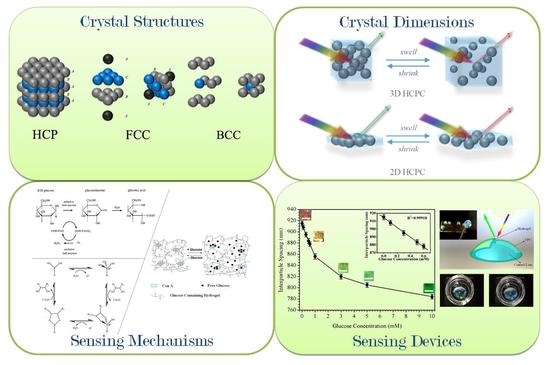
Hydrogel-Based Colloidal Photonic Crystal Devices for Glucose Sensing
Abstract
:1. Introduction
2. Preparation of CPCs and HCPCs
2.1. CPCs by Self-Assembly of Colloidal Particles
2.1.1. Opaline 3D CPCs
2.1.2. Inverted Opaline 3D CPCs
2.1.3. D CPCs
2.2. Hydrogel-Based CPCs (HCPCs)
3. HCPC Glucose-Sensing Materials and Devices
3.1. History of HCPC Glucose Sensor Materials
3.1.1. GOx-Immobilized HCPC
3.1.2. Con A Complex HCPC
3.1.3. BA-derived HCPC
3.2. Current and Emerging Devices
3.2.1. D HCPC Glucose Sensors
3.2.2. Contact Lens
3.2.3. Other Emerging Devices
4. Summary and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Yablonovitch, E. Inhibited spontaneous emission in solid-state physics and electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef] [Green Version]
- John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486–2489. [Google Scholar] [CrossRef] [Green Version]
- Noda, S.; Tomoda, K.; Yamamoto, N.; Chutinan, A. Full three-dimensional photonic bandgap crystals at near-infrared wavelengths. Science 2000, 289, 604–606. [Google Scholar] [CrossRef]
- Juodkazis, S.; Rosa, L.; Bauerdick, S.; Peto, L.; El-Ganainy, R.; John, S. Sculpturing of photonic crystals by ion beam lithography: Towards complete photonic bandgap at visible wavelengths. Opt. Express 2011, 19, 5802–5810. [Google Scholar] [CrossRef]
- Subramania, G.; Lee, Y.J.; Fischer, A.J.; Koleske, D.D. Log-pile TiO2 photonic crystal for light control at near-UV and visible wavelengths. Adv. Mater. 2010, 22, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, X.L.; Li, Z.H.; Zhu, Z.G.; Qian, S.H.; Flewitt, A.J. Current and emerging technology for continuous glucose monitoring. Sensors 2017, 17, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Kurihara, S. Photochemical on-off switching of one-dimensional photonic crystals consisting of azo-functionalized liquid crystal polymer and polyvinyl alcohol. Crystals 2019, 9, 610. [Google Scholar] [CrossRef] [Green Version]
- Arsenault, A.C.; Puzzo, D.P.; Manners, I.; Ozin, G.A. Photonic-crystal full-colour displays. Nat. Photonics 2007, 1, 468–472. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Dong, Z.Q.; Chen, H.W.; Chen, Y.; Zhu, Z.G.; Shih, W.H. Two-dimensional photonic crystals. Prog. Chem. 2018, 30, 775–784. [Google Scholar] [CrossRef]
- Chen, S.L.; Wang, A.J.; Dai, C.; Benziger, J.B.; Liu, X.C. The effect of photonic band gap on the photo-catalytic activity of nc-TiO2/SnO2 photonic crystal composite membranes. Chem. Eng. J. 2014, 249, 48–53. [Google Scholar] [CrossRef]
- Akram, B.; Wang, X. Self-assembly of ultrathin nanocrystals to multidimensional superstructures. Langmuir 2019, 35, 10246–10266. [Google Scholar] [CrossRef]
