Polymeric Carbon Nitride Armored Centimeter-Wide Organic Droplets in Water for All-Liquid Heterophase Emission Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of CMp-vTA
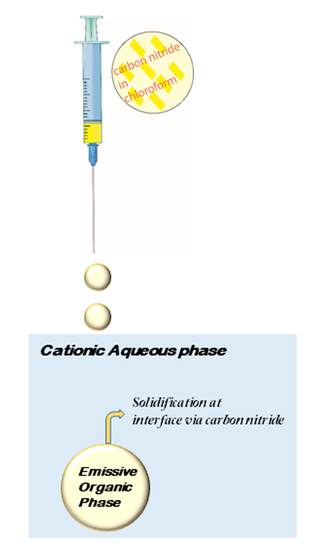
2.3. Droplet Formation Experiments
2.4. Characterization
3. Results
3.1. Synthesis and Characterization of CMp-vTA
3.2. Formation of Organic Droplets in Aqueous Phase via Injection
3.3. Multicolor Emission
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thejokalyani, N.; Dhoble, S. Novel approaches for energy efficient solid state lighting by RGB organic light emitting diodes—A review. Renew. Sustain. Energy Rev. 2014, 32, 448–467. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burn, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Mikosch, A.; Ciftci, S.; Tainter, G.D.; Shivanna, R.; Haehnle, B.; Deschler, F.; Kuehne, A.J.C.; Hähnle, B. Laser emission from self-assembled colloidal crystals of conjugated polymer particles in a metal-halide perovskite matrix. Chem. Mater. 2019, 31, 2590–2596. [Google Scholar] [CrossRef]
- Romero-Servin, S.; Lozano-Hernández, L.-A.; Maldonado, J.-L.; Carriles, R.; Ramos-Ortiz, G.; Pérez-Gutiérrez, E.; Scherf, U.; Zolotukhin, M.G. light emission properties of a cross-conjugated fluorene polymer: Demonstration of its use in electro-luminescence and lasing devices. Polymers 2016, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.; Zhu, S.; Feng, T.; Zheng, C.; Yang, B. Crosslink-enhanced emission effect on luminescence in polymers: Advances and perspectives. Angew. Chem. Int. 2020, 59, 9826–9840. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef] [Green Version]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [Green Version]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, Y.; Pan, Q.; Qiu, J. A fantastic graphitic carbon nitride (g-C3N4) material: Electronic structure, photocatalytic and photoelectronic properties. J. Photochem. Photobiol. C Photochem. Rev. 2014, 20, 33–50. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Antonietti, M. Graphitic carbon nitride “reloaded”: Emerging applications beyond (photo)catalysis. Chem. Soc. Rev. 2016, 45, 2308–2326. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Wang, S.; Ren, H.; Hou, Y.; Wang, X. Photocatalytic reduction of CO2 by graphitic carbon nitride polymers derived from urea and barbituric acid. Appl. Catal. B Environ. 2015, 179, 1–8. [Google Scholar] [CrossRef]
- Vu, N.-N.; Nguyen, C.-C.; Kaliaguine, S.; Do, T.-O. Synthesis of g-C 3 N 4 nanosheets by using a highly condensed lamellar crystalline melamine–cyanuric acid supramolecular complex for enhanced solar hydrogen generation. ChemSusChem 2018, 12, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, B.; Zhao, Y.; Hu, C.; Qu, L. A Graphitic-C3N4“seaweed” architecture for enhanced hydrogen evolution. Angew. Chem. Int. Ed. 2015, 54, 11433–11437. [Google Scholar] [CrossRef]
- Dante, R.C.; Martín-Ramos, P.; Navas-Gracia, L.M.; Sánchez-Arévalo, F.; Martín-Gil, J. Polymeric carbon nitride nanosheets. J. Macromol. Sci. Part B 2012, 52, 623–631. [Google Scholar] [CrossRef]
