Synthesis and Investigation of Thermo-Induced Gelation of Partially Cross-Linked Poly-2-isopropyl-2-oxazoline in Aqueous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Methods
2.1.1. Synthesis of Cross-Linker
2.1.2. Synthesis of Cross-Linked Polyoxazoline
2.1.3. Determination of Molecular Weight Rh-D and Hydrodynamic Characteristics
2.1.4. Thermoresponsive Behavior Study
2.2. Materials and Instruments
3. Results
3.1. Synthesis and Characterization
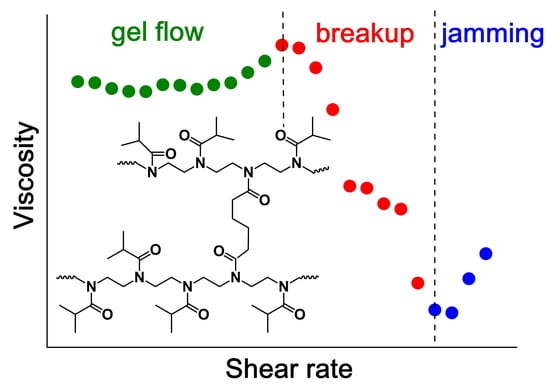
3.2. Thermoresponsive Behavior of CR-PiPrOx
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef]
- Wu, D.-C.; Loh, X.J.; Wu, Y.-L.; Lay, C.L.; Liu, Y. ‘Living’ controlled in situ gelling systems: Thiol-disulfide exchange method toward tailor-made biodegradable hydrogels. J. Am. Chem. Soc. 2010, 132, 15140–15143. [Google Scholar] [CrossRef]
- Moon, H.J.; Ko, D.Y.; Park, M.H.; Joo, M.K.; Jeong, B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem. Soc. Rev. 2012, 41, 4860–4883. [Google Scholar] [CrossRef]
- Cojocaru, F.-D.; Botezat, D.; Gardikiotis, I.; Uritu, C.-M.; Dodi, G.; Trandafir, L.; Rezus, C.; Rezus, E.; Tamba, B.-I.; Mihai, C.-T. Nanomaterials designed for antiviral drug delivery transport across biological barriers. Pharmaceutics 2020, 12, 171. [Google Scholar] [CrossRef] [Green Version]
- Winter, H.H.; Chambon, F. Analysis of linear viscoelasticity of a crosslinking polymer at the gel point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Rogovina, L.Z.; Vasil’ev, V.G.; Braudo, E.E. Definition of the concept of polymer gel. Polym. Sci. Ser. C 2008, 50, 85–92. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Lee, D.S. In situ gelling pH- and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J. Control. Release 2014, 193, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-S.; Chiang, P.-R.; Hong, W.-H.; Chiao, C.-C.; Chu, I.-M.; Hsiue, G.-H.; Shen, C.-R. Study In vivo intraocular biocompatibility of in situ gelation hydrogels: Poly(2-Ethyl Oxazoline)-block-poly(ε-Caprolactone)-block-poly(2-Ethyl Oxazoline) copolymer, matrigel and pluronic F127. PLoS ONE 2013, 8, e67495. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.; Abdul Karim, A.; Loh, X.J. Modification of thermal and mechanical properties of PEG-PPG-PEG copolymer (F127) with MA-POSS. Polymers 2016, 8, 341. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, S.D.; Mazumder, M.A.J.; Lasowski, F.; Fitzpatrick, L.E.; Sheardown, H. PNIPAAm-grafted-collagen as an injectable, In situ gelling, bioactive cell delivery scaffold. Biomacromolecules 2010, 11, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Ajazuddin; Alexander, A.; Khan, J.; Giri, T.K.; Tripathi, D.K.; Saraf, S.; Saraf, S. Advancement in stimuli triggered in situ gelling delivery for local and systemic route. Expert Opin. Drug Deliv. 2012, 9, 1573–1592. [Google Scholar] [CrossRef] [PubMed]
- Liow, S.S.; Dou, Q.; Kai, D.; Abdul Karim, A.; Zhang, K.; Xu, F.; Loh, X.J. Thermogels: In situ gelling biomaterial. ACS Biomater. Sci. Eng. 2016, 2, 295–316. [Google Scholar] [CrossRef]