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-assembly of colloidal nanocrystals: From intricate structures to functional materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.B.; Ravaine, S. Bottom-up assembly and applications of photonic materials. Crystals 2016, 5, 54. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.B.; Sanders, J.V.; Segnit, E.R. Structure of opal. Nature 1964, 204, 990–991. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Wang, J.X.; Wen, Y.Q.; Ge, H.L.; Sun, Z.W.; Zheng, Y.M.; Song, Y.L.; Jiang, L. Simple fabrication of full color colloidal crystal films with tough mechanical strength. Macromol. Chem. Phys. 2006, 207, 596–604. [Google Scholar] [CrossRef]
- Cui, L.Y.; Zhang, Y.Z.; Wang, J.X.; Ren, Y.B.; Song, Y.L.; Jiang, L. Ultra-fast fabrication of colloidal photonic crystals by spray coating. Macromol. Rapid Commun. 2009, 30, 598–603. [Google Scholar] [CrossRef]
- Li, Q.; Jonas, U.; Zhao, X.S.; Kappl, M. The forces at work in colloidal self-assembly: A review on fundamental interactions between colloidal particles. Asia-Pac. J. Chem. Eng. 2008, 3, 255–268. [Google Scholar] [CrossRef]
- Woodcock, L.V. Entropy difference between the face-centred cubic and hexagonal close-packed crystal structures. Nature 1997, 385, 141–143. [Google Scholar] [CrossRef]
- Bolhuis, P.G.; Frenkel, D.; Mau, S.C.; Huse, D.A. Entropy difference between crystal phases. Nature 1997, 388, 235–236. [Google Scholar] [CrossRef]
- Mau, S.C.; Huse, D.A. Stacking entropy of hard-sphere crystals. Phys. Rev. E 1999, 59, 4396–4401. [Google Scholar] [CrossRef] [Green Version]
- Norris, D.J.; Arlinghaus, E.G.; Meng, L.L.; Heiny, R.; Scriven, L.E. Opaline photonic crystals: How does self-assembly work? Adv. Mater. 2004, 16, 1393–1399. [Google Scholar] [CrossRef]
- Rundquist, P.A.; Photinos, P.; Jagannathan, S.; Asher, S.A. Dynamical Bragg diffraction from crystalline colloidal arrays. J. Chem. Phys. 1989, 91, 4932–4941. [Google Scholar] [CrossRef]
- Cong, H.L.; Cao, W.X. Array patterns of binary colloidal crystals. J. Phys. Chem. B 2005, 109, 1695–1698. [Google Scholar] [CrossRef]
- Li, J.L.; Zhang, S.; Chen, H.H.; Gu, Z.Z.; Lu, Z.H. Three-dimensional non-close-packed arrays formed by soft PMMA spheres. Colloid Surf. A 2007, 299, 54–57. [Google Scholar] [CrossRef]
- Meseguer, F.; Blanco, A.; Míguez, H.; García–Santamaría, F.; Ibisate, M.; López, C. Synthesis of inverse opals. Colloid Surf. A 2002, 202, 281–290. [Google Scholar] [CrossRef]
- Zhou, J.M.; Li, H.L.; Ye, L.; Liu, J.; Wang, J.X.; Zhao, T.; Jiang, L.; Song, Y.L. Facile fabrication of tough SiC inverse opal photonic crystals. J. Phys. Chem. C 2014, 114, 22303–22308. [Google Scholar] [CrossRef]
- Waterhouse, G.I.N.; Chen, W.T.; Chan, A.; Sun-Waterhouse, D.X. Achieving color and function with structure: Optical and catalytic support properties of ZrO2 inverse opal thin films. ACS Omega 2018, 3, 9658–9674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.H.; Xia, Y.N. Macroporous membranes with highly ordered and three-dimensionally interconnected spherical pores. Adv. Mater. 1998, 10, 1045–1048. [Google Scholar] [CrossRef]
- Jiang, H.L.; Yang, X.L.; Chen, C.; Zhu, Y.H.; Li, C.Z. Facile and controllable fabrication of three-dimensionally quasi-ordered macroporous TiO2 for high performance lithium-ion battery applications. New J. Chem. 2013, 37, 1578–1583. [Google Scholar] [CrossRef]
