-based biopolymers: Learn from the nature. J. Polym. Res. 2018, 25, 45. [Google Scholar] [CrossRef]
- Pojanavaraphan, T.; Magaraphan, R. Prevulcanized natural rubber latex/clay aerogel nanocomposites. Eur. Polym. J. 2008, 44, 1968–1977. [Google Scholar] [CrossRef]
- Phuhiangpa, N.; Ponloa, W.; Phongphanphanee, S.; Smitthipong, W. Performance of Nano- and Microcalcium Carbonate in Uncrosslinked Natural Rubber Composites: New Results of Structure–Properties Relationship. Polymers 2020, 12, 2002. [Google Scholar] [CrossRef]
- Maria, H.J.; Lyczko, N.; Nzihou, A.; Joseph, K.; Mathew, C.; Thomas, S. Stress relaxation behavior of organically modified montmorillonite filled natural rubber/nitrile rubber nanocomposites. Appl. Clay Sci. 2014, 87, 120–128. [Google Scholar] [CrossRef]












| Sample Name | Control NRF | NRF/ 2 Ch | NRF/ 4 Ch | NRF/ 6 Ch | NRF/ 8 Ch | NRF/ 2 Si | NRF/ 4 Si | NRF/ 6 Si | NRF/ 8 Si |
|---|---|---|---|---|---|---|---|---|---|
| Chemicals | (phr 1) | ||||||||
| NRL | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| KO | 3.63 | 3.63 | 3.63 | 3.63 | 3.63 | 3.63 | 3.63 | 3.63 | 3.63 |
| Vulcanizing chemicals | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 | 4.00 |
| WingL | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| ZnO | 2.80 | 2.80 | 2.80 | 2.80 | 2.80 | 2.80 | 2.80 | 2.80 | 2.80 |
| DPG | 0.67 | 0.67 | 0.67 | 0.67 | 0.67 | 0.67 | 0.67 | 0.67 | 0.67 |
| SSF | 1.66 | 1.66 | 1.66 | 1.66 | 1.66 | 1.66 | 1.66 | 1.66 | 1.66 |
| Charcoal | - | 2.00 | 4.00 | 6.00 | 8.00 | - | - | - | - |
| Silica | - | - | - | - | - | 2.00 | 4.00 | 6.00 | 8.00 |
| Sample Name | Average Pore Size (±0.300 mm) | Porosity (±1.00%) | Cell Density (±150 cm−3) |
|---|---|---|---|
| Control NRF | 0.836 | 31.39 | 3041 |
| NRF/2 Ch | 0.860 | 31.69 | 2778 |
| NRF/4 Ch | 0.988 | 27.58 | 1807 |
| NRF/6 Ch | 1.081 | 25.46 | 1379 |
| NRF/8 Ch | 1.092 | 25.04 | 1333 |
| NRF/2 Si | 1.079 | 28.57 | 1412 |
| NRF/4 Si | 0.909 | 28.09 | 2316 |
| NRF/6 Si | 0.876 | 27.49 | 2546 |
| NRF/8 Si | 0.673 | 26.34 | 5606 |
| Sample Name | Compression Strain (%) | Compression Limit (λ) | Fu (N) | F (N) | Fu/F |
|---|---|---|---|---|---|
| Control NRF | 20 | 0.8 | 1.70 | 2.47 | 0.6865 |
| 30 | 0.7 | 3.21 | 4.94 | 0.6500 | |
| 40 | 0.6 | 5.43 | 8.62 | 0.6300 | |
| 50 | 0.5 | 10.07 | 16.66 | 0.6044 | |
| 60 | 0.4 | 17.51 | 29.31 | 0.5972 | |
