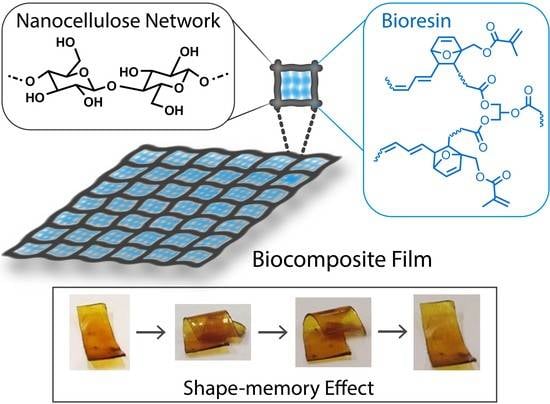
Hydrophobic Shape-Memory Biocomposites from Tung-Oil-Based Bioresin and Onion-Skin-Derived Nanocellulose Networks
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Furfuryl Methacrylated TO Resins
2.3. Extraction of Nanocellulose from Onion Skins and Preparation of Nanocellulose Films
2.4. Preparation of Biocomposites
2.5. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, K.; Åkesson, D.; Skrifvars, M. Synthesis of reactive soybean oils for use as a biobased thermoset resins in structural natural fiber composites. J. Appl. Polym. Sci. 2010, 115, 3137–3145. [Google Scholar] [CrossRef]
- Cakmakli, B.; Hazer, B.; Tekin, I.O.; Kizgut, S.; Koksal, M.; Menceloglu, Y. Synthesis and characterization of polymeric linseed oil grafted methyl methacrylate or styrene. Macromol. Biosci. 2004, 4, 649–655. [Google Scholar] [CrossRef] [Green Version]
- Çakmaklı, B.; Hazer, B.; Tekin, İ.Ö.; Cömert, F.B. Synthesis and characterization of polymeric soybean oil-g-methyl methacrylate (and n-butyl methacrylate) graft copolymers: Biocompatibility and bacterial adhesion. Biomacromolecules 2005, 6, 1750–1758. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Renewable polymeric materials from vegetable oils: A perspective. Mater. Today 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Scholten, P.B.V.; Detrembleur, C.; Meier, M.A.R. Plant-Based Nonactivated Olefins: A New Class of Renewable Monomers for Controlled Radical Polymerization. ACS Sustain. Chem. Eng. 2019, 7, 2751–2762. [Google Scholar] [CrossRef]
- Scholz, A.; Lewis, R.L.; Bachan, M.A.; Stewart, A.L.; Quirino, R.L. Biocomposites from the reinforcement of a tung oil-based thermosetting resin with collagen. Mater. Chem. Front. 2017, 1, 1795–1803. [Google Scholar] [CrossRef]
- Brunner, H.; Tucker, D.R. The nature of the products obtained by refluxing styrene and drying oils in xylol solution. II. Additional data on the styrene-tung oil reaction. J. Appl. Chem. 1951, 1, 563–568. [Google Scholar] [CrossRef]
- Ge, Q.; Wang, H.; She, Y.; Jiang, S.; Cao, M.; Zhai, L.; Jiang, S. Synthesis, characterization, and properties of acrylate-modified tung-oil waterborne insulation varnish. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Li, F.; Larock, R.C. Thermosetting polymers from cationic copolymerization of tung oil: Synthesis and characterization. J. Appl. Polym. Sci. 2000, 78, 1044–1056. [Google Scholar] [CrossRef]
- Li, F.; Larock, R.C. Synthesis, structure and properties of new tung oil-styrene-divinylbenzene copolymers prepared by thermal polymerization. Biomacromolecules 2003, 4, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Shang, Q.; Jia, P.; Dai, Y.; Zhou, Y.; Liu, Z. Tung oil-based unsaturated co-ester macromonomer for thermosetting polymers: Synergetic synthesis and copolymerization with styrene. ACS Sustain. Chem. Eng. 2016, 4, 3437–3449. [Google Scholar] [CrossRef]
- Trumbo, D.L.; Mote, B.E. Synthesis of tung oil-diacrylate copolymers via the Diels-Alder reaction and properties of films from the copolymers. J. Appl. Polym. Sci. 2001, 80, 2369–2375. [Google Scholar] [CrossRef]
- Sain, S.; Åkesson, D.; Skrifvars, M. Synthesis and properties of thermosets from tung oil and furfuryl methacrylate. Polymers 2020, 12, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meiorin, C.; Aranguren, M.I.; Mosiewicki, M.A. Smart and structural thermosets from the cationic copolymerization of a vegetable oil. J. Appl. Polym. Sci. 2012, 124, 5071–5078. [Google Scholar] [CrossRef]