- Jiang, P.; McFarland, M.J. Wafer-scale periodic nanohole arrays templated from two-dimensional nonclose-packed colloidal crystals. J. Am. Chem. Soc. 2005, 127, 3710–3711. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Hong, G.S.; Qi, L.M. Nanosphere lithography at the gas/liquid interface: A general approach toward free-standing high-quality nanonets. Chem. Mater. 2010, 22, 476–481. [Google Scholar] [CrossRef]
- Vogel, N.; Retsch, M.; Fustin, C.; del Campo, A.; Jonas, U. Advances in colloidal assembly: The design of structure and hierarchy in two and three dimensions. Chem. Rev. 2015, 115, 6265–6311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, Z.J.; Yu, Z.Y.; Ling, L.T.; Wang, C.F.; Chen, S. Large-scale colloidal films with robust structural colors. Mater. Horiz. 2019, 6, 90–96. [Google Scholar] [CrossRef]
- Cagnani, G.R.; Spada, E.R.; Cagnani, L.D.; Torres, B.B.M.; Balogh, D.T.; Bardosova, M.; Faria, R.M. Large-area flexible 2D-colloidal crystals produced directly using roll-to-roll processing. Colloid Surf. A 2020, 588, 124389. [Google Scholar] [CrossRef]
- Villaescusa, L.A.; Mihi, A.; Rodríguez, I.; García-Bennett, A.E.; Míguez, H. Growth of mesoporous materials within colloidal crystal films by spin-coating. J. Phys. Chem. B 2005, 109, 19643–19649. [Google Scholar] [CrossRef] [PubMed]
- Alfrey, T.; Bradford, E.B.; Vanderhoff, J.W.; Oster, G. Optical properties of uniform particle-size latexes. J. Opt. Soc. Am. 1954, 44, 603–609. [Google Scholar] [CrossRef]
- Velev, O.D.; Denkov, N.D.; Kralchevsky, P.A.; Ivanovl, I.B.; Yoshimura, H.; Nagayama, K. Mechanism of formation of two-dimensional crystals from later particles on substrata. Prog. Colloid Polym. Sci. 1993, 93, 366–367. [Google Scholar] [CrossRef]
- Dimitrov, A.S.; Nagayama, K. Continuous convective assembling of fine particles into two-dimensional arrays on solid surfaces. Langmuir 1996, 12, 1303–1311. [Google Scholar] [CrossRef]
- Scriven, L.E.; Sternling, C.V. The marangoni effects. Nature 1960, 187, 186–188. [Google Scholar] [CrossRef]
- Zhang, J.T.; Wang, L.L.; Lamont, D.N.; Velankar, S.S.; Asher, S.A. Fabrication of large-area two-dimensional colloidal crystals. Angew. Chem. Int. Ed. 2012, 51, 6117. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.Q.; He, J.; Zhou, S.Q.; Yang, X.; Li, S.Z.; Sheng, J.; Wang, D.; Yu, T.B.; Ye, J.C.; Cui, Y. Large-area nanosphere self-assembly by a micro-propulsive injection method for high throughput periodic surface nanotexturing. Nano Lett. 2015, 15, 4591–4598. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 49–60. [Google Scholar] [CrossRef]
- Ge, J.P.; Yin, Y.D. Responsive photonic crystals. Angew. Chem. Int. Ed. 2011, 50, 1492–1522. [Google Scholar] [CrossRef]
- Zheng, D.; Sun, L.G.; Xie, Z.Y.; Xiong, G.R.; Xu, H.; Gu, Z.Z. Preparation of tunable colloidal crystals based on temperature-sensitive hydrogel. Chem. J. Chin. Univ. 2008, 29, 618–622. [Google Scholar]
