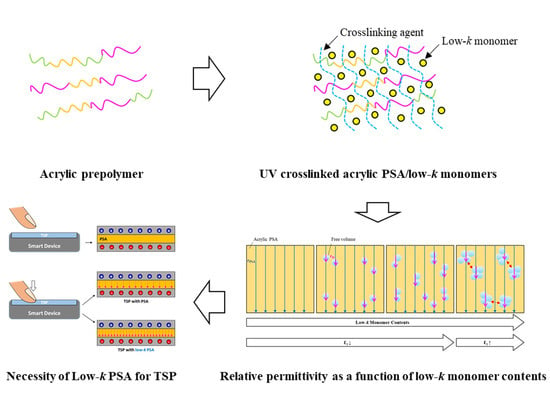
Free Volume Effect via Various Chemical Structured Monomers on Adhesion Property and Relative Permittivity in Acrylic Pressure Sensitive Adhesives
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Chemicals
2.2. Acrylic Pre-Polymer Synthesis
2.3. UV Crosslinking of Acrylic PSAs with Monomers
2.4. Characterizations
3. Results and Discussion
3.1. Gel Fraction
3.2. Transmittance
3.3. Adhesion Performances
3.4. Relative Permittivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benedek, I.; Feldstein, M.M. Technology of pressure-sensitive adhesives and products. In Handbook of Pressure-Sensitive Adhesive and Products, 1st ed.; Benedek, I., Feldstein, M.M., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Satas, D. Pressure sensitive adhesives and adhesive products in the United States. In Handbook of Pressure-Sensitive Adhesive Technology, 3rd ed.; Satas, D., Ed.; Van Nostrand-Reinhold: New York, NY, USA, 1999; Volume 1, pp. 1–21. [Google Scholar]
- Lee, J.-H.; Lee, T.-H.; Shim, K.-S.; Park, J.-W.; Kim, H.-J.; Kim, Y.; Jung, S. Effect of crosslinking density on adhesion performance and flexibility properties of acrylic pressure sensitive adhesives for flexible display applications. Int. J. Adhes. Adhes. 2017, 74, 137–143. [Google Scholar] [CrossRef]
- Keizai, F. Special Adhesive and Passivation Materials Market Outlook and Application 2012; Fuji Keizai: Tokyo, Japan, 2011. [Google Scholar]
- Lee, S.-W.; Park, J.-W.; Park, C.-H.; Kim, H.-J. Enhanced optical properties and thermal stability of optically clear adhesives. Int. J. Adhes. 2014, 50, 93–95. [Google Scholar] [CrossRef]
- Hummel, R.E. Electronic Properties of Materials, 4th ed.; Springer: Dordrecht, The Netherlands, 2001. [Google Scholar]
- Shamiryan, D.; Abell, T.; Iacopi, F.; Maex, K. Low-k dielectric materials. Mater. Today 2004, 7, 34–39. [Google Scholar] [CrossRef]
- Maex, K.; Baklanov, M.R.; Shamiryan, D.; Iacopi, F.; Brongersma, S.H.; Yanovitskaya, Z.S. Low dielectric constant materials for microelectronics. J. Appl. Phys. 2003, 93, 8793–8841. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, S.; Lee, S.; Lee, J.; Kho, D. Low Dielectric Pressure-Sensitive Adhesive Film for Capacitive Touch Screen Panel. Patent No. KR101572010B1, 5 February 2014. [Google Scholar]
- Farrell, R.; Goshal, T.; Cvelbar, U.; Petkov, N.; Morris, M.A. Advances in ultra low dielectric constant ordered porous materials. ECS Interface 2011, 20, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Volksen, W.; Miller, R.D.; Dubois, G. Low dielectric constant materials. Chem. Rev. 2010, 110, 56–110. [Google Scholar] [CrossRef]
- Bucholz, T.L.; Li, S.P.; Loo, Y.-L. Ultra-low-k materials derived from poly(D,L-lactide-b-pentafluorostyrene) diblock copolymers. J. Mater. Chem. 2008, 18, 530–536. [Google Scholar] [CrossRef]
- Huang, Q.R.; Kim, H.-C.; Huang, E.; Mecerreyes, D.; Hedrick, J.L.; Volksen, W.; Frank, C.W.; Miller, R.D. Miscibility in organic/inorganic hybrid nanocomposites suitable for microelectronic applications: Comparison of modulated differential scanning calorimetry and fluorescence spectroscopy. Macromolecules 2004, 36, 7661–7671. [Google Scholar] [CrossRef]
- Long, T.M.; Swager, T.M. Molecular design of free volume as a route to low-k dielectric materials. J. Am. Chem. Soc. 2003, 125, 14113–14119. [Google Scholar] [CrossRef]
- Eslava, S.; Irrutia, J.; Busawon, A.N.; Baklanov, M.R.; Iacopi, F.; Aldea, S.; Maex, K.; Martens, J.A.; Kirschhock, C.E.A. Zeolite-inspired low-k dielectrics overcoming limitations of zeolite films. J. Am. Chem. Soc. 2008, 130, 17528–17536. [Google Scholar] [CrossRef]