- Meiorin, C.; Aranguren, M.I.; Mosiewicki, M.A. Polymeric networks based on tung oil: Reaction and modification with green oil monomers. Eur. Polym. J. 2015, 67, 551–560. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Yang, G.; Zheng, X.; Zhou, S. Multi-Stimulus-Responsive Shape-Memory Polymer Nanocomposite Network Cross-Linked by Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 4118–4126. [Google Scholar] [CrossRef]
- Kavitha, A.A.; Singha, N.K. Smart “All Acrylate” ABA Triblock Copolymer Bearing Reactive Functionality via Atom Transfer Radical Polymerization (ATRP): Demonstration of a “Click Reaction” in Thermoreversible Property. Macromolecules 2010, 43, 3193–3205. [Google Scholar] [CrossRef]
- Vilela, C.; Silvestre, A.J.D.; Gandini, A. Thermoreversible nonlinear Diels-Alder polymerization of furan/plant oil monomers. J. Polym. Sci. A Polym. Chem. 2013, 51, 2260–2270. [Google Scholar] [CrossRef]
- Huang, Y.; Pang, L.; Wang, H.; Zhong, R.; Zeng, Z.; Yang, J. Synthesis and properties of UV-curable tung oil based resins via modification of Diels-Alder reaction, nonisocyanate polyurethane and acrylates. Prog. Org. Coat. 2013, 76, 654–661. [Google Scholar] [CrossRef]
- Xu, X.; Chen, L.; Guo, J.; Cao, X.; Wang, S. Synthesis and characteristics of tung oil-based acrylated-alkyd resin modified by isobornyl acrylate. RSC Adv. 2017, 7, 30439–30445. [Google Scholar] [CrossRef] [Green Version]
- Auad, M.L.; Contos, V.S.; Nutt, S.; Aranguren, M.I.; Marcovich, N.E. Characterization of nanocellulose- reinforced shape memory polyurethanes. Polymer International 2008, 57, 651–659. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Dehghannya, J.; Entezami, A.A.; Asl, A.K. Novel nanocomposites based on fatty acid modified cellulose nanofibers/poly(lactic acid): Morphological and physical properties. Food Packag. Shelf Life 2015, 5, 21–31. [Google Scholar] [CrossRef]
- Garces, I.T.; Aslanzadeh, S.; Boluk, Y.; Ayranci, C. Cellulose nanocrystals (CNC) reinforced shape memory polyurethane ribbons for future biomedical applications and design. J. Thermoplast. Compos. Mater. 2020, 33, 377–392. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, Z.; Liu, Z.; Kang, H.; Liu, Y. Cellulose nanofibers/polyurethane shape memory composites with fast water-responsivity. J. Mater. Chem. B. 2018, 6, 1668–1677. [Google Scholar] [CrossRef]
- Wu, M.; Sukyai, P.; Lv, D.; Zhang, F.; Wang, P.; Liu, C.; Li, B. Water and humidity-induced shape memory cellulose nanopaper with quick response, excellent wet strength and folding resistance. Chem. Eng. J. 2020, 392, 123673. [Google Scholar] [CrossRef]
- Sabo, R.C.; Elhajjar, R.F.; Clemons, C.M.; Pillai, K.M. Characterization and Processing of Nanocellulose Thermosetting Composites. In Handbook of Polymer Nanocomposites. Processing, Performance and Application: Volume C: Polymer Nanocomposites of Cellulose Nanoparticles; Pandey, J.K., Takagi, H., Nakagaito, A.N., Kim, H.-J., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 265–295. [Google Scholar] [CrossRef]
- Ansari, F.; Galland, S.; Johansson, M.; Plummer, C.J.G.; Berglund, L.A. Cellulose nanofiber network for moisture stable, strong and ductile biocomposites and increased epoxy curing rate. Compos. Part A Appl. Sci. Manuf. 2014, 63, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Ansari, F.; Skrifvars, M.; Berglund, L. Nanostructured biocomposites based on unsaturated polyester resin and a cellulose nanofiber network. Compos. Sci. Technol. 2015, 117, 298–306. [Google Scholar] [CrossRef] [Green Version]
- Henriksson, M.; Berglund, L.A. Structure and properties of cellulose nanocomposite films containing melamine formaldehyde. J. Appl. Polym. Sci. 2007, 106, 2817–2824. [Google Scholar] [CrossRef]