- Zheng, D.; Chen, H.H.; Zhang, S.; Wang, J.; Gu, Z.Z. Crystallization of colloidal crystal on hydrogel surface. Colloid Surf. A 2007, 292, 63–68. [Google Scholar] [CrossRef]
- Asher, S.A.; Holtz, J.; Liu, L.; Wu, Z.J. Self-assembly motif for creating submicron periodic materials. Polymerized crystalline colloidal arrays. J. Am. Chem. Soc. 1994, 116, 4997–4998. [Google Scholar] [CrossRef]
- Liu, L.; Li, P.S.; Asher, S.A. Entropic trapping of macromolecules by mesoscopic periodic voids in a polymer hydrogel. Nature 1999, 397, 141–144. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, Y.H.; Bao, H.; Yang, X.L.; Li, C.Z. Physically controlled cross-linking in gelated crystalline colloidal array photonic crystals. ACS Appl. Mater. Interfaces 2010, 2, 1499–1504. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, Y.H.; Bao, H.; Zhao, P.; Jiang, H.L.; Peng, L.M.; Yang, X.L.; Li, C.Z. Solvent-assisted poly (vinyl alcohol) gelated crystalline colloidal array photonic crystals. Soft Matter 2011, 7, 915–921. [Google Scholar] [CrossRef]
- Cui, Q.Z.; Wang, W.; Gu, B.H.; Liang, L.Y. A combined physical-chemical polymerization process for fabrication of nanoparticle-hydrogel sensing materials. Macromolecules 2012, 45, 8382–8386. [Google Scholar] [CrossRef]
- Coukouma, A.E.; Smith, N.L.; Asher, S.A. Removable interpenetrating network enables highly-responsive 2-D photonic crystal hydrogel sensors. Analyst 2015, 140, 6517–6521. [Google Scholar] [CrossRef]
- Holtz, J.H.; Asher, S.A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 1997, 389, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Witt, S.; Wohlfahrt, G.; Schomburg, D.; Hecht, H.J.; Kalisz, H.M. Conserved arginine-516 of Penicillium Amagasakiense glucose oxidase is essential for the efficient binding of β-D-glucose. Biochem. J. 2000, 347, 553–559. [Google Scholar] [CrossRef]
- Kamenjicki, M.; Asher, S.A. Epoxide functionalized polymerized crystalline colloidal arrays. Sens. Actuator B Chem. 2005, 106, 373–377. [Google Scholar] [CrossRef]
- Yin, R.X.; Tong, Z.; Yang, D.Z.; Nie, J. Glucose and pH dual-responsive concanavalin A based microhydrogels for insulin delivery. Int. J. Biol. Macromol. 2011, 49, 1137–1142. [Google Scholar] [CrossRef]
- Steiner, M.S.; Duerkop, A.; Wolfbeis, O.S. Optical methods for sensing glucose. Chem. Soc. Rev. 2011, 40, 4805–4839. [Google Scholar] [CrossRef]
- Alexeev, V.L.; Das, S.; Finegold, D.N.; Asher, S.A. Photonic crystal glucose-sensing material for noninvasive monitoring of glucose in tear fluid. Clin. Chem. 2004, 50, 2353–2360. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Guan, Y.; Zhang, Y.J. Thin hydrogel films based on lectin-saccharide biospecific interaction for label-free optical glucose sensing. Sens. Actuator B Chem. 2018, 272, 243–251. [Google Scholar] [CrossRef]
- Dai, Y.; Bao, H.; Lin, J.P.; Foulger, S.H. Synthesis of polymerized crystalline colloidal array hydrogel film with glucose stimulated stop band shift. J. East China Univ. Sci. Technol. 2007, 33, 350–353. [Google Scholar]