- Cao, Q.; Kumru, B.; Antonietti, M.; Schmidt, B.V.K.J. Graphitic carbon nitride and polymers: A mutual combination for advanced properties. Mater. Horizons 2020, 7, 762–786. [Google Scholar] [CrossRef] [Green Version]
- Chaubey, S.; Singh, P.; Singh, C.; Shambhavi; Sharma, K.; Yadav, R.K.; Kumar, A.; Baeg, J.O.; Dwivedi, D.; Singh, A.P. Self-assembled protein/carbon nitride/sulfur hydrogel photocatalyst for highly selective solar chemical production. Mater. Lett. 2020, 259, 126752. [Google Scholar] [CrossRef]
- Fang, Y.; Li, X.; Wang, X. Synthesis of Polymeric Carbon Nitride Films with Adhesive Interfaces for Solar Water Splitting Devices. ACS Catal. 2018, 8, 8774–8780. [Google Scholar] [CrossRef]
- Schwinghammer, K.; Tuffy, B.; Mesch, M.B.; Wirnhier, E.; Martineau-Corcos, C.; Taulelle, F.; Schnick, W.; Senker, J.; Lotsch, B.V.; Senker, J. Triazine-based carbon nitrides for visible-light-driven hydrogen evolution. Angew. Chem. Int. Ed. 2013, 52, 2435–2439. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic carbon nitride (g–C3N4)–based metal-free photocatalysts for water splitting: A review. Carbon 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Pan, Z.; Zheng, Y.; Guo, F.; Niu, P.; Wang, X. Decorating CoP and Pt Nanoparticles on graphitic carbon nitride nanosheets to promote overall water splitting by conjugated polymers. ChemSusChem 2016, 10, 87–90. [Google Scholar] [CrossRef]
- Zhang, G.; Li, G.; Heil, T.; Zafeiratos, S.; Lai, F.; Savateev, A.; Antonietti, M.; Wang, X. Tailoring the grain boundary chemistry of polymeric carbon nitride for enhanced solar hydrogen production and CO2 reduction. Angew. Chem. Int. Ed. 2019, 58, 3433–3437. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Peng, T.; Zhang, X.; Li, K.; Ye, L.; Zan, L. Effect of graphitic carbon nitride microstructures on the activity and selectivity of photocatalytic CO2 reduction under visible light. Catal. Sci. Technol. 2013, 3, 1253. [Google Scholar] [CrossRef]
- Wan, S.; Ou, M.; Zhong, Q.; Cai, W. Haloid acid induced carbon nitride semiconductors for enhanced photocatalytic H2 evolution and reduction of CO2 under visible light. Carbon 2018, 138, 465–474. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, L.; Wang, M.; Tian, J.; Jin, X.; Guo, L.; Wang, L.; Shi, J. Carbon-vacancy modified graphitic carbon nitride: Enhanced CO2 photocatalytic reduction performance and mechanism probing. J. Mater. Chem. A 2019, 7, 1556–1563. [Google Scholar] [CrossRef]
- Kaya, K.; Kiskan, B.; Kumru, B.; Schmidt, B.V.K.J.; Yagci, Y. An oxygen-tolerant visible light induced free radical polymerization using mesoporous graphitic carbon nitride. Eur. Polym. J. 2020, 122, 109410. [Google Scholar] [CrossRef]
- Al-Naji, M.; Puértolas, B.; Kumru, B.; Cruz, D.; Bäumel, M.; Schmidt, B.V.K.J.; Tarakina, N.V.; Pérez-Ramírez, J.; Puértola, B. Sustainable continuous flow valorization of γ-Valerolactone with Trioxane to α-Methylene-γ-Valerolactone over basic Beta Zeolites. ChemSusChem 2019, 12, 2628–2636. [Google Scholar] [CrossRef]
- Kumru, B.; Molinari, V.; Hilgart, M.; Rummel, F.; Schäffler, M.; Schmidt, B.V.K.J. Polymer grafted graphitic carbon nitrides as precursors for reinforced lubricant hydrogels. Polym. Chem. 2019, 10, 3647–3656. [Google Scholar] [CrossRef] [Green Version]