- Nakagaito, A.N.; Yano, H. Novel high-strength biocomposites based on microfibrillated cellulose having nano-order-unit web-like network structure. Appl. Phys. A 2005, 80, 155–159. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Reddy, J.P.; Luo, X. Isolation of cellulose nanocrystals from onion skin and their utilization for the preparation of agar-based bio-nanocomposites films. Cellulose 2015, 22, 407–420. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Haafiz, M.K.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Hietala, M.; Sain, S.; Oksman, K. Highly redispersible sugar beet nanofibers as reinforcement in bionanocomposites. Cellulose 2017, 24, 2177–2189. [Google Scholar] [CrossRef]
- Tanpichai, S.; Witayakran, S.; Srimarut, Y.; Woraprayote, W.; Malila, Y. Porosity, density and mechanical properties of the paper of steam exploded bamboo microfibers controlled by nanofibrillated cellulose. J. Mater. Res. Technol. 2019, 8, 3612–3622. [Google Scholar] [CrossRef]
- Huang, K.; Liu, Z.; Zhang, J.; Li, S.; Li, M.; Xia, J.; Zhou, Y. Epoxy Monomers Derived from Tung Oil Fatty Acids and Its Regulable Thermosets Cured in Two Synergistic Ways. Biomacromolecules 2014, 15, 837–843. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, T.; Ma, G.; Li, P.; Ling, L.; Wang, B. Synthesis of a tung oil-rosin adduct via the Diels-Alder reaction: Its reaction mechanism and properties in an ultraviolet-curable adhesive. J. Appl. Polym. Sci. 2013, 130, 4201–4208. [Google Scholar] [CrossRef]
- Eren, T.; Küsefoğlu, S.H.; Wool, R. Polymerization of maleic anhydride–modified plant oils with polyols. J. Appl. Polym. Sci. 2003, 90, 197–202. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Tammelin, T.; Schulfter, K.; Kiiskinen, H.; Samela, J.; Bismarck, A. High performance cellulose nanocomposites: Comparing the reinforcing ability of bacterial cellulose and nanofibrillated cellulose. ACS Appl. Mater. Interfaces 2012, 4, 4078–4086. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Hsieh, Y.-L. Cellulose nanocrystal isolation from tomato peels and assembled nanofibers. Carbohydr. Polym. 2015, 122, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, C.C.S.; Michelin, M.; Cerqueira, M.A.; Gonçalves, C.; Tonon, R.V.; Pastrana, L.M.; Freitas-Silva, O.; Vicente, A.A.; Cabral, L.M.C.; Teixeira, J.A. Cellulose nanocrystals from grape pomace: Production, properties and cytotoxicity assessment. Carbohydr. Polym. 2018, 192, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Łojewska, J.; Miśkowiec, P.; Łojewski, T.; Proniewicz, L.M. Cellulose oxidative and hydrolytic degradation: In situ FTIR approach. Polym. Degrad. Stab. 2005, 88, 512–520. [Google Scholar] [CrossRef]
- Sato, A.; Kabusaki, D.; Okumura, H.; Nakatani, T.; Nakatsubo, F.; Yano, H. Surface modification of cellulose nanofibers with alkenyl succinic anhydride for high-density polyethylene reinforcement. Compos. Part A Appl. Sci. Manuf. 2016, 83, 72–79. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Gorecki, W.; Sanchez, J.-Y.; Dufresne, A. Nanocomposite polymer electrolytes based on poly(oxyethylene) and cellulose nanocrystals. J. Phys. Chem. B. 2004, 108, 10845–10852. [Google Scholar] [CrossRef]
- Malho, J.-M.; Ouellet-Plamondon, C.; Rüggeberg, M.; Laaksonen, P.; Ikkala, O.; Burgert, I.; Linder, M.B. Enhanced plastic deformations of nanofibrillated cellulose film by adsorbed moisture and protein-mediated interactions. Biomacromolecules 2015, 16, 311–318. [Google Scholar] [CrossRef]
- Sain, S.; Ray, D.; Mukhopadhyay, A. Improved mechanical and moisture resistance property of in situ polymerized transparent PMMA/Cellulose composites. Polym. Compos. 2015, 36, 1748–1758. [Google Scholar] [CrossRef]