- Cui, Q.Z.; Muscatello, M.M.W.; Asher, S.A. Photonic crystal borax competitive binding carbohydrate sensing motif. Analyst 2009, 134, 875–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Fang, H.; Wang, B.H. Boronolectins and fluorescent boronolectins: An examination of the detailed chemistry issues important for the design. Med. Res. Rev. 2005, 25, 490–520. [Google Scholar] [CrossRef] [PubMed]
- Asher, S.A.; Alexeev, V.L.; Goponenko, A.V.; Sharma, A.C.; Lednev, I.K.; Wilcox, C.S.; Finegold, D.N. Photonic crystal carbohydrate sensors: Low ionic strength sugar sensing. J. Am. Chem. Soc. 2003, 125, 3322–3329. [Google Scholar] [CrossRef] [PubMed]
- Alexeev, V.L.; Sharma, A.C.; Goponenko, A.V.; Das, S.; Lednev, I.K.; Wilcox, C.S.; Finegold, D.N.; Asher, S.A. High ionic strength glucose-sensing photonic crystal. Anal. Chem. 2003, 75, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Pruzinsky, S.A.; Braun, P.V. Glucose-sensitive inverse opal hydrogels: Analysis of optical diffraction response. Langmuir 2004, 20, 3096–3106. [Google Scholar] [CrossRef]
- Nakayama, D.; Takeoka, Y.; Watanabe, M.; Kataoka, K. Simple and precise preparation of a porous gel for a colorimetric glucose sensor by a templating technique. Angew. Chem. Int. Ed. 2003, 42, 4197–4200. [Google Scholar] [CrossRef]
- Lane, J.D.; Krumholz, D.M.; Sack, R.A.; Morris, C. Tear glucose dynamics in diabetes mellitus. Curr. Eye Res. 2006, 31, 895–901. [Google Scholar] [CrossRef]
- Pirnstill, C.W.; Malik, B.H.; Gresham, V.C.; Coté, G.L. In vivo glucose monitoring using dual-wavelength polarimetry to overcome corneal birefringence in the presence of motion. Diabetes Technol. Ther. 2012, 14, 819–827. [Google Scholar] [CrossRef]
- Rico-Yuste, A.; Carrasco, S. Molecularly imprinted polymer-based hybrid materials for the development of optical sensors. Polymers 2019, 11, 1173. [Google Scholar] [CrossRef] [Green Version]
- Xue, F.; Duan, T.R.; Huang, S.Y.; Wang, Q.H.; Xue, M.; Meng, Z.H. A covalently imprinted photonic crystal for glucose sensing. J. Nanomater. 2013, 530701. [Google Scholar] [CrossRef]
- Feng, X.Q.; Xu, J.; Liu, Y.X.; Zhao, W.P. Visual sensors of an inverse opal hydrogel for the colorimetric detection of glucose. J. Mater. Chem. B 2019, 7, 3576–3581. [Google Scholar] [CrossRef]
- Huang, C.; Cheng, Y.; Gao, Z.W.; Zhang, H.B.; Wei, J. Portable label-free inverse opal photonic hydrogel particles serve as facile pesticides colorimetric monitoring. Sens. Actuator B Chem. 2018, 273, 1705–1712. [Google Scholar] [CrossRef]
- Prevo, B.G.; Velev, O.D. Controlled, rapid deposition of structured coatings from micro- and nanoparticle suspensions. Langmuir 2004, 20, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, X.H.; Dong, Z.Q.; Chen, G.; Han, L.X.; Zhu, Z.G. Anti-counterfeiting layer of 2D colloidal crystal based photonic material. Mater. Sci. Forum. 2019, 972, 185–190. [Google Scholar] [CrossRef]