- Cruz, D.; Cerrillo, J.G.; Kumru, B.; Li, N.; Perea, J.D.; Schmidt, B.V.K.J.; Lauermann, I.; Brabec, C.J.; Antonietti, M. Influence of Thiazole-Modified Carbon Nitride Nanosheets with Feasible Electronic properties on inverted Perovskite solar cells. J. Am. Chem. Soc. 2019, 141, 12322–12328. [Google Scholar] [CrossRef]
- Xu, J.; Brenner, T.J.K.; Chabanne, L.; Neher, D.; Antonietti, M.; Shalom, M. Liquid-Based Growth of Polymeric Carbon Nitride Layers and Their Use in a Mesostructured Polymer solar cell with Voc exceeding 1 V. J. Am. Chem. Soc. 2014, 136, 13486–13489. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, Y.; Yu, W.; Guo, X.; Yu, S.; Zhang, J.; Li, C. Ultrathin two-dimensional graphitic carbon nitride as a solution-processed cathode interfacial layer for inverted polymer solar cells. J. Mater. Chem. A 2016, 4, 8000–8004. [Google Scholar] [CrossRef]
- Cui, Q.; Xu, J.; Wang, X.; Li, L.; Antonietti, M.; Shalom, M. Phenyl-Modified Carbon Nitride Quantum Dots with Distinct Photoluminescence Behavior. Angew. Chem. Int. Ed. 2016, 55, 3672–3676. [Google Scholar] [CrossRef]
- Cui, Q.; Xu, J.; Shen, G.; Zhang, C.; Li, L.; Antonietti, M. Hybridizing Carbon Nitride Colloids with a shell of water-soluble conjugated Polymers for tunable full-color emission and synergistic cell imaging. ACS Appl. Mater. Interfaces 2017, 9, 43966–43974. [Google Scholar] [CrossRef] [PubMed]
- Kumru, B.; Cruz, D.; Heil, T.; Schmidt, B.V.K.J.; Antonietti, M. Electrostatic stabilization of Carbon Nitride Colloids in organic solvents enables stable dispersions and transparent homogeneous CN Films for Optoelectronics. J. Am. Chem. Soc. 2018, 140, 17532–17537. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Cui, Q.; Meng, P.; Waclawik, E.R.; Yang, H.; Xu, J. Direct observation of carbon nitride-stabilized Pickering emulsions. Langmuir 2018, 34, 10135–10143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Meng, P.; Waclawik, E.R.; Zhang, C.; Li, X.-H.; Yang, H.; Antonietti, M.; Xu, J. Palladium/Graphitic Carbon Nitride (g-C3N4) Stabilized Emulsion Microreactor as a Store for Hydrogen from Ammonia Borane for Use in Alkene Hydrogenation. Angew. Chem. Int. Ed. 2018, 57, 14857–14861. [Google Scholar] [CrossRef]
- Cao, Q.; Heil, T.; Kumru, B.; Antonietti, M.; Schmidt, B.V.K.J. Visible-light induced emulsion photopolymerization with carbon nitride as a stabilizer and photoinitiator. Polym. Chem. 2019, 10, 5315–5323. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Cui, Q.; Yang, Y.; Xu, J.; Han, C.; Li, L. Graphitic carbon Nitride as a distinct solid stabilizer for emulsion Polymerization. Chem. A Eur. J. 2018, 24, 2286–2291. [Google Scholar] [CrossRef]
- Liu, M.; Ishida, Y.; Ebina, Y.; Sasaki, T.; Hansen, J.; Takata, M.; Aida, T. An anisotropic hydrogel with electrostatic repulsion between cofacially aligned nanosheets. Nature 2014, 517, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.T.; Hwang, Y.; Chen, A.C.; Varghese, S.; Sah, R. Cartilage-like mechanical properties of poly (ethylene glycol)-diacrylate hydrogels. Biomaterials 2012, 33, 6682–6690. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Kumru, B.; Al Nakeeb, N.; Willersinn, J.; Schmidt, B.V.K.J. Thermoadaptive supramolecular α-Cyclodextrin Crystallization-Based Hydrogels via Double Hydrophilic block Copolymer templating. Polymers 2018, 10, 576. [Google Scholar] [CrossRef] [Green Version]
