Coumarin-Containing Light-Responsive Carboxymethyl Chitosan Micelles as Nanocarriers for Controlled Release of Pesticide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of 7-Diethylamino-4-Hydroxymethylcoumarin (1)
2.3. Synthesis of (7-Diethylaminocoumarin-4-Yl)Methyl Succinate (DEACMS)
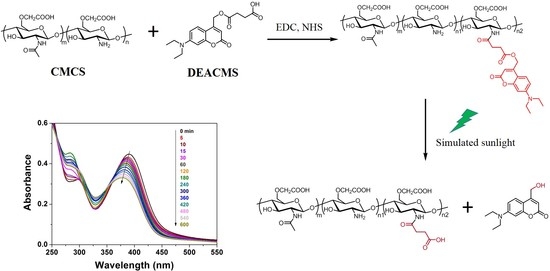
2.4. Synthesis of Carboxymethyl Chitosan-(7-Diethylaminocoumarin-4-Yl)Methyl Succinate (CMCS-DEACMS)
2.5. Preparation of CMCS-DEACMS Micelles
2.6. Characterization of the CMCS-DEACMS Micelles
2.7. Photoresponse and Stability of the CMCS-DEACMS Micelles
2.8. Preparation of 2,4-D Loaded Micelles
2.9. Controlled Release of 2,4-D Loaded Micelles
2.10. Bioactivity of 2,4-D Loaded Micelles
3. Results and Discussion
3.1. Synthesis and Characterization of CMCS-DEACMS
3.2. Self-Assembly Behavior of CMCS-DEACMS Micelles
3.3. Light-Responsive Property of the Micelles
3.4. Light-Controlled Release of 2,4-D
3.5. Bioactivity Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, C.; Zhang, G.; Dai, Z.; Xiang, Y.; Liu, B.; Bian, P.; Zheng, K.; Wu, Z.; Cai, D. Fabrication of light-responsively controlled-release herbicide using a nanocomposite. Chem. Eng. J. 2018, 349, 101–110. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, G.; Chi, Y.; Cai, D.; Wu, Z. Fabrication of a controllable nanopesticide system with magnetic collectability. Chem. Eng. J. 2017, 328, 320–330. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Hassan, A.A.; Kim, K.H. Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release 2019, 294, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cui, H.; Wang, Y.; Sun, C.; Cui, B.; Zeng, Z. Development strategies and prospects of nano-based smart pesticide formulation. J. Agric. Food Chem. 2018, 66, 6504–6512. [Google Scholar] [CrossRef]
- Liu, Z.; Qie, R.; Li, W.; Hong, N.; Li, Y.; Li, C.; Wang, R.; Shi, Y.; Guo, X.; Jia, X. Preparation of avermectin microcapsules with anti-photodegradation and slow-release by the assembly of lignin derivatives. New J. Chem. 2017, 41, 3190–3195. [Google Scholar] [CrossRef]
- Chauhan, N.; Dilbaghi, N.; Gopal, M.; Kumar, R.; Kim, K.-H.; Kumar, S. Development of chitosan nanocapsules for the controlled release of hexaconazole. Int. J. Biol. Macromol. 2017, 97, 616–624. [Google Scholar] [CrossRef]
- Grillo, R.; Pereira, A.E.S.; Nishisaka, C.S.; De Lima, R.; Oehlke, K.; Greiner, R.; Fraceto, L.F. Chitosan/tripolyphosphate nanoparticles loaded with paraquat herbicide: An environmentally safer alternative for weed control. J. Hazard. Mater. 2014, 278, 163–171. [Google Scholar] [CrossRef]
- Dailey, O.D. Volatilization of alachlor from polymeric formulations. J. Agric. Food Chem. 2004, 52, 6742–6746. [Google Scholar] [CrossRef]
- Fernández-Pérez, M.; Villafranca-Sánchez, M.; González-Pradas, E.; Flores-Céspedes, F. Controlled release of diuron from an alginate-bentonite formulation: Water release kinetics and soil mobility study. J. Agric. Food Chem. 1999, 47, 791–798. [Google Scholar] [CrossRef]
- Lao, S.B.; Zhang, Z.X.; Xu, H.H.; Jiang, G.B. Novel amphiphilic chitosan derivatives: Synthesis, characterization and micellar solubilization of rotenone. Carbohydr. Polym. 2010, 82, 1136–1142. [Google Scholar] [CrossRef]
