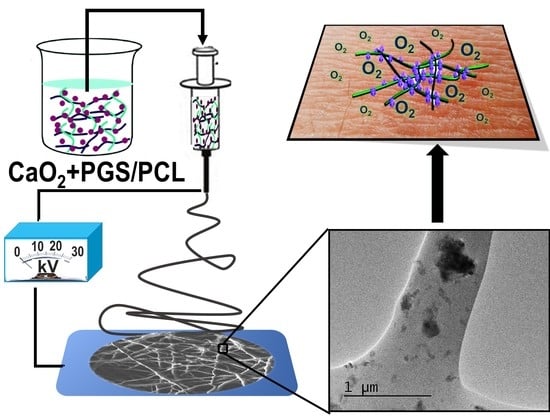
Oxygen-Releasing Antibacterial Nanofibrous Scaffolds for Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning
2.3. Characterization
2.4. Degradation and Oxygen Release
2.5. Antibacterial Performance and Metabolic Activity
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in Regenerative Medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [Green Version]
- Suvarnapathaki, S.; Wu, X.; Lantigua, D.; Nguyen, M.A.; Camci-Unal, G. Breathing life into engineered tissues using oxygen-releasing biomaterials. NPG Asia Mater. 2019, 11, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.; Memic, A.; Annabi, N.; Hossain, M.; Paul, A.; Dokmeci, M.R.; Dehghani, F.; Khademhosseini, A. Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater. 2013, 10, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Barua, S.; Chattopadhyay, P.; Aidew, L.; Buragohain, A.K.; Karak, N. Infection-resistant hyperbranched epoxy nanocomposite as a scaffold for skin tissue regeneration. Polym. Int. 2014, 64, 303–311. [Google Scholar] [CrossRef]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Combining Stem Cells and Biomaterial Scaffolds for Constructing Tissues and Cell Delivery. StemJournal 2019, 1, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Ahadian, S.; Radisic, M. Biochemical and Biophysical Cues in Matrix Design for Chronic and Diabetic Wound Treatment. Tissue Eng. Part B Rev. 2017, 23, 9–26. [Google Scholar] [CrossRef] [Green Version]
- Camci-Unal, G.; Alemdar, N.; Annabi, N.; Khademhosseini, A. Oxygen Releasing Biomaterials for Tissue Engineering. Polym. Int. 2013, 62, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholipourmalekabadi, M.; Zhao, S.; Harrison, B.S.; Mozafari, M.; Seifalian, A. Oxygen-Generating Biomaterials: A New, Viable Paradigm for Tissue Engineering? Trends Biotechnol. 2016, 34, 1010–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarker, M.; Chen, X.; Schreyer, D. Experimental approaches to vascularisation within tissue engineering constructs. J. Biomater. Sci. Polym. Ed. 2015, 26, 683–734. [Google Scholar] [CrossRef] [PubMed]
- Becquart, P.; Cambon-Binder, A.; Monfoulet, L.-E.; Bourguignon, M.; Vandamme, K.; Bensidhoum, M.; Petite, H.; Logeart-Avramoglou, D. Ischemia Is the Prime but Not the Only Cause of Human Multipotent Stromal Cell Death in Tissue-Engineered Constructs In Vivo. Tissue Eng. Part A 2012, 18, 2084–2094. [Google Scholar] [CrossRef]
- LaVan, F.B.; Hunt, T.K. Oxygen and wound healing. Clin. Plast. Surg. 1990, 17, 463–472. [Google Scholar] [PubMed]
- Shiekh, P.A.; Singh, A.; Kumar, A. Oxygen-Releasing Antioxidant Cryogel Scaffolds with Sustained Oxygen Delivery for Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2018, 10, 18458–18469. [Google Scholar] [CrossRef] [PubMed]
- Nomi, M.; Atala, A.; De Coppi, P.; Soker, S. Principals of neovascularization for tissue engineering. Mol. Asp. Med. 2002, 23, 463–483. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Aghabaglou, F.; McCarthy, A.; Mostafavi, A.; Wiseman, C.; Bonick, Z.; Ghanavati, I.; Harris, S.; Kreikemeier-Bower, C.; Basri, S.M.M.; et al. A Wirelessly Controlled Smart Bandage with 3D-Printed Miniaturized Needle Arrays. Adv. Funct. Mater. 2020, 30, 1905544. [Google Scholar] [CrossRef]