- Brewer, K.; Gundsambuu, B.; Facal Marina, P.; Barry, S.C.; Blencowe, A. Thermoresponsive poly(ε-Caprolactone)-poly(Ethylene/Propylene Glycol) copolymers as injectable hydrogels for cell therapies. Polymers 2020, 12, 367. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, X.; Li, Y.; Zhou, Y.; Fan, C.; Li, W.; Ma, S.; Fan, Y.; Huang, Y.; Li, N.; et al. Synthesis, characterization and biocompatibility of poly(2-ethyl-2-oxazoline)–poly(d,l-lactide)–poly(2-ethyl-2-oxazoline) hydrogels. Acta Biomater. 2011, 7, 4149–4159. [Google Scholar] [CrossRef]
- Kronek, J.; Kroneková, Z.; Lustoň, J.; Paulovičová, E.; Paulovičová, L.; Mendrek, B. In vitro bio-immunological and cytotoxicity studies of poly(2-oxazolines). J. Mater. Sci. Mater. Med. 2011, 22, 1725–1734. [Google Scholar] [CrossRef]
- Kelly, A.M.; Hecke, A.; Wirnsberger, B.; Wiesbrock, F. Synthesis of poly(2-oxazoline)-based hydrogels with tailor-made swelling degrees capable of stimuli-triggered compound release. Macromol. Rapid Commun. 2011, 32, 1815–1819. [Google Scholar] [CrossRef]
- Dargaville, T.R.; Forster, R.; Farrugia, B.L.; Kempe, K.; Voorhaar, L.; Schubert, U.S.; Hoogenboom, R. Poly(2-oxazoline) hydrogel monoliths via thiol-ene coupling. Macromol. Rapid Commun. 2012, 33, 1695–1700. [Google Scholar] [CrossRef]
- Hartlieb, M.; Schubert, S.; Kempe, K.; Windhab, N.; Schubert, U.S. Stabilization of factor VIII by poly(2-oxazoline) hydrogels. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 10–14. [Google Scholar] [CrossRef]
- Zahoranová, A.; Kroneková, Z.; Zahoran, M.; Chorvát, D., Jr.; Janigová, I.; Kronek, J. Poly(2-oxazoline) hydrogels crosslinked with aliphatic bis(2-oxazoline)s: Properties, cytotoxicity, and cell cultivation. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1548–1559. [Google Scholar] [CrossRef]
- Fimberger, M.; Tsekmes, I.-A.; Kochetov, R.; Smit, J.J.; Wiesbrock, F. Crosslinked poly(2-oxazoline)s as “green” materials for electronic applications. Polymers 2016, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Šrámková, P.; Zahoranová, A.; Kroneková, Z.; Šišková, A.; Kronek, J. Poly(2-oxazoline) hydrogels by photoinduced thiol-ene “click” reaction using different dithiol crosslinkers. J. Polym. Res. 2017, 24, 82. [Google Scholar] [CrossRef]
- Van der Heide, D.J.; Verbraeken, B.; Hoogenboom, R.; Dargaville, T.R.; Hickey, D.K. Porous poly (2-oxazoline) scaffolds for developing 3D primary human tissue culture. Biomater. Tissue Technol. 2017, 1, 1–5. [Google Scholar]
- Park, J.-R.; Van Guyse, J.F.R.; Podevyn, A.; Bolle, E.C.L.; Bock, N.; Linde, E.; Celina, M.; Hoogenboom, R.; Dargaville, T.R. Influence of side-chain length on long-term release kinetics from poly(2-oxazoline)-drug conjugate networks. Eur. Polym. J. 2019, 120, 109217. [Google Scholar] [CrossRef]
- Jerca, F.A.; Anghelache, A.M.; Ghibu, E.; Cecoltan, S.; Stancu, I.-C.; Trusca, R.; Vasile, E.; Teodorescu, M.; Vuluga, D.M.; Hoogenboom, R.; et al. Poly(2-isopropenyl-2-oxazoline) hydrogels for biomedical applications. Chem. Mater. 2018, 30, 7938–7949. [Google Scholar] [CrossRef] [Green Version]
- Wloka, T.; Czich, S.; Kleinsteuber, M.; Moek, E.; Weber, C.; Gottschaldt, M.; Liefeith, K.; Schubert, U.S. Microfabrication of 3D-hydrogels via two-photon polymerization of poly(2-ethyl-2-oxazoline) diacrylates. Eur. Polym. J. 2020, 122, 109295. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, J. A Thermosensitive and biodegradable physical gel with chemically crosslinked nanogels as the building block. Macromol. Rapid Commun. 2008, 29, 751–756. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, G.; Zeng, X.; Li, J.; Li, G.; Huang, W.; Sun, R.; Wong, C. High-strength, tough, fatigue resistant, and self-healing hydrogel based on dual physically cross-linked network. ACS Appl. Mater. Interfaces 2016, 8, 24030–24037. [Google Scholar] [CrossRef]