- Campos, E.V.R.; De Oliveira, J.L.; Fraceto, L.F.; Singh, B. Polysaccharides as safer release systems for agrochemicals. Agron. Sustain. Dev. 2015, 35, 47–66. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, J. Encapsulation of the herbicide picloram by using polyelectrolyte biopolymers as layer-by-layer materials. J. Agric. Food Chem. 2013, 61, 3789–3796. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Zhang, W.; Ding, G.; Guo, D.; Zhu, J.; Wang, B.; Punyapitak, D.; Cao, Y. Preparation and characterization of enzyme-responsive emamectin benzoate microcapsules based on a copolymer matrix of silica-epichlorohydrin-carboxymethylcellulose. RSC Adv. 2015, 5, 93170–93179. [Google Scholar] [CrossRef]
- Atta, S.; Paul, A.; Banerjee, R.; Bera, M.; Ikbal, M.; Dhara, D.; Singh, N.D.P. Photoresponsive polymers based on a coumarin moiety for the controlled release of pesticide 2,4-D. RSC Adv. 2015, 5, 99968–99975. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, N.; Gopal, M.; Kumar, R.; Dilbaghi, N. Development and evaluation of alginate-chitosan nanocapsules for controlled release of acetamiprid. Int. J. Biol. Macromol. 2015, 81, 631–637. [Google Scholar] [CrossRef]
- Binder, C.R.; García-Santos, G.; Andreoli, R.; Diaz, J.; Feola, G.; Wittensoeldner, M.; Yang, J. Simulating human and environmental exposure from hand-held knapsack pesticide application: Be-wetspa-pest, an integrative, spatially explicit modeling approach. J. Agric. Food Chem. 2016, 64, 3999–4008. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Chen, C.; Wang, R.; Dong, S.; Li, J.; Zhang, G.; Cai, D.; Zhai, S.; Wu, Z. Near-infrared light-responsively controlled-release herbicide using biochar as a photothermal agent. ACS Sustain. Chem. Eng. 2019, 7, 14924–14932. [Google Scholar] [CrossRef]
- Camara, M.C.; Campos, E.V.R.; Monteiro, R.A.; Do Espirito Santo Pereira, A.; De Freitas Proença, P.L.; Fraceto, L.F. Development of stimuli-responsive nano-based pesticides: Emerging opportunities for agriculture. J. Nanobiotechnol. 2019, 17, 100. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.B.; Pan, Y.F.; Xiao, H.N.; Liu, J. Controlled release of agrochemicals using pH and redox dual-responsive cellulose nanogels. J. Agric. Food Chem. 2019, 67, 6700–6707. [Google Scholar] [CrossRef]
- Sarkar, D.J.; Singh, A. Base triggered release of insecticide from bentonite reinforced citric acid crosslinked carboxymethyl cellulose hydrogel composites. Carbohydr. Polym. 2017, 156, 303–311. [Google Scholar] [CrossRef]
- Mattos, B.D.; Tardy, B.L.; Pezhman, M.; Kämäräinen, T.; Linder, M.; Schreiner, W.H.; Magalhães, W.L.E.; Rojas, O.J. Controlled biocide release from hierarchically-structured biogenic silica: Surface chemistry to tune release rate and responsiveness. Sci. Rep. 2018, 8, 5555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Z.; Guo, J.; Wu, D.; Tan, M.; Xiong, X.; Yin, Y.; He, G. Photo-responsive shell cross-linked micelles based on carboxymethyl chitosan and their application in controlled release of pesticide. Carbohydr. Polym. 2015, 132, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Huang, W.; Wang, D.; Huang, X.; Zhu, X.; Yan, D. Chitosan-based nanocarriers with pH and light dual response for anticancer drug delivery. Biomacromolecules 2013, 14, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Fomina, N.; Mcfearin, C.; Sermsakdi, M.; Edigin, O.; Almutairi, A. UV and near-IR triggered release from polymeric nanoparticles. J. Am. Chem. Soc. 2010, 132, 9540–9542. [Google Scholar] [CrossRef] [Green Version]