- Memic, A.; Abudula, T.; Mohammed, H.S.; Navare, K.J.; Colombani, T.; Bencherif, S.A. Latest Progress in Electrospun Nanofibers for Wound Healing Applications. ACS Appl. Bio Mater. 2019, 2, 952–969. [Google Scholar] [CrossRef]
- Abudula, T.; Saeed, U.; Memic, A.; Gauthaman, K.; Hussain, M.A.; Al-Turaif, H. Electrospun cellulose Nano fibril reinforced PLA/PBS composite scaffold for vascular tissue engineering. J. Polym. Res. 2019, 26, 110. [Google Scholar] [CrossRef]
- Rinoldi, C.; Fallahi, A.; Yazdi, I.K.; Paras, J.C.; Kijeńska-Gawrońska, E.; Santiago, G.T.-D.; Tuoheti, A.; Demarchi, D.; Annabi, N.; Khademhosseini, A.; et al. Mechanical and Biochemical Stimulation of 3D Multilayered Scaffolds for Tendon Tissue Engineering. ACS Biomater. Sci. Eng. 2019, 5, 2953–2964. [Google Scholar] [CrossRef]
- Khalili, S.; Khorasani, S.N.; Razavi, S.M.; Hashemibeni, B.; Tamayol, A. Nanofibrous Scaffolds with Biomimetic Composition for Skin Regeneration. Appl. Biochem. Biotechnol. 2018, 187, 1193–1203. [Google Scholar] [CrossRef]
- Khan, F.; Aldhahri, M.; Hussain, M.A.; Gauthaman, K.; Memic, A.; Abuzenadah, A.; Kumosani, T.; Barbour, E.; Alothmany, N.S.; Aldhaheri, R. Encapsulation of 5-Flurouracil into PLGA Nanofibers and Enhanced Anticancer Effect in Combination with Ajwa-Dates-Extract (Phoenix dactylifera L.). J. Biomed. Nanotechnol. 2018, 14, 553–563. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Rinoldi, C.; Kijeńska-Gawrońska, E.; Chlanda, A.; Choińska, E.; Khenoussi, N.; Tamayol, A.; Khademhosseini, A.; Swieszkowski, W. Nanobead-on-string composites for tendon tissue engineering. J. Mater. Chem. B 2018, 6, 3116–3127. [Google Scholar] [CrossRef] [PubMed]
- Abudula, T.; Saeed, U.; Salah, N.; Memic, A.; Al-Turaif, H. Study of Electrospinning Parameters and Collection Methods on Size Distribution and Orientation of PLA/PBS Hybrid Fiber Using Digital Image Processing. J. Nanosci. Nanotechnol. 2018, 18, 8240–8251. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Ward, C.L.; Atala, A.; Yoo, J.J.; Harrison, B.S. Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials 2009, 30, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.S.; Eberli, D.; Lee, S.J.; Atala, A.; Yoo, J.J. Oxygen producing biomaterials for tissue regeneration. Biomaterials 2007, 28, 4628–4634. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, E.; Coronel, M.M.; Fraker, C.A.; Ricordi, C.; Stabler, C.L. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc. Natl. Acad. Sci. USA 2012, 109, 4245–4250. [Google Scholar] [CrossRef] [Green Version]
- Fraker, C.; Mendez, A.J.; Stabler, C.L. Complementary Methods for the Determination of Dissolved Oxygen Content in Perfluorocarbon Emulsions and Other Solutions. J. Phys. Chem. B 2011, 115, 10547–10552. [Google Scholar] [CrossRef] [PubMed]
- Northup, A.; Cassidy, D.P. Calcium peroxide (CaO2) for use in modified Fenton chemistry. J. Hazard. Mater. 2008, 152, 1164–1170. [Google Scholar] [CrossRef]
