Curing Kinetic Analysis of Acrylate Photopolymer for Additive Manufacturing by Photo-DSC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
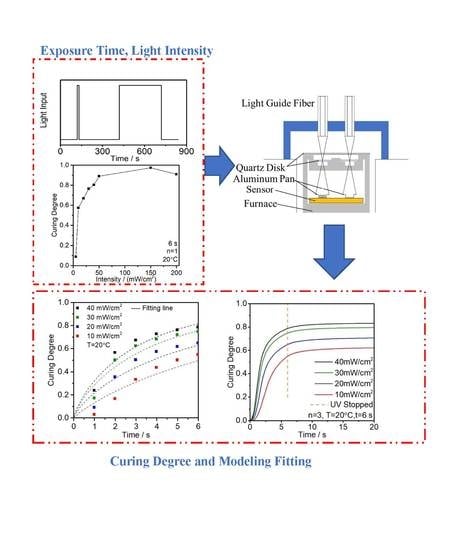
2.2. Methods
2.3. Parameter Selection
2.3.1. UV Light Intensity
2.3.2. Exposure Time
3. Results and Discussion
3.1. Effects of UV Light Intensity on the Enthalpy and Curing Degree
3.2. Effect of UV Exposure Time on the Enthalpy and Curing Degree
3.3. UV Curing Kinetic Model Parameters Analysis
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsui, L.; Maines, E.; Evans, L.; David, K.; Lavin, J.M. Additive Manufacturing of Alumina Components by Extrusion of in-Situ UV-Cured Pastes; Sandia National Lab.(SNL-NM): Albuquerque, NM, USA, 2018.
- Vatani, M.; Choi, J.-W. Direct-print photopolymerization for 3D printing. Rapid Prototyp. J. 2017, 23, 337–343. [Google Scholar] [CrossRef]
- Lewis, J.A.; Gratson, G.M. Direct writing in three dimensions. Mat. Today 2004, 7, 32–39. [Google Scholar] [CrossRef]
- Pieke, S. Experimental Studies on the Efficient Cross-Linking of Surface Coatings with UV Radiation; Karlsruhe Institute of Technology: Karlsruhe, Germany, 2009. [Google Scholar]
- Lovell, L.G.; Elliott, B.J.; Brown, J.R.; Bowman, C.N. The effect of wavelength on the polymerization of multi(meth)acrylates with disulfide/benzilketal combinations. Polymer 2001, 42, 421–429. [Google Scholar] [CrossRef]
- Manoharan, V.; Chou, S.M.; Forrester, S.; Chai, G.B.; Kong, P.W. Application of additive manufacturing techniques in sports footwear. Virtual Phys. Prototyp. 2013, 8, 249–252. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Rapid Manufacturing: An Industrial Revolution for the Digital Age; Hopkinson, N.; Hague, R.J.M.; Dickens, P.M. (Eds.) John Wiley: Chichester, UK, 2006; ISBN 978-0-470-01613-8. [Google Scholar]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.-D.; Hong, J.-W. Photo-curing kinetics for the UV-initiated cationic polymerization of a cycloaliphatic diepoxide system photosensitized by thioxanthone. Eur. Polym. J. 2005, 41, 367–374. [Google Scholar] [CrossRef]
- Hadjou Belaid, Z.; Mechernene, L.; Abdoune, F.Z.; Berrayah, A.; Maschke, U. Photo-differential Scanning Calorimetry Study on Photopolymerization of Polyacrylate/E7 Liquid Crystal Blends. J. Macromol. Sci. Part B 2019, 58, 1–15. [Google Scholar] [CrossRef]
- Rusu, M.C.; Block, C.; Van Assche, G.; Van Mele, B. Influence of temperature and UV intensity on photo-polymerization reaction studied by photo-DSC. J. Therm. Anal. Calorim. 2012, 110, 287–294. [Google Scholar] [CrossRef]
- Golaz, B.; Michaud, V.; Leterrier, Y.; Månson, J.-A.E. UV intensity, temperature and dark-curing effects in cationic photo-polymerization of a cycloaliphatic epoxy resin. Polymer 2012, 53, 2038–2048. [Google Scholar] [CrossRef]
- Lin, J.-T.; Liu, H.-W.; Chen, K.-T.; Cheng, D.-C. Modeling the Optimal Conditions for Improved Efficacy and Crosslink Depth of Photo-Initiated Polymerization. Polymers 2019, 11, 217. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.-T.; Liu, H.-W.; Chen, K.-T.; Cheng, D.-C. Modeling the Kinetics, Curing Depth, and Efficacy of Radical-Mediated Photopolymerization: The Role of Oxygen Inhibition, Viscosity, and Dynamic Light Intensity. Front. Chem. 2019, 7, 760. [Google Scholar] [CrossRef] [PubMed]
- Montserrat, S.; Flaque, C.; Pages, P.; Malek, J. Effect of the crosslinking degree on curing kinetics of an epoxy–anhydride system. J. Appl. Polym. Sci. 1995, 56, 1413–1421. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Ramanujan, D.; Ramani, K.; Chen, Y.; Williams, C.B.; Wang, C.C.L.; Shin, Y.C.; Zhang, S.; Zavattieri, P.D. The status, challenges, and future of additive manufacturing in engineering. Comput. Aided Des. 2015, 69, 65–89. [Google Scholar] [CrossRef]
- Saha, S.K.; Wang, D.; Nguyen, V.H.; Chang, Y.; Oakdale, J.S.; Chen, S.-C. Scalable submicrometer additive manufacturing. Science 2019, 366, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Tumbleston, J.R.; Shirvanyants, D.; Ermoshkin, N.; Janusziewicz, R.; Johnson, A.R.; Kelly, D.; Chen, K.; Pinschmidt, R.; Rolland, J.P.; Ermoshkin, A.; et al. Continuous liquid interface production of 3D objects. Science 2015, 347, 1349–1352. [Google Scholar] [CrossRef]
- IGM RESINS: PHOTOMER 4017. Available online: https://www.igmresins.com/en/product/photomer_4017 (accessed on 2 December 2019).