- Witte, H.; Seeliger, W. Cyclische imidsäureester aus nitrilen und aminoalkoholen. Lieb. Ann. 1974, 6, 996–1009. [Google Scholar] [CrossRef]
- Amirova, A.; Golub, O.; Kirila, T.; Razina, A.; Tenkovtsev, A.; Filippov, A. Influence of arm length on aqueous solution behavior of thermosensitive poly(2-isopropyl-2-oxazoline) stars. Colloid Polym. Sci. 2017, 295, 117–124. [Google Scholar] [CrossRef]
- Chujo, Y.; Sada, K.; Matsumoto, K.; Saegusa, T. Synthesis of nonionic hydrogel, lipogel, and amphigel by copolymerization of 2-oxazolines and a bisoxazoline. Macromolecules 1990, 23, 1234–1237. [Google Scholar] [CrossRef]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Daoud, M.; Bouchaud, E.; Jannink, G. Swelling of polymer gels. Macromolecules 1986, 19, 1955–1960. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular size distribution in three dimensional polymers. VI. branched polymers containing A—R—Bf-1 type units. J. Am. Chem. Soc. 1952, 74, 2718–2723. [Google Scholar] [CrossRef]
- Rosen, S.L.; Rodriguez, F. Flow properties of a linear–gel polymer system. J. Appl. Polym. Sci. 1965, 9, 1601–1613. [Google Scholar] [CrossRef]
- Rosalina, I.; Bhattacharya, M. Flow curves, stress relaxation and creep measurements of starch gels. J. Texture Stud. 2007, 32, 247–269. [Google Scholar] [CrossRef]
- Tempel, M.; Isenberg, G.; Sackmann, E. Temperature-induced sol-gel transition and microgel formation in α-actinin cross-linked actin networks: A rheological study. Phys. Rev. E 1996, 54, 1802–1810. [Google Scholar] [CrossRef]
- Steed, J.W. Supramolecular gel chemistry: Developments over the last decade. Chem. Commun. 2011, 47, 1379–1383. [Google Scholar] [CrossRef]
- Bromberg, L. Scaling of rheological properties of hydrogels from associating polymers. Macromolecules 1998, 31, 6148–6156. [Google Scholar] [CrossRef]
- Glassner, M.; Vergaelen, M.; Hoogenboom, R. Poly(2-oxazoline)s: A comprehensive overview of polymer structures and their physical properties. Polym. Int. 2018, 67, 32–45. [Google Scholar] [CrossRef]
- Diehl, C.; Černoch, P.; Zenke, I.; Runge, H.; Pitschke, R.; Hartmann, J.; Tiersch, B.; Schlaad, H. Mechanistic study of the phase separation/crystallization process of poly(2-isopropyl-2-oxazoline) in hot water. Soft Matter 2010, 6, 3784–3788. [Google Scholar] [CrossRef]
- Katsumoto, Y.; Tsuchiizu, A.; Qiu, X.; Winnik, F.M. Dissecting the mechanism of the heat-induced phase separation and crystallization of poly(2-isopropyl-2-oxazoline) in water through vibrational spectroscopy and molecular orbital calculations. Macromolecules 2012, 45, 3531–3541. [Google Scholar] [CrossRef]
- Nishimura, T.; Sumi, N.; Mukai, S.; Sasaki, Y.; Akiyoshi, K. Supramacromolecular injectable hydrogels by crystallization-driven self-assembly of carbohydrateconjugated poly(2-isopropyloxazoline)s for biomedical applications. J. Mater. Chem. B 2019, 7, 6362–6369. [Google Scholar] [CrossRef]
- Amirova, A.; Rodchenko, S.; Milenin, S.; Tatarinova, E.; Kurlykin, M.; Tenkovtsev, A.; Filippov, A. Influence of a hydrophobic core on thermoresponsive behavior of dendrimer-based star-shaped poly(2-isopropyl-2-oxazoline) in aqueous solutions. J. Polym. Res. 2017, 24, 124. [Google Scholar] [CrossRef]