- Murase, N.; Mukawa, T.; Sunayama, H.; Takeuchi, T. Molecularly imprinted polymers bearing spiropyran-based photoresponsive binding sites capable of photo-triggered switching for molecular recognition activity. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1637–1644. [Google Scholar] [CrossRef]
- Beauté, L.; Mcclenaghan, N.; Lecommandoux, S. Photo-triggered polymer nanomedicines: From molecular mechanisms to therapeutic applications. Adv. Drug Deliv. Rev. 2019, 138, 148–166. [Google Scholar] [CrossRef]
- Gandioso, A.; Contreras, S.; Melnyk, I.; Oliva, J.; Nonell, S.; Velasco, D.; García-Amorós, J.; Marchán, V. Development of green/red-absorbing chromophores based on a coumarin scaffold that are useful as caging groups. J. Org. Chem. 2017, 82, 5398–5408. [Google Scholar] [CrossRef] [Green Version]
- Atta, S.; Jana, A.; Ananthakirshnan, R.; Narayana Dhuleep, P.S. Fluorescent caged compounds of 2,4-dichlorophenoxyacetic acid (2,4-D): Photorelease technology for controlled release of 2,4-D. J. Agric. Food Chem. 2010, 58, 11844–11851. [Google Scholar] [CrossRef]
- Klán, P.; Šolomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable protecting groups in chemistry and biology: Reaction mechanisms and efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef]
- Iturmendi, A.; Theis, S.; Maderegger, D.; Monkowius, U.; Teasdale, I. Coumarin-caged polyphosphazenes with a visible-light driven on-demand degradation. Macromol. Rapid Commun. 2018, 39, 1800377. [Google Scholar] [CrossRef]
- Xu, Z.P.; Gao, Z.H.; Shao, X.S. Light-triggered release of insecticidally active spirotetramat-enol. Chin. Chem. Lett. 2018, 29, 1648–1650. [Google Scholar] [CrossRef]
- Pang, Y.; Li, X.; Wang, S.; Qiu, X.; Yang, D.; Lou, H. Lignin-polyurea microcapsules with anti-photolysis and sustained-release performances synthesized via pickering emulsion template. React. Funct. Polym. 2018, 123, 115–121. [Google Scholar] [CrossRef]
- Kamari, A.; Aljafree, N.F.A.; Yusoff, S.N.M. N,N-dimethylhexadecyl carboxymethyl chitosan as a potential carrier agent for rotenone. Int. J. Biol. Macromol. 2016, 88, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, M.; Pang, Y.; Qiu, X. Lignin-based microsphere: Preparation and performance on encapsulating the pesticide avermectin. ACS Sustain. Chem. Eng. 2017, 5, 3321–3328. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Dong, H. Controlled release of herbicide acetochlor from clay/carboxylmethylcellulose gel formulations. J. Agric. Food Chem. 2008, 56, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, J.D.; Tiera, M.J.; Tiera, V.A.D. Synthesis, characterization, and antifungal activities of amphiphilic derivatives of diethylaminoethyl citosan against Aspergillus flavus. J. Agric. Food Chem. 2015, 63, 5725–5731. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: A review. Indian J. Plant Physiol. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Mujtaba, M.; Khawar, K.M.; Camara, M.C.; Carvalho, L.B.; Fraceto, L.F.; Morsi, R.E.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Ullah, H.; et al. Chitosan-based delivery systems for plants: A brief overview of recent advances and future directions. Int. J. Biol. Macromol. 2020, 154, 683–697. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, H.; Chen, L.; Hao, L.; Chen, H.; Zhou, X. Carboxymethyl chitosan grafted trisiloxane surfactant nanoparticles with pH sensitivity for sustained release of pesticide. Carbohydr. Polym. 2020, 243, 116433. [Google Scholar] [CrossRef]
- Abigail M, E.A.; Samuel S, M.; Chidambaram, R. Application of rice husk nanosorbents containing 2,4-dichlorophenoxyacetic acid herbicide to control weeds and reduce leaching from soil. J. Taiwan Inst. Chem. Eng. 2016, 63, 318–326. [Google Scholar] [CrossRef]
- Wu, Y.G.; Chan, W.L.; Szeto, Y.S. Preparation of O-carboxymethyl chitosans and their effect on color yield of acid dyes on silk. J. Appl. Polym. Sci. 2003, 90, 2500–2502. [Google Scholar] [CrossRef]
- Huang, Q.; Bao, C.; Ji, W.; Wang, Q.; Zhu, L. Photocleavable coumarin crosslinkers based polystyrene microgels: Phototriggered swelling and release. J. Mater. Chem. 2012, 22, 18275–18282. [Google Scholar] [CrossRef]
- Jena, S.K.; Sangamwar, A.T. Polymeric micelles of amphiphilic graft copolymer of α-tocopherol succinate-g-carboxymethyl chitosan for tamoxifen delivery: Synthesis, characterization and in vivo pharmacokinetic study. Carbohydr. Polym. 2016, 151, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Y.; Qiu, L.; Wang, X.; Li, T.; Han, L.; Ouyang, H.; Xu, W.; Chu, K. Preparation and evaluation of carboxymethyl chitosan-rhein polymeric micelles with synergistic antitumor effect for oral delivery of paclitaxel. Carbohydr. Polym. 2019, 206, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tan, J.; Kang, H.; Ma, L.; Jin, X.; Liu, R.; Huang, Y. Synthesis, self-assembly and drug release behaviors of pH-responsive copolymers ethyl cellulose-graft-PDEAEMA through ATRP. Carbohydr. Polym. 2011, 84, 195–202. [Google Scholar] [CrossRef]
- Zhong, H.Q.; Liu, C.C.; Ge, W.J.; Sun, R.C.; Huang, F.; Wang, X.H. Self-assembled conjugated polymer/chitosan-graft-oleic acid micelles for fast visible detection of aliphatic biogenic amines by “turn-on” FRET. ACS App. Mater. Interfaces 2017, 9, 22875–22884. [Google Scholar] [CrossRef]
- Shen, F.; Zhong, H.; Ge, W.; Ren, J.; Wang, X. Quercetin/chitosan-graft-alpha lipoic acid micelles: A versatile antioxidant water dispersion with high stability. Carbohydr. Polym. 2020, 234, 115927. [Google Scholar] [CrossRef]
- Cao, L.D.; Zhou, Z.L.; Niu, S.J.; Cao, C.; Li, X.H.; Shan, Y.P.; Huang, Q.L. Positive-charge functionalized mesoporous silica nanoparticles as nanocarriers for controlled 2,4-dichlorophenoxy acetic acid sodium salt release. J. Agric. Food Chem. 2018, 66, 6594–6603. [Google Scholar] [CrossRef]
- Hermosín, M.C.; Celis, R.; Facenda, G.; Carrizosa, M.J.; Ortega-Calvo, J.J.; Cornejo, J. Bioavailability of the herbicide 2,4-D formulated with organoclays. Soil Biol. Biochem. 2006, 38, 2117–2124. [Google Scholar] [CrossRef]
- Rege, P.D.; Tian, Y.; Corey, E.J. Studies of new indole alkaloid coupling methods for the synthesis of haplophytine. Org. Lett. 2006, 8, 3117–3120. [Google Scholar] [CrossRef]