- Kanai, T.; Sawada, T.; Kitamura, K. Optical determination of the lattice constants of colloidal crystals without use of the refractive index. Langmuir 2003, 19, 1984–1986. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Luck, L.A.; Punihaole, D.; Madura, J.D.; Asher, S.A. Photonic crystal protein hydrogel sensor materials enabled by conformationally induced volume phase transition. Chem. Sci. 2016, 7, 4557–4562. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.Q.; Xue, M.; He, Q.; Lu, W.; Meng, Z.H.; Yan, D.; Qiu, L.L.; Zhou, L.J.; Yu, Y.J. A non-enzymatic urine glucose sensor with 2-D photonic crystal hydrogel. Anal. Bioanal. Chem. 2016, 408, 8317–8323. [Google Scholar] [CrossRef]
- Lan, Y.H.; Xue, M.; Qiu, L.L.; Meng, Z.H. Clinical evaluation of a photonic crystal sensor for glucose monitoring in urine. ChemistrySelect 2019, 4, 6547–6551. [Google Scholar] [CrossRef]
- Xue, F.; Meng, Z.H.; Wang, F.Y.; Wang, Q.H.; Xue, M.; Xu, Z.B. A 2-D photonic crystal hydrogel for selective sensing of glucose. J. Mater. Chem. A 2014, 2, 9559–9565. [Google Scholar] [CrossRef]
- Chen, C.; Dong, Z.Q.; Shen, J.H.; Chen, H.W.; Zhu, Y.H.; Zhu, Z.G. 2D photonic crystal hydrogel sensor for tear glucose monitoring. ACS Omega 2018, 3, 3211–3217. [Google Scholar] [CrossRef]
- Li, W.J.; Xiang, J.H.; Men, D.D.; Zhang, H.H. 2D Au nanosphere arrays/PVA-PBA-modified-hydrogel composite film for glucose detection with strong diffraction intensity and linear response. Nanomaterials 2019, 9, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. A glucose sensing contact lens: A non-invasive technique for continuous physiological glucose monitoring. J. Fluoresc. 2003, 13, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Lakdawala, D.H.; Shaikh, A.A.; Desai, A.R.; Choksi, H.H.; Vaidya, R.J.; Ranch, K.M.; Koli, A.R.; Vyas, B.A.; Shah, D.O. In vitro and in vivo evaluation of novel implantation technology in hydrogel contact lenses for controlled drug delivery. J. Controll. Release 2016, 226, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.R.; Hung, C.C.; Chiu, H.Y.; Chang, P.H.; Li, B.R.; Cheng, S.J.; Yang, J.W.; Lin, S.F.; Chen, G.Y. Noninvasive glucose monitoring with a contact lens and smartphone. Sensors 2018, 18, 3208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreddu, R.; Vigolo, D.; Yetisen, A.K. Contact lens technology: From fundamentals to applications. Adv. Healthc. Mater. 2019, 8, 1900368. [Google Scholar] [CrossRef]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Wearable contact lens biosensors for continuous glucose monitoring using smartphones. ACS Nano 2018, 12, 5452–5462. [Google Scholar] [CrossRef]
- Xie, Z.Y.; Li, L.L.; Liu, P.M.; Zheng, F.Y.; Guo, L.Y.; Zhao, Y.J.; Jin, L.; Li, T.T.; Gu, Z.Z. Self-assembled coffee-ring colloidal crystals for structurally colored contact lenses. Small 2015, 11, 926–930. [Google Scholar] [CrossRef]
- Lai, C.F.; Li, J.S.; Fang, Y.T.; Chien, C.J.; Lee, C.H. UV and blue-light anti-reflective structurally colored contact lenses based on a copolymer hydrogel with amorphous array nanostructures. RSC Adv. 2018, 8, 4006–4013. [Google Scholar] [CrossRef] [Green Version]