- Ro, H.W.; Char, K.; Jeon, E.-C.; Kim, H.-J.; Kwon, D.; Lee, H.-J.; Lee, J.-K.; Rhee, H.-W.; Soles, C.L.; Yoon, D.Y. High-modulus spin-on organosilicate glasses for nanoporous applications. Adv. Mater. 2007, 19, 705–710. [Google Scholar] [CrossRef]
- Rathore, J.S.; Interrante, L.V.; Dubois, G. Ultra low-k films derived from hyperbranched polycarbosilanes (HBPCS). Adv. Funct. Mater. 2008, 18, 4022–4028. [Google Scholar] [CrossRef]
- Dubois, G.; Volkse, W.; Magbitang, T.M.; Miller, R.D.; Gage, D.M.; Dauskardt, R.H. Molecular network reinforcement of sol-gel glasses. Adv. Mater. 2007, 19, 3989–3994. [Google Scholar] [CrossRef]
- Hedrick, J.L.; Miller, R.D.; Hawker, C.J.; Carter, K.R.; Volkse, W.; Yoon, D.Y.; Trollsas, M. Templating nanoporosity in thin-film dielectric insulators. Adv. Mater. 1998, 10, 1049–1053. [Google Scholar] [CrossRef]
- Fu, G.-D.; Yuan, Z.; Kang, E.-T.; Neoh, K.-G.; Lai, D.M.; Huan, A.C.H. Nanoporous ultra-low-dielectric-constant fluoropolymer films via selective UV decomposition of poly(pentafluorostyrene)-block-poly(methyl methacrylate) copolymers prepared using atom transfer radical polymerization. Adv. Funct. Mater. 2005, 15, 315–322. [Google Scholar] [CrossRef]
- Lee, B.; Park, Y.-H.; Hwang, Y.-T.; Oh, W.; Yoon, J.; Ree, M. Ultralow-k nanoporous organosilicate dielectric films imprinted with dendritic spheres. Nat. Mater. 2005, 4, 147–151. [Google Scholar] [CrossRef]
- Connor, E.F.; Sundberg, L.K.; Kim, H.-C.; Cornelissen, J.L.; Magbitang, T.; Rice, P.M.; Lee, V.Y.; Hawker, C.J.; Volksen, W.; Hedrick, J.L.; et al. Templating of silsesquioxane cross-linking using unimolecular self-organizing polymers. Angew. Chem. Int. Ed. 2003, 42, 3785–3788. [Google Scholar] [CrossRef]
- Ding, S.-J.; Wang, P.-F.; Zhang, D.W.; Wang, J.-T.; Lee, W.W. Anovel structural amorphous fluoropolymer film with an ultra-low dielectric constant. Mater. Lett. 2001, 49, 154–159. [Google Scholar] [CrossRef]
- Lew, C.M.; Li, Z.; Shuang, L.; Hwang, S.-J.; Liu, Y.; Medina, D.I.; Sun, M.; Wang, J.; Davis, M.E.; Yan, Y.S. Pure-silica-zeolite MFI and MEL low-dielectric-constant films with fluoro-organic functionalization. Adv. Funct. Mater. 2008, 18, 3454–3460. [Google Scholar] [CrossRef]
- Yuan, C.; Jin, K.; Li, K.; Diao, S.; Tong, J.; Fang, Q. Non-porous low-k dielectric films based on a new structural amorphous fluoropolymer. Adv. Mater. 2013, 25, 4875–4878. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.; Jin, K.; Wang, L.; Sun, J.; Fang, Q. A new fluorinated polysiloxane with good optical properties and low dielectric constant at high frequency based on easily available tetraethoxysilane (TEOS). Macromolecules 2017, 50, 9394–9402. [Google Scholar] [CrossRef]
- Zhang, K.; Han, L.; Froimowicz, P.; Ishida, H. A smart latent catalyst containing o-trifluoroacetamide functional benzoxazine: Precursor for low temperature formation of very high performance polybenzoxazole with low dielectric constant and high thermal stability. Macromolecules 2017, 50, 6552–6560. [Google Scholar] [CrossRef]
- Chern, Y.-T.; Shiue, H.-C. Low dielectric constants of soluble polyimides based on adamantine. Macromolecules 1997, 30, 4646–4651. [Google Scholar] [CrossRef]
- Chern, Y.-T.; Shiue, H.-C. High sunglass transition temperatures and low dielectric constants of polyimides derived from 4, 9-Bis(4-aminophenyl) diamantine. Chem. Mater. 1998, 10, 210–216. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, T.-H.; Shim, K.-S.; Park, J.-W.; Kim, H.-J.; Kim, Y.; Jung, S. Molecular weight and crosslinking on the adhesion performance and flexibility of acrylic PSAs. J. Adhes. Sci. Technol. 2016, 30, 2316–2328. [Google Scholar] [CrossRef]