- Appel, E.A.; Loh, X.J.; Jones, S.T.; Biedermann, F.; Dreiss, C.A.; Scherman, O.A. Ultrahigh-water-content supramolecular Hydrogels exhibiting multistimuli responsiveness. J. Am. Chem. Soc. 2012, 134, 11767–11773. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Liu, X.; Li, Y.; Wu, X.; Wang, D.; Forth, J.; Russell, T.P. Liquid Letters. Adv. Mater. 2018, 30, 1705800. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Liu, T.; Sun, H.; Wang, B.; Shi, S.; Russell, T.P. Interfacial Assembly and Jamming of Polyelectrolyte surfactants: A Simple route to print liquids in low-viscosity solution. ACS Appl. Mater. Interfaces 2020, 12, 18116–18122. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.-Y.; Chai, Y.; Hou, H.; Xie, G.; Jiang, Y.; Xu, Q.-F.; Liu, F.; Ashby, P.D.; Lu, J.-M.; Russell, T.P. Stabilizing liquids using interfacial supramolecular polymerization. Angew. Chem. Int. Ed. 2019, 58, 12112–12116. [Google Scholar] [CrossRef]
- Qian, B.; Shi, S.; Wang, H.; Russell, T.P. Reconfigurable Liquids Stabilized by DNA Surfactants. ACS Appl. Mater. Interfaces 2020, 12, 13551–13557. [Google Scholar] [CrossRef]
- Huang, C.; Sun, Z.; Cui, M.; Liu, F.; Helms, B.A.; Russell, T.P. Structured liquids with pH-triggered reconfigurability. Adv. Mater. 2016, 28, 6612–6618. [Google Scholar] [CrossRef]
- Sciortino, A.; Cannizzo, A.; Messina, F. Carbon nanodots: A Review—From the current understanding of the fundamental photophysics to the full control of the optical response. J. Carbon Res. 2018, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Li, J.-X.; Zeng, M.; Xu, J.; Wang, X.; Hu, W. Polymer nanodots of graphitic carbon nitride as effective fluorescent probes for the detection of Fe3+ and Cu2+ ions. Nanoscale 2014, 6, 4157. [Google Scholar] [CrossRef]
- Chen, T.; Chen, C.; Liu, Q.; Zhang, Z.; Fang, X. A one-step process for preparing a phenyl-modified g-C3N4 green phosphor with a high quantum yield. RSC Adv. 2017, 7, 51702–51710. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.; Radhika, S.; Yoon, M. Graphitic carbon nitride coupled with perylene nanoparticles as efficient solar photocatalyst. Mol. Catal. 2017, 433, 274–281. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Q.; Kumru, B. Polymeric Carbon Nitride Armored Centimeter-Wide Organic Droplets in Water for All-Liquid Heterophase Emission Technology. Polymers 2020, 12, 1626. https://doi.org/10.3390/polym12081626
Cao Q, Kumru B. Polymeric Carbon Nitride Armored Centimeter-Wide Organic Droplets in Water for All-Liquid Heterophase Emission Technology. Polymers. 2020; 12(8):1626. https://doi.org/10.3390/polym12081626
Chicago/Turabian StyleCao, Qian, and Baris Kumru. 2020. "Polymeric Carbon Nitride Armored Centimeter-Wide Organic Droplets in Water for All-Liquid Heterophase Emission Technology" Polymers 12, no. 8: 1626. https://doi.org/10.3390/polym12081626