- Riaz, R.S.; Elsherif, M.; Moreddu, R.; Rashid, I.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Anthocyanin-functionalized contact lens sensors for ocular pH monitoring. ACS Omega 2019, 4, 21792–21798. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.Z.; Chen, S.; Chen, J.L.; Ding, H.L.; Deng, D.W.; Xie, Z.Y. Self-reporting colorimetric analysis of drug release by molecular imprinted structural color contact lens. ACS Appl. Mater. Interface 2018, 10, 34611–34617. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Zhao, Q.L.; Du, X.M. Structurally coloured contact lens sensor for point-of-care ophthalmic health monitoring. J. Mater. Chem. B 2020. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.L.; Chen, C.; Shen, J.H.; Zhao, X.L.; Qian, S.H.; Zhu, Z.G. A gelated colloidal crystal attached lens for noninvasive continuous monitoring of tear glucose. Polymers 2017, 9, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, F.; Asher, S.A.; Meng, Z.H.; Wang, F.Y.; Lu, W.; Xue, M.; Qi, F.L. Two-dimensional colloidal crystal heterostructures. RSC Adv. 2015, 5, 18939–18944. [Google Scholar] [CrossRef]
- Men, D.D.; Zhang, H.H.; Hang, L.F.; Liu, D.L.; Li, X.Y.; Cai, W.P.; Xiong, Q.H.; Li, Y. Optical sensor based on hydrogel films with 2D colloidal arrays attached on both the surfaces: Anti-curling performance and enhanced optical diffraction intensity. J. Mater. Chem. C 2015, 3, 3659–3665. [Google Scholar] [CrossRef]
- Deng, C.S.; Pan, T.T.; Du, L.; Wang, M.; Ni, H.B.; Ni, X.Q. Fabrication of monolayer crystalline films on optical fiber end by micro-flow injection method. Chem. J. Chin. Univ. 2018, 9, 708–713. [Google Scholar] [CrossRef]
- Chen, X.J.; Liu, G.Q.; Ren, C.R.; Gao, M.J.; Fan, X.D. Investigation on sulfamethazine molecularly imprinted two-dimensional photonic crystal hydrogel sensor. Chem. J. Chin. Univ. 2018, 39, 212–218. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Fu, Q.Q.; Ge, J.P. Test-paper-like photonic crystal viscometer. Small 2017, 13, 1603351. [Google Scholar] [CrossRef]
- Fu, Q.Q.; Zhu, B.T.; Ge, J.P. Hierarchically structured photonic crystals for integrated chemical separation and colorimetric detection. Nanoscale 2017, 9, 2457–2463. [Google Scholar] [CrossRef]
- Diouf, A.; Bouchikhi, B.; El Bari, N. A nonenzymatic electrochemical glucose sensor based on molecularly imprinted polymer and its application in measuring saliva glucose. Mater. Sci. Eng. C Mater. 2019, 98, 1196–1209. [Google Scholar] [CrossRef]
- Elsherif, M.; Hassan, M.U.; Yetisen, A.K.; Butt, H. Glucose sensing with phenylboronic acid functionalized hydrogel-based optical diffusers. ACS Nano 2018, 12, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Moon, H.; Kim, H.; Lee, G.H.; Kwon, W.S.; Yoo, S.; Myung, D.; Yun, S.H.; Bao, Z.N.; Hahn, S.K. Multifunctional materials for implantable and wearable photonic healthcare devices. Nat. Rev. Mater. 2020, 5, 149–165. [Google Scholar] [CrossRef]