- Cassidy, D.P.; Irvine, R.L. Use of calcium peroxide to provide oxygen for contaminant biodegradation in a saturated soil. J. Hazard. Mater. 1999, 69, 25–39. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Bawa, H.K.; Ng, G.; Wu, Y.; Libera, M.; Van Der Mei, H.; Busscher, H.; Yu, X. Oxygen-Generating Nanofiber Cell Scaffolds with Antimicrobial Properties. ACS Appl. Mater. Interfaces 2010, 3, 67–73. [Google Scholar] [CrossRef]
- Jeffries, E.M.; Allen, R.; Gao, J.; Pesce, M.; Wang, Y. Highly elastic and suturable electrospun poly(glycerol sebacate) fibrous scaffolds. Acta Biomater. 2015, 18, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Sundback, C.A.; Shyu, J.Y.; Wang, Y.; Faquin, W.C.; Langer, R.S.; Vacanti, J.P.; Hadlock, T.A. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials 2005, 26, 5454–5464. [Google Scholar] [CrossRef] [PubMed]
- Tamayol, A.; Najafabadi, A.H.; Mostafalu, P.; Yetisen, A.K.; Commotto, M.; Aldhahri, M.; Abdel-Wahab, M.S.; Najafabadi, Z.I.; Latifi, S.; Akbari, M.; et al. Biodegradable elastic nanofibrous platforms with integrated flexible heaters for on-demand drug delivery. Sci. Rep. 2017, 7, 9220. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Aldhahri, M.; Tamayol, A.; Mostafalu, P.; Abdel-Wahab, M.S.; Samandari, M.; Moghaddam, K.M.; Annabi, N.; Bencherif, S.A.; Khademhosseini, A. Nanofibrous Silver-Coated Polymeric Scaffolds with Tunable Electrical Properties. Nanomaterials 2017, 7, 63. [Google Scholar] [CrossRef]
- Abudula, T.; Gzara, L.; Simonetti, G.; Alshahrie, A.; Salah, N.; Morganti, P.; Chianese, A.; Fallahi, A.; Tamayol, A.; Bencherif, S.A.; et al. The Effect of Poly (Glycerol Sebacate) Incorporation within Hybrid Chitin–Lignin Sol–Gel Nanofibrous Scaffolds. Materials 2018, 11, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalakonda, P.; Aldhahri, M.A.; Abdel-Wahab, M.S.; Tamayol, A.; Moghaddam, K.M.; Benrached, F.; Pain, A.; Khademhosseini, A.; Memic, A.; Chaieb, S. Microfibrous silver-coated polymeric scaffolds with tunable mechanical properties. RSC Adv. 2017, 7, 34331–34338. [Google Scholar] [CrossRef] [Green Version]
- Najafabadi, A.H.; Tamayol, A.; Annabi, N.; Ochoa, M.; Mostafalu, P.; Akbari, M.; Nikkhah, M.; Rahimi, R.; Dokmeci, M.R.; Sonkusale, S.; et al. Biodegradable nanofibrous polymeric substrates for generating elastic and flexible electronics. Adv. Mater. 2014, 26, 5823–5830. [Google Scholar] [CrossRef] [PubMed]
- Salah, N.; Habib, S.S.; Khan, Z.H.; Memic, A.; Azam, A.; Alarfaj, E.; Zahed, N.; Al-Hamedi, S. High-energy ball milling technique for ZnO nanoparticles as antibacterial material. Int. J. Nanomed. 2011, 6, 863–869. [Google Scholar] [CrossRef] [Green Version]
- Stojilovic, N. Why Can’t We See Hydrogen in X-ray Photoelectron Spectroscopy? J. Chem. Educ. 2012, 89, 1331–1332. [Google Scholar] [CrossRef]
- Hasan, A.; Soliman, S.; El Hajj, F.; Tseng, Y.-T.; Yalcin, H.C.; Marei, H. Fabrication and In Vitro Characterization of a Tissue Engineered PCL-PLLA Heart Valve. Sci. Rep. 2018, 8, 8187. [Google Scholar] [CrossRef]