- Wudy, K.; Budde, T. Reaction kinetics and curing behavior of epoxies for use in a combined selective laser beam melting process of polymers. J. Appl. Polym. Sci. 2019, 136, 46850. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhai, Z.; Drummer, D. Thermal Conductivity of Aluminosilicate- and Aluminum Oxide-Filled Thermosets for Injection Molding: Effect of Filler Content, Filler Size and Filler Geometry. Polymers 2018, 10, 457. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.Z.; He, P.S.; Pan, L.J. Cure kinetics of epoxy-based nanocomposites analyzed by Avrami theory of phase change. Polym. Test. 2003, 22, 689–697. [Google Scholar] [CrossRef]
- Murias, P.; Byczyński, Ł.; Maciejewski, H.; Galina, H. A quantitative approach to dynamic and isothermal curing of an epoxy resin modified with oligomeric siloxanes. J. Therm. Anal. Calorim. 2015, 122, 215–226. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Bao, S.; Shen, S.; Wang, W.; Hang, G.; He, P. Differential scanning calorimetric study on the curing behavior of epoxy resin/diethylenetriamine/organic montmorillonite nanocomposite. J. Polym. Sci. B Polym. Phys. 2003, 41, 378–386. [Google Scholar] [CrossRef]
- Scherzer, T. Depth Profiling of the Degree of Cure during the Photopolymerization of Acrylates Studied by Real-Time FT-IR Attenuated Total Reflection Spectroscopy. Appl. Spectrosc. 2002, 56, 1403–1412. [Google Scholar] [CrossRef]
- Habib, E.; Zhu, X.X. Photo-calorimetry method optimization for the study of light-initiated radical polymerization of dental resins. Polymer 2018, 135, 178–184. [Google Scholar] [CrossRef]
- Fan, P.L.; Schumacher, R.M.; Azzolin, K.; Geary, R.; Eichmiller, F.C. Curing-light intensity and depth of cure of resin-based composites tested according to international standards. J. Am. Dent. Assoc. 2002, 133, 429–434. [Google Scholar] [CrossRef] [PubMed]
- De Beer, M.P.; van der Laan, H.L.; Cole, M.A.; Whelan, R.J.; Burns, M.A.; Scott, T.F. Rapid, continuous additive manufacturing by volumetric polymerization inhibition patterning. Sci. Adv. 2019, 5, eaau8723. [Google Scholar] [CrossRef] [Green Version]
- Bennett, J. Measuring UV curing parameters of commercial photopolymers used in additive manufacturing. Addit. Manuf. 2017, 18, 203–212. [Google Scholar] [CrossRef]








| Light Intensity (mW/cm2) | (J/g) | (J/g) | Peak Time (s) | Curing Degree (%) |
|---|---|---|---|---|
| 5 | 8.01 | 259.62 | 6 | 3.1 |
| 10 | 242.3 | 541.76 | 2.28 | 67.09 |
| 20 | 275.47 | 548.29 | 1.48 | 75.33 |
| 30 | 475.67 | 568.91 | 1.18 | 83.63 |
| 40 | 424.21 | 571.54 | 1.08 | 87.07 |
| Exposure Time (s) | (J/g) | (J/g) | Peak Time (s) | Curing Degree (%) |
|---|---|---|---|---|
| 1.2 | 182 | 568.38 | 1.18 | 26.85 |
| 3 | 398.15 | 560.79 | 1.48 | 70.98 |
| 6 | 275.47 | 548.29 | 1.48 | 75.33 |
| 9 | 462.82 | 568.28 | 1.48 | 81.44 |
| 12 | 461.08 | 564.1 | 1.38 | 81.74 |
| 10 mW/cm2 | 20 mW/cm2 | 30 mW/cm2 | 40 mW/cm2 | |
|---|---|---|---|---|
| a | 0.179 | 0.246 | 0.338 | 0.39 |
| b | 0.878 | 0.775 | 0.794 | 0.818 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; Drummer, D. Curing Kinetic Analysis of Acrylate Photopolymer for Additive Manufacturing by Photo-DSC. Polymers 2020, 12, 1080. https://doi.org/10.3390/polym12051080
Jiang F, Drummer D. Curing Kinetic Analysis of Acrylate Photopolymer for Additive Manufacturing by Photo-DSC. Polymers. 2020; 12(5):1080. https://doi.org/10.3390/polym12051080
Chicago/Turabian StyleJiang, Fengze, and Dietmar Drummer. 2020. "Curing Kinetic Analysis of Acrylate Photopolymer for Additive Manufacturing by Photo-DSC" Polymers 12, no. 5: 1080. https://doi.org/10.3390/polym12051080