- Gordon, G.V.; Perz, S.V.; Tabler, R.L.; Stasser, J.L.; Owen, M.J.; Tonge, J.S. Silicone Release Coatings: A Closer Look at Release Mechanisms; No. 26-016-98; Dow Corning Corporation; 1988. [Google Scholar]
- Czech, Z. Multifuctional propyleneimines-new generation of crosslinkers for solvent-based pressure-sensitive adhesives. Int. J. Adhes. Adhes. 2004, 24, 502–511. [Google Scholar]
- Czech, Z. New generation of crosslinking agents Abased on multifunctional methylaziridines. Int. J. Adhes. Adhes. 2007, 27, 49–58. [Google Scholar] [CrossRef]
- Lee, S.-W.; Park, J.-W.; Lee, S.-H.; Lee, Y.-J.; Bae, K.-R.; Kim, H.-J.; Kim, K.-M.; Kim, H.-I.; Ryu, J.-M. Adhesion performance of UV-curable debonding acrylic PSAs with different thickness in thin Si-wafer manufacture process. J. Adhes. Interface 2010, 11, 120–125. [Google Scholar]
- Lee, S.-W.; Park, J.-W.; Kim, H.-J.; Kim, K.-M.; Kim, H.-I.; Ryu, J.-M. Adhesion performance and microscope morphology of UV-curable semi-interpenetrated dicing acrylic PSAs in Si-wafer manufacture process for MCP. J. Adhes. Sci. Technol. 2012, 26, 317–329. [Google Scholar] [CrossRef]
- Czech, Z.; Kurzawa, R. Acrylic pressure-sensitive adhesive for transdermal drug delivery systems. J. Appl. Polym. Sci. 2007, 106, 2398–2404. [Google Scholar] [CrossRef]
- Park, C.-H.; Lee, S.-J.; Lee, T.-H.; Kim, H.-J. Charaterization of an acrylic polymer under hygrothermal aging as an optically clear adhesive for touch screen panels. Int. J. Adhes. Adhes. 2015, 63, 137–144. [Google Scholar] [CrossRef]
- Lu, Z.; Lanagan, M.; Manias, E.; Macdonald, D.D. Two-port transmission line technique for dielectric property characterization of polymer electrolyte membranes. J. Phys. Chem. B 2009, 113, 13551–13559. [Google Scholar] [CrossRef]
- Geyer, R.G.; Krupka, J. Microwave dielectric-properties of anisotropic materials at cryogenic temperatures. IEEE Trans. Instrum. Meas. 1995, 44, 329–331. [Google Scholar] [CrossRef]
- Chang, K.; Luo, H.; Geise, G.M. Water content, relative permittivity, and ion sorption properties of polymers for membrane desalination. J. Membr. Sci. 2019, 574, 24–32. [Google Scholar] [CrossRef]












| Sample Names | Reactive Monomers | ||
|---|---|---|---|
| 2-HEA (wt.%) | 2-EHA (wt.%) | IBA (wt.%) | |
| Acrylic Pre-Polymer | 20 | 60 | 20 |
| Sample Names | Acrylic Pre-Polymer (wt.%) | Monomers | |||
|---|---|---|---|---|---|
| HMDS (phr) | NVC (phr) | TBA (phr) | ISTA (phr) | ||
| PSA-HMDS | 100 | 2/4/6/8/10 | - | - | - |
| PSA-NVC | - | 2/4/6/8/10 | - | - | |
| PSA-TBA | - | - | 2/4/6/8/10 | - | |
| PSA-ISTA | - | - | - | 2/4/6/8/10 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Kim, J.-S.; Kim, H.-J.; Park, K.; Moon, J.; Lee, J.; Park, Y. Free Volume Effect via Various Chemical Structured Monomers on Adhesion Property and Relative Permittivity in Acrylic Pressure Sensitive Adhesives. Polymers 2020, 12, 2633. https://doi.org/10.3390/polym12112633
Lee J-H, Kim J-S, Kim H-J, Park K, Moon J, Lee J, Park Y. Free Volume Effect via Various Chemical Structured Monomers on Adhesion Property and Relative Permittivity in Acrylic Pressure Sensitive Adhesives. Polymers. 2020; 12(11):2633. https://doi.org/10.3390/polym12112633
Chicago/Turabian StyleLee, Jung-Hun, Ji-Soo Kim, Hyun-Joong Kim, Kyujong Park, Jungwoo Moon, Jinyoung Lee, and Youngju Park. 2020. "Free Volume Effect via Various Chemical Structured Monomers on Adhesion Property and Relative Permittivity in Acrylic Pressure Sensitive Adhesives" Polymers 12, no. 11: 2633. https://doi.org/10.3390/polym12112633