- Ferrer, A.; Filpponen, I.; Rodríguez, A.; Laine, J.; Rojas, O.J. Valorization of residual Empty Palm Fruit Bunch Fibers (EPFBF) by microfluidization: Production of nanofibrillated cellulose and EPFBF nanopaper. Bioresour. Technol. 2012, 125, 249–255. [Google Scholar] [CrossRef]
- Cataldi, A.; Esposito Corcione, C.; Frigione, M.; Pegoretti, A. Photocurable resin/nanocellulose composite coatings for wood protection. Prog. Org. Coat. 2017, 106, 128–136. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, C.; Zheng, Y.; Xie, Y.; He, W.; Wang, Y.; Qiao, K.; Yue, L. The effects of two biocompatible plasticizers on the performance of dry bacterial cellulose membrane: A comparative study. Cellulose 2018, 25, 5893–5908. [Google Scholar] [CrossRef]
- Murawski, A.; Quirino, R.L. Bio-based composites with enhanced matrix-reinforcement interactions from the polymerization of α-eleostearic acid. Coatings 2019, 9, 447. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wu, Q.; An, R.; Shang, Q.; Feng, G.; Hu, Y.; Jia, P.; Zhou, Y.; Lei, W. Synthesis and properties of tung oil-based unsaturated co-ester resins bearing steric hindrance. Polymers 2019, 11, 826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Ding, H.; Yang, X.; Xu, L.; Xia, J.; Li, S. Preparation and Properties of Self-Healing Polyurethane Elastomer Derived from Tung-Oil-Based Polyphenol. ACS Omega 2020, 5, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Handoko, H. Bio-Based Thermosetting Copolymers of Eugenol and Tung Oil. Master’s Thesis, Iowa State University, Ames, IA, USA, 2014. [Google Scholar]
- Sylvain, G. Compression-moulded and multifunctional cellulose network materials. Doctoral Thesis, KTH School of Chemical Science and Engineering, Stockholm, Sweden, 2013. [Google Scholar]
- McKeen, L. 10-Elastomers. In The effect of sterilization methods on plastics and elastomers (fourth edition); McKeen, L., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 305–351. [Google Scholar] [CrossRef]
- Lim, D.B.K.; Gong, H. Highly stretchable and transparent films based on cellulose. Carbohydr. Polym. 2018, 201, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Thapaliya, B.P.; Do-Thanh, C.-L.; Jafta, C.J.; Tao, R.; Lyu, H.; Borisevich, A.Y.; Yang, S.-z.; Sun, X.-G.; Dai, S. Simultaneously boosting the ionic conductivity and mechanical strength of polymer gel electrolyte membranes by confining ionic liquids into hollow silica nanocavities. Batteries Supercaps 2019, 2, 985–991. [Google Scholar] [CrossRef]
- Karim, Z.; Svedberg, A.; Lee, K.-Y.; Khan, M.J. Processing-structure-property correlation understanding of microfibrillated cellulose based dimensional structures for ferric ions removal. Sci. Rep. 2019, 9, 10277. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, D.; Zhang, R. Preparation of ultralong cellulose nanofibers and optically transparent nanopapers derived from waste corrugated paper pulp. Bioresources 2013, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Lindström, S.B.; Karabulut, E.; Kulachenko, A.; Sehaqui, H.; Wågberg, L. Mechanosorptive creep in nanocellulose materials. Cellulose 2012, 19, 809–819. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Villar, M.A. Thermal and mechanical characterization of linear low-density polyethylene/wood flour composites. J. Appl. Polym. Sci. 2003, 90, 2775–2784. [Google Scholar] [CrossRef]
- Kalita, H.; Karak, N. Biobased hyperbranched shape-memory polyurethanes: Effect of different vegetable oils. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Anthamatten, M.; Roddecha, S.; Li, J. Energy storage capacity of shape-memory polymers. Macromolecules 2013, 46, 4230–4234. [Google Scholar] [CrossRef]