- Lee, J.; Park, J.M.; Jang, W.-D. Fructose-sensitive thermal transition behaviour of boronic ester-bearing telechelic poly(2-isopropyl-2-oxazoline). Chem. Commun. 2019, 55, 3343–3346. [Google Scholar] [CrossRef]
- Wang, C.-H.; Hwang, Y.-S.; Chiang, P.-R.; Shen, C.-R.; Hong, W.-H.; Hsiue, G.-H. Extended release of bevacizumab by thermosensitive biodegradable and biocompatible hydrogel. Biomacromolecules 2012, 13, 40–48. [Google Scholar] [CrossRef]
- Amirova, A.I.; Blokhin, A.N.; Razina, A.B.; Tenkovtsev, A.V.; Filippov, A.P. The behavior of thermoresponsive star-shaped poly-2-isopropyl-2-oxazoline in saline media. Mendeleev Commun. 2019, 29, 472–474. [Google Scholar] [CrossRef]
- Mishra, P.C.; Mukherjee, S.; Nayak, S.K.; Panda, A. A brief review on viscosity of nanofluids. Int. Nano Lett. 2014, 4, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Kromin, A.; Ulmer, M.P.; Wessels, B.W.; Backman, V. Nanoparticle sizing with a resolution beyond the diffraction limit using UV light scattering spectroscopy. Opt. Commun. 2003, 228, 1–7. [Google Scholar] [CrossRef]
- Adam, M.; Delsanti, M. Viscosity of semi-dilute polymer solutions. J. Phys. (Paris) 1982, 43, 549–557. [Google Scholar] [CrossRef]
- Croucher, M.D.; Milkie, T.H. Temperature dependence of the shear viscosity of sterically stabilised polymer colloids. Faraday Discuss. Chem. Soc. 1983, 76, 261–276. [Google Scholar] [CrossRef]
- Yu, L.; Chang, G.; Zhang, H.; Ding, J. Temperature-induced spontaneous sol-gel transitions of poly(d,l-lactic acid-co-glycolic acid)-b-poly(ethylene glycol)-b-poly(D,L-lactic acid-co-glycolic acid) triblock copolymers and their end-capped derivatives in water. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 1122–1133. [Google Scholar] [CrossRef]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Krasnou, I.; Tarabukina, E.; Melenevskaya, E.; Filippov, A.; Aseyev, V.; Hietala, S.; Tenhu, H. Rheological behavior of poly(vinylpyrrolidone)/fullerene c60 complexes in aqueous medium. J. Macromol. Sci. B 2008, 47, 500–510. [Google Scholar] [CrossRef]
- Chassenieux, C.; Nicolai, T.; Benyahia, L. Rheology of associative polymer solutions. Curr. Opin. Colloid Interface Sci. 2011, 16, 18–26. [Google Scholar] [CrossRef]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amirova, A.; Rodchenko, S.; Kurlykin, M.; Tenkovtsev, A.; Krasnou, I.; Krumme, A.; Filippov, A. Synthesis and Investigation of Thermo-Induced Gelation of Partially Cross-Linked Poly-2-isopropyl-2-oxazoline in Aqueous Media. Polymers 2020, 12, 698. https://doi.org/10.3390/polym12030698
Amirova A, Rodchenko S, Kurlykin M, Tenkovtsev A, Krasnou I, Krumme A, Filippov A. Synthesis and Investigation of Thermo-Induced Gelation of Partially Cross-Linked Poly-2-isopropyl-2-oxazoline in Aqueous Media. Polymers. 2020; 12(3):698. https://doi.org/10.3390/polym12030698
Chicago/Turabian StyleAmirova, Alina, Serafim Rodchenko, Mikhail Kurlykin, Andrey Tenkovtsev, Illia Krasnou, Andres Krumme, and Alexander Filippov. 2020. "Synthesis and Investigation of Thermo-Induced Gelation of Partially Cross-Linked Poly-2-isopropyl-2-oxazoline in Aqueous Media" Polymers 12, no. 3: 698. https://doi.org/10.3390/polym12030698