- Salehi, S.; Bahners, T.; Gutmann, J.S.; Gao, S.; Mader, E.; Fuchsluger, T.A. Characterization of structural, mechanical and nano-mechanical properties of electrospun PGS/PCL fibers. RSC Adv. 2014, 4, 16951–16957. [Google Scholar] [CrossRef]
- Madan, S.; Wasewar, K.L.; Kumar, C.R. Optimization of adsorptive removal of α-toluic acid by CaO2 nanoparticles using response surface methodology. Resour. Technol. 2017, 3, 329–336. [Google Scholar] [CrossRef]
- Rez, M.F.A.; Binobaid, A.; Alghosen, A.; Mirza, E.H.; Alam, J.; Fouad, H.; Hashem, M.; Alsalman, H.; Almalak, H.M.; Mahmood, A. Tubular poly (ε-caprolactone)/chitosan nanofibrous scaffold prepared by electrospinning for vascular tissue engineering applications. J. Biomater. Tissue Eng. 2017, 7, 427–436. [Google Scholar] [CrossRef]
- Corrêa, A.C.; Carmona, V.B.; Simão, J.A.; Mattoso, L.H.C.; Marconcini, J.M. Biodegradable blends of urea plasticized thermoplastic starch (UTPS) and poly(ε-caprolactone) (PCL): Morphological, rheological, thermal and mechanical properties. Carbohydr. Polym. 2017, 167, 177–184. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Wang, S.; Li, R.; Shi, D.; Gao, Y.; Song, L. Effect of different nucleating agent on crystallization kinetics and morphology of polypropylene. Polymers 2019, 19, 32–39. [Google Scholar] [CrossRef]
- Liu, W.J.; Yang, H.; Wang, Z.; Dong, L.S.; Liu, J.J. Effect of nucleating agents on the crystallization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate). J. Appl. Polym. Sci. 2002, 86, 2145–2152. [Google Scholar] [CrossRef]
- Symons, M.; Rusakiewicz, S.; Rees, R.; Ahmad, S. Hydrogen peroxide: A potent cytotoxic agent effective in causing cellular damage and used in the possible treatment for certain tumours. Med. Hypotheses 2001, 57, 56–58. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Lopes, H.P. Mechanisms of antimicrobial activity of calcium hydroxide: A critical review. Int. Endod. J. 1999, 32, 361–369. [Google Scholar] [CrossRef]
- Zhang, H.; Dalisson, B.; Tran, S.; E Barralet, J. Preservation of Blood Vessels with an Oxygen Generating Composite. Adv. Health Mater. 2018, 7, 1701338. [Google Scholar] [CrossRef]
- Crespy, D.; Friedemann, K.; Popa, A.M. Colloid-Electrospinning: Fabrication of Multicompartment Nanofibers by the Electrospinning of Organic or/and Inorganic Dispersions and Emulsions. Macromol. Rapid Commun. 2012, 33, 1978–1995. [Google Scholar] [CrossRef]
- Yin, C.-G.; Ma, Y.; Liu, Z.-J.; Fan, J.-C.; Shi, P.; Xu, Q.-J.; Min, Y.-L. Multifunctional boron nitride nanosheet/polymer composite nanofiber membranes. Polymer 2019, 162, 100–107. [Google Scholar] [CrossRef]
- Wu, X.; Stroll, S.I.; Lantigua, D.; Suvarnapathaki, S.; Camci-Unal, G. Eggshell particle-reinforced hydrogels for bone tissue engineering: An orthogonal approach. Biomater. Sci. 2019, 7, 2675–2685. [Google Scholar] [CrossRef]