- Saralegi, A.; Gonzalez, M.L.; Valea, A.; Eceiza, A.; Corcuera, M.A. The role of cellulose nanocrystals in the improvement of the shape-memory properties of castor oil-based segmented thermoplastic polyurethanes. Compos. Sci. Technol. 2014, 92, 27–33. [Google Scholar] [CrossRef]
- Yao, Z.; Wu, D.; Chen, C.; Zhang, M. Creep behavior of polyurethane nanocomposites with carbon nanotubes. Compos. Part A Appl. Sci. Manuf. 2013, 50, 65–72. [Google Scholar] [CrossRef]
- Tobushi, H.; Hashimoto, T.; Ito, N.; Hayashi, S.; Yamada, E. Shape fixity and shape recovery in a film of shape memory polymer of polyurethane series. J. Intell. Mater. Syst. Struct. 1998, 9, 127–136. [Google Scholar] [CrossRef]
- Li, G.; Yu, D.; Song, Z.; Wang, H.; Liu, W. Facile fabrication of transparent paper with tunable wettability for use in biodegradable substrate. ACS Sustain. Chem. Eng. 2020, 8, 2176–2185. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Venditti, R.; Jur, J.; Gorga, R.E.; Pawlak, J.J. Cellulose-lignin biodegradable and flexible uv protection film. ACS Sustain. Chem. Eng. 2017, 5, 625–631. [Google Scholar] [CrossRef]
- Casado, U.; Marcovich, N.E.; Aranguren, M.I.; Mosiewicki, M.A. High-strength composites based on tung oil polyurethane and wood flour: Effect of the filler concentration on the mechanical properties. Polym. Eng. Sci. 2009, 49, 713–721. [Google Scholar] [CrossRef]
- Nicharat, A.; Shirole, A.; Foster, E.J.; Weder, C. Thermally activated shape memory behavior of melt-mixed polyurethane/cellulose nanocrystal composites. J. Appl. Polym. Sci. 2017, 134, 45033. [Google Scholar] [CrossRef]
- Saralegi, A.; Johan Foster, E.; Weder, C.; Eceiza, A.; Corcuera, M.A. Thermoplastic shape-memory polyurethanes based on natural oils. Smart Mater. Struct. 2014, 23, 025033. [Google Scholar] [CrossRef]












| Resins | TO:FMA Mole Ratio | 10% CNF-Reinforced Biocomposites | 10% CNC-Reinforced Biocomposites |
|---|---|---|---|
| T1F3 | 1:3 | T1F3_CNF | T1F3_CNC |
| T1F6 | 1:6 | T1F6_CNF | T1F6_CNC |
| T1F9 | 1:9 | T1F9_CNF | T1F9_CNC |
| Sample | E′ at 25 °C (MPa) a | Tg (°C) b | Loss Factor (Tan δ) | n (103 mol m−3) c | Strength (MPa) | Tensile Modulus (MPa) d | Ti (°C) e | T50 (°C) e |
|---|---|---|---|---|---|---|---|---|
| T1F3 | 15 | 25 | 0.54 | 1.49 | 0.09 | 0.34 | 262 | 410 |
| T1F6 | 63 | 39 | 0.73 | 2.98 | 0.84 | 1.83 | 259 | 399 |
| T1F9 | 199 | 49 | 0.64 | 5.59 | 1.2 | 3.08 | 259 | 402 |
| T1F3_CNF | 490 | 36 | 0.14 | 100 | 17.28 | 288 | 217 | 375 |
| T1F6_CNF | 1492 | 41; 94 | 0.1 | 305 | 19.01 | 637 | 230 | 385 |
| T1F9_CNF | 2250 | 52; 95 | 0.1 | 442 | 16.09 | 805 | 255 | 408 |
| T1F3_CNC | 1317 | 77; 105 | 0.11 | 206 | 20.85 | 630 | 242 | 402 |
| T1F6_CNC | 1723 | 58; 110 | 0.09 | 300 | 21 | 780 | 239 | 402 |
| T1F9_CNC | 2047 | 32; 107 | 0.09 | 322 | 11.42 | 814 | 248 | 408 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sain, S.; Åkesson, D.; Skrifvars, M.; Roy, S. Hydrophobic Shape-Memory Biocomposites from Tung-Oil-Based Bioresin and Onion-Skin-Derived Nanocellulose Networks. Polymers 2020, 12, 2470. https://doi.org/10.3390/polym12112470
Sain S, Åkesson D, Skrifvars M, Roy S. Hydrophobic Shape-Memory Biocomposites from Tung-Oil-Based Bioresin and Onion-Skin-Derived Nanocellulose Networks. Polymers. 2020; 12(11):2470. https://doi.org/10.3390/polym12112470
Chicago/Turabian StyleSain, Sunanda, Dan Åkesson, Mikael Skrifvars, and Souvik Roy. 2020. "Hydrophobic Shape-Memory Biocomposites from Tung-Oil-Based Bioresin and Onion-Skin-Derived Nanocellulose Networks" Polymers 12, no. 11: 2470. https://doi.org/10.3390/polym12112470