- Yi, W.; Xiong, D.B.; Zhang, D. Biomimetic and bioinspired photonic structures. Nano Adv. 2016, 1, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.H.; Choi, T.M.; Kim, B.; Han, S.H.; Lee, J.M.; Kim, S.H. Chameleon-inspired mechanochromic photonic films composed of non-close-packed colloidal arrays. ACS Nano 2017, 11, 11350–11357. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.X.; Bazrafshan, A.; Pokutta, A.; Sulejmani, F.; Sun, W.; Combs, J.D.; Clarke, K.C.; Salaita, K. Chameleon-inspired strain-accommodating smart skin. ACS Nano 2019, 13, 9918–9926. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, H.; Ho, J.F.; Ng, R.C.; Ng, R.J.H.; Hall-Chen, V.H.; Koay, E.H.H.; Dong, Z.G.; Liu, H.L.; Qiu, C.W.; et al. Structural color three-dimensional printing by shrinking photonic crystals. Nat. Commun. 2019, 10, 4340. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Tang, W.W.; Wang, X.H.; Zhao, X.L.; Chen, C.; Zhu, Z.G. Applications of hydrogels with special physical properties in biomedicine. Polymers 2019, 11, 1420. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yu, F.; Chen, G.; Liu, J.; Li, X.L.; Cheng, B.; Mo, X.M.; Chen, C.; Pan, J.F. Moist-retaining, self-recoverable, bioadhesive, and transparent in situ forming hydrogels to accelerate wound healing. ACS Appl. Mater. Interface 2020, 12, 2023–2038. [Google Scholar] [CrossRef]






| Type | Hydrogel | Detection Limit (mM) | PBG Shift (nm) | Linearity Range (mM) | Sensitivity (mM/nm) 1 | Response Time | Detection Medium | Ref |
|---|---|---|---|---|---|---|---|---|
| GOx | PAM | 0.1–0.5 | ~115 | NA | NA | <2 min | NA | [57] |
| epoxy-PAM | 0.1–1 | 86 | 0–0.2 | 430 | <5 min | NA | [53] | |
| Con A | PAM/Con A | 5–25 | 20 | 0–10 | ~2 | NA | PBS | [59] |
| BA | PVA/borax | 0.33–40 | ~62 | 0–40 | ~1.55 | <2~3 min | PBS | [60] |
| APBA-PAM | 0.05–100 | 128 | 0–5 | ~6.2 | NA | Tris-HCl | [62] | |
| APBA-PEG-co-PAM | 0.2–50 | 64 B-shift 20 R-shift | 0–8 8–50 | 8 0.48 | NA | Tris-HCl | [63] | |
| APBA-PHEMA | 0.1–100 | 120 | NA | NA | ~45 S | CHES | [64] | |
| APBA-PNIPAM | 5–20 | ~83 | 5–20 | ~5.53 | NA | CHES | [65] | |
| AFPBA-PEG-co-PAM | 0.001–40 | 234 | NA | NA | NA | ATF | [66] |
| Hydrogel | Detection Limit (mM) | PBG Shift (nm) | Linearity Range (mM) | Response Time | Detection Medium | Ref |
|---|---|---|---|---|---|---|
| protein hydrogel | 1.24 × 10−5-10 | 20 | NA | ~20 min | PBS | [76] |
| APBA-PAM-co-AA | 0–15 | NA | 0-1 | <230 S | artificial urea | [77] |
| APBA-PAM-co-AA | 0.4–53.3 | 80 (0.1–10 mM) | 0.1–2 | 20 min | Clinical urine | [78] |
| APBA-PAM-co-AA | 0–20 | NA | NA | <3 min | CHES and ATF | [79] |
| BBA-PVA | 0.1–10 | 25 (0.1–0.6 mM) | 01–0.6 | <200 S | ATF | [80] |
| APBA-PAM-PVA | 2–80 | 120 | 0–20 | NA | CHES | [81] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Chen, C. Hydrogel-Based Colloidal Photonic Crystal Devices for Glucose Sensing. Polymers 2020, 12, 625. https://doi.org/10.3390/polym12030625
Tang W, Chen C. Hydrogel-Based Colloidal Photonic Crystal Devices for Glucose Sensing. Polymers. 2020; 12(3):625. https://doi.org/10.3390/polym12030625
Chicago/Turabian StyleTang, Wenwei, and Cheng Chen. 2020. "Hydrogel-Based Colloidal Photonic Crystal Devices for Glucose Sensing" Polymers 12, no. 3: 625. https://doi.org/10.3390/polym12030625