- Mao, D.; Li, Q.; Li, D.; Chen, Y.; Chen, X.; Xu, X. Fabrication of 3D porous poly(lactic acid)-based composite scaffolds with tunable biodegradation for bone tissue engineering. Mater. Des. 2018, 142, 1–10. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, Y.; Xiao, C.; Zhao, W.; Wu, H.; Tang, J.; Li, Z.; Yu, S.; Li, X.; Min, L.; et al. Application of hydroxyapatite nanoparticles in tumor-associated bone segmental defect. Sci. Adv. 2019, 5, eaax6946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, T.; Wang, C.; Wang, Y.; Xu, W.; Hu, J.; Cheng, Y. A Nanocomposite Hydrogel with Potent and Broad-Spectrum Antibacterial Activity. ACS Appl. Mater. Interfaces 2018, 10, 15163–15173. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Lai, B.F.; Ding, C.; Kizhakkedathu, J.N.; Hancock, R.E.; Wang, R. Multilayered coating on titanium for controlled release of antimicrobial peptides for the prevention of implant-associated infections. Biomaterials 2013, 34, 5969–5977. [Google Scholar] [CrossRef]
- Li, P.; Poon, Y.F.; Li, W.; Zhu, H.-Y.; Yeap, S.H.; Cao, Y.; Qi, X.; Zhou, C.; Lamrani, M.; Beuerman, R.W.; et al. A polycationic antimicrobial and biocompatible hydrogel with microbe membrane suctioning ability. Nat. Mater. 2010, 10, 149–156. [Google Scholar] [CrossRef]
- Schlecht, L.M.; Peters, B.M.; Krom, B.P.; Freiberg, J.A.; Hänsch, G.M.; Filler, S.G.; Jabra-Rizk, M.A.; Shirtliff, M. Systemic Staphylococcus aureus infection mediated by Candida albicans hyphal invasion of mucosal tissue. Microbiology 2015, 161, 168–181. [Google Scholar] [CrossRef] [Green Version]
- Estrela, C.; Pimenta, F.C.; Ito, I.Y.; Bammann, L.L. Antimicrobial evaluation of calcium hydroxide in infected dentinal tubules. J. Endod. 1999, 25, 416–418. [Google Scholar] [CrossRef]
- Estrela, C.; Pimenta, F.C.; Ito, I.Y.; Bammann, L.L. In vitro determination of direct antimicrobial effect of calcium hydroxide. J. Endod. 1998, 24, 15–17. [Google Scholar] [CrossRef]





| Sample Name | PGS (% wt./v) | PCL (% wt./v) | CaO2 (% wt./v) | Catalase (mg/mL) |
|---|---|---|---|---|
| CP | 0 | 0 | 100 | - |
| CP0 | 10 | 10 | 0 | - |
| CP1 | 10 | 10 | 1 | - |
| CP2.5 | 10 | 10 | 2.5 | - |
| CP5 | 10 | 10 | 5 | - |
| CP10 | 10 | 10 | 10 | - |
| CP2.5C | 10 | 10 | 2.5 | 1 |
| CP5C | 10 | 10 | 5 | 1 |
| Sample Name | Carbon (%) | Oxygen (%) | Calcium (%) |
|---|---|---|---|
| CP | 8.19 | 68.05 | 23.76 |
| CP0 | 76.78 | 23.22 | 0 |
| CP1 | 75.8 | 24.77 | 0.2 |
| CP2.5 | 70.39 | 28.95 | 0.66 |
| CP5 | 69.35 | 29.09 | 1.59 |
| CP10 | 62.02 | 34.7 | 3.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, T.; Gauthaman, K.; Hammad, A.H.; Joshi Navare, K.; Alshahrie, A.A.; Bencherif, S.A.; Tamayol, A.; Memic, A. Oxygen-Releasing Antibacterial Nanofibrous Scaffolds for Tissue Engineering Applications. Polymers 2020, 12, 1233. https://doi.org/10.3390/polym12061233
Abdullah T, Gauthaman K, Hammad AH, Joshi Navare K, Alshahrie AA, Bencherif SA, Tamayol A, Memic A. Oxygen-Releasing Antibacterial Nanofibrous Scaffolds for Tissue Engineering Applications. Polymers. 2020; 12(6):1233. https://doi.org/10.3390/polym12061233
Chicago/Turabian StyleAbdullah, Turdimuhammad, Kalamegam Gauthaman, Ahmed H. Hammad, Kasturi Joshi Navare, Ahmed A. Alshahrie, Sidi A. Bencherif, Ali Tamayol, and Adnan Memic. 2020. "Oxygen-Releasing Antibacterial Nanofibrous Scaffolds for Tissue Engineering Applications" Polymers 12, no. 6: 1233. https://doi.org/10.3390/polym12061233