- Yan, F.; Chen, L.; Tang, Q.; Wang, R. Synthesis and characterization of a photocleavable cross-linker and its application on tunable surface modification and protein photodelivery. Bioconj. Chem. 2004, 15, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Han, B.; Li, H.; Yang, Y.; Liu, W. Carboxymethyl chitosan represses tumor angiogenesis in vitro and in vivo. Carbohydr. Polym. 2015, 129, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zheng, H.; Hu, R.; Liao, J.; Fei, Z.; Wei, X.; Xiong, X.; Zhang, F.; Zheng, H.; Li, D. pH-Responsive nanoparticles based on covalently grafted conjugates of carboxymethyl chitosan and daunorubicin for the delivery of anti-cancer drugs. J. Biomed. Nanotechnol. 2017, 13, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Hsieh, Y.-L. Nanofibrous membranes from aqueous electrospinning of carboxymethyl chitosan. Nanotechnology 2008, 19, 125707. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.; Dung, P.; Gey, C.; Milas, M. Substituent distribution on O, N-carboxymethylchitosans by 1H and 13C NMR. Int. J. Biol. Macromol. 1992, 14, 122–128. [Google Scholar] [CrossRef]
- Du, Y.-Z.; Wang, L.; Yuan, H.; Wei, X.-H.; Hu, F.-Q. Preparation and characteristics of linoleic acid-grafted chitosan oligosaccharide micelles as a carrier for doxorubicin. Colloids Surf. B 2009, 69, 257–263. [Google Scholar] [CrossRef]
- Suzuki, A.Z.; Watanabe, T.; Kawamoto, M.; Nishiyama, K.; Yamashita, H.; Ishii, M.; Iwamura, M.; Furuta, T. Coumarin-4-ylmethoxycarbonyls as phototriggers for alcohols and phenols. Org. Lett. 2003, 5, 4867. [Google Scholar] [CrossRef]
- Roche, A.; Terriac, E.; Tejedor, R.M.; Oriol, L.; Del Campo, A.; Piñol, M. Supramolecular block copolymers as novel UV and NIR responsive nanocarriers based on a photolabile coumarin unit. Eur. Polym. J. 2020, 126, 109561. [Google Scholar] [CrossRef]
- Li, H.B.; Chen, C.M.; An, Q.; Huo, G.Y.; Run, M.T. Photo-responsive nanoparticles for beta-lapachone delivery in vitro. Chin. Chem. Lett. 2018, 29, 1347–1349. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Chen, W.; Jing, M.M.; Liu, S.Z.; Feng, J.T.; Wu, H.; Zhou, Y.W.; Zhang, X.; Ma, Z.Q. Self-assembled mixed micelle loaded with natural pyrethrins as an intelligent nano-insecticide with a novel temperature-responsive release mode. Chem. Eng. J. 2019, 361, 1381–1391. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Wang, J.; Zhang, L.; Chen, Q.; Yue, W.; Ke, N.; Xie, H. Coumarin-Containing Light-Responsive Carboxymethyl Chitosan Micelles as Nanocarriers for Controlled Release of Pesticide. Polymers 2020, 12, 2268. https://doi.org/10.3390/polym12102268
Feng S, Wang J, Zhang L, Chen Q, Yue W, Ke N, Xie H. Coumarin-Containing Light-Responsive Carboxymethyl Chitosan Micelles as Nanocarriers for Controlled Release of Pesticide. Polymers. 2020; 12(10):2268. https://doi.org/10.3390/polym12102268
Chicago/Turabian StyleFeng, Song, Junqin Wang, Lihua Zhang, Qin Chen, Wang Yue, Ni Ke, and Haibo Xie. 2020. "Coumarin-Containing Light-Responsive Carboxymethyl Chitosan Micelles as Nanocarriers for Controlled Release of Pesticide" Polymers 12, no. 10: 2268. https://doi.org/10.3390/polym12102268