| 70 | 0.3 | 33.37 | 56.15 | 0.5943 | |
| NRF/8 Ch | 20 | 0.8 | 1.05 | 1.23 | 0.8550 |
| 30 | 0.7 | 1.87 | 2.43 | 0.7672 | |
| 40 | 0.6 | 3.02 | 4.16 | 0.7275 | |
| 50 | 0.5 | 5.43 | 7.96 | 0.6817 | |
| 60 | 0.4 | 10.54 | 16.11 | 0.6540 | |
| 70 | 0.3 | 25.73 | 41.12 | 0.6258 | |
| NRF/8 Si | 20 | 0.8 | 3.41 | 4.34 | 0.7868 |
| 30 | 0.7 | 5.07 | 6.56 | 0.7727 | |
| 40 | 0.6 | 7.09 | 9.15 | 0.7752 | |
| 50 | 0.5 | 10.60 | 13.79 | 0.7686 | |
| 60 | 0.4 | 17.00 | 21.86 | 0.7777 | |
| 70 | 0.3 | 32.94 | 42.51 | 0.7749 |
| Sample Name | Swelling Ratio | Volume Fraction of Rubber (Vr) | ΔG (J/mol) | ΔS (J/mol·K) |
|---|---|---|---|---|
| Control NRF | 2.83 | 0.2377 | −29.15 | 0.0971 |
| NRF/2 Ch | 2.33 | 0.2755 | −42.85 | 0.1428 |
| NRF/4 Ch | 2.24 | 0.2823 | −45.74 | 0.1524 |
| NRF/6 Ch | 2.25 | 0.2790 | −44.33 | 0.1477 |
| NRF/8 Ch | 2.23 | 0.2843 | −46.61 | 0.1553 |
| NRF/2 Si | 2.33 | 0.2739 | −42.21 | 0.1406 |
| NRF/4 Si | 2.30 | 0.2732 | −41.91 | 0.1396 |
| NRF/6 Si | 2.24 | 0.2777 | −43.77 | 0.1458 |
| NRF/8 Si | 2.19 | 0.2839 | −46.41 | 0.1546 |
| Sample Name | Eg @ −70 °C (MPa) | Er @ 0 °C (MPa) | Tg (°C) | tan δ max | tA | (∆Ha)avg (kJ·K/mol) |
|---|---|---|---|---|---|---|
| Control NRF | 184.05 | 0.42 | −49.08 | 1.39 | 31.08 | 128.33 |
| NRF/2 Ch | 193.35 | 0.49 | −40.75 | 1.75 | 39.04 | 107.81 |
| NRF/4 Ch | 248.15 | 0.51 | −37.17 | 1.82 | 40.87 | 109.92 |
| NRF/6 Ch | 240.76 | 0.54 | −35.17 | 1.78 | 40.24 | 112.16 |
| NRF/8 Ch | 251.01 | 0.52 | −36.17 | 1.69 | 38.13 | 118.76 |
| NRF/2 Si | 250.74 | 0.39 | −48.17 | 1.54 | 28.25 | 151.17 |
| NRF/4 Si | 283.10 | 0.44 | −45.50 | 1.41 | 30.51 | 143.53 |
| NRF/6 Si | 294.05 | 0.45 | −43.00 | 1.39 | 25.73 | 174.06 |
| NRF/8 Si | 280.63 | 0.44 | −44.67 | 1.32 | 27.60 | 159.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasopdee, T.; Smitthipong, W. Effect of Fillers on the Recovery of Rubber Foam: From Theory to Applications. Polymers 2020, 12, 2745. https://doi.org/10.3390/polym12112745
Prasopdee T, Smitthipong W. Effect of Fillers on the Recovery of Rubber Foam: From Theory to Applications. Polymers. 2020; 12(11):2745. https://doi.org/10.3390/polym12112745
Chicago/Turabian StylePrasopdee, Thridsawan, and Wirasak Smitthipong. 2020. "Effect of Fillers on the Recovery of Rubber Foam: From Theory to Applications" Polymers 12, no. 11: 2745. https://doi.org/10.3390/polym12112745