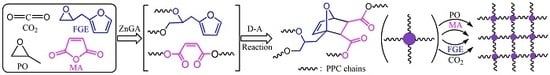
A Novel One-Pot Synthesis of Poly(Propylene Carbonate) Containing Cross-Linked Networks by Copolymerization of Carbon Dioxide, Propylene Oxide, Maleic Anhydride, and Furfuryl Glycidyl Ether
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Copolymerization Procedure
2.3. Characterization and Measurements
3. Results and discussions
3.1. Synthesis
3.2. Thermal Properties
3.3. Dimensional Stability
3.4. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Beckman, E.J. Making polymers from carbon dioxide. Science 1999, 283, 946–947. [Google Scholar] [CrossRef]
- Xu, Y.H.; Lin, L.M.; Xiao, M.; Wang, S.J.; Smith, A.T.; Sun, L.Y.; Meng, Y.Z. Synthesis and properties of CO2-based plastics: Environmentally-friendly, energy-saving and biomedical polymeric materials. Prog. Polym. Sci. 2018, 80, 163–182. [Google Scholar] [CrossRef]
- Luinstra, G.A.; Borchardt, E. Material properties of poly(propylene carbonates). Adv. Polym. Sci. 2011, 245, 29–48. [Google Scholar] [CrossRef]
- Chen, L.J.; Qin, Y.S.; Wang, X.H.; Li, Y.S.; Zhao, X.J.; Wang, F.S. Toughening of poly(propylene carbonate) by hyperbranched poly(ester-amide) via hydrogen bonding interaction. Polym. Int. 2011, 60, 1697–1704. [Google Scholar] [CrossRef]
- Nakano, K.; Hashimoto, S.I.; Nakamura, M.; Kamada, T.; Nozaki, K. Stereocomplex of poly (propylene carbonate): Synthesis of stereogradient poly(propylene carbonate) by regio-and enantioselective copolymerization of propylene oxide with carbon dioxide. Angew. Chem. Int. Ed. 2011, 150, 4868–4871. [Google Scholar] [CrossRef]
- Li, B.; Wu, G.P.; Ren, W.M.; Wang, Y.M.; Rao, D.Y.; Lu, X.B. Asymmetric, region-and stereo-selective alternating copolymerization of CO2 and propylene oxide catalyzed by chiral chromium Salan complexes. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6102–6113. [Google Scholar] [CrossRef]
- Peng, S.W.; An, Y.X.; Chen, C.; Fei, B.; Zhuang, Y.G.; Dong, L.S. Thermal degradation kinetics of uncapped and end-capped poly (propylene carbonate). Polym. Degrad. Stab. 2003, 80, 141–147. [Google Scholar] [CrossRef]
- An, J.J.; Ke, Y.C.; Cao, X.Y.; Ma, Y.M.; Wang, F.S. A novel method to improve the thermal stability of poly (propylene carbonate). Polym. Chem. 2014, 5, 4245–4250. [Google Scholar] [CrossRef]
- Shi, L.; Lu, X.B.; Zhang, R.; Peng, X.J.; Zhang, C.Q.; Li, J.F.; Peng, X.M. Asymmetric alternating copolymerization and terpolymerization of epoxides with carbon dioxide at mild conditions. Macromolecules 2006, 39, 5679–5685. [Google Scholar] [CrossRef]
- Song, P.F.; Xiao, M.; Du, F.G.; Wang, S.J.; Gan, L.Q.; Liu, G.Q.; Meng, Y.Z. Synthesis and properties of aliphatic polycarbonates derived from carbon dioxide, propylene oxide and maleic anhydride. J. Appl. Polym. Sci. 2008, 109, 4121–4129. [Google Scholar] [CrossRef]
- Seong, J.E.; Na, S.J.; Cyriac, A.; Kim, B.W.; Lee, B.Y. Terpolymerizations of CO2, propylene oxide, and various epoxides using a Cobalt (III) complex of salen-type ligand tethered by four quaternary ammonium salts. Macromolecules 2010, 43, 903–908. [Google Scholar] [CrossRef]
- Song, P.F.; Xu, H.D.; Mao, X.D.; Liu, X.J.; Wang, L. A one-step strategy for aliphatic poly(carbonate-ester)s with high performance derived from CO2, propylene oxide and L-lactide. Polym. Adv. Technol. 2017, 28, 736–741. [Google Scholar] [CrossRef]
- Tao, Y.H.; Wang, X.H.; Zhao, X.J.; Li, J.; Wang, F.S. Crosslinkable poly(propylene carbonate): High-yield synthesis and performance improvement. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5329–5336. [Google Scholar] [CrossRef]
- Song, P.F.; Wang, S.J.; Xiao, M.; Du, F.G.; Gan, L.Q.; Liu, G.Q.; Meng, Y.Z. Cross-linkable and thermally stable aliphatic polycarbonates derived from CO2, propylene oxide and maleic anhydride. J. Polym. Res. 2009, 16, 91–97. [Google Scholar] [CrossRef]
- Cyriac, A.; Lee, S.H.; Lee, B.Y. Connection of polymer chains using diepoxide in CO2/propylene oxide copolymerizations. Polym. Chem. 2011, 2, 950–956. [Google Scholar] [CrossRef]
- Zeng, S.S.; Wang, S.J.; Xiao, M.; Han, D.M.; Meng, Y.Z. Preparation and properties of biodegradable blend containing poly(propylene carbonate) and starch acetate with different degrees of substitution. Carbohydr. Polym. 2011, 86, 1260–1265. [Google Scholar] [CrossRef]
- Kuang, T.R.; Li, K.C.; Chen, B.Y.; Peng, X.F. Poly(propylene carbonate)-based in situ nanofibrillar biocomposites with enhanced miscibility, dynamic mechanical properties, rheological behavior and extrusion foaming ability. Compos. Part B Eng. 2017, 123, 112–123. [Google Scholar] [CrossRef]
- Enriquez, E.; Mohanty, A.K.; Misra, M. Biobased blends of poly(propylene carbonate) and poly(hydroxybutyrate-co-hydroxyvalerate): Fabrication and characterization. J. Appl. Polym. Sci. 2017, 134, 44420. [Google Scholar] [CrossRef]
- Chen, S.S.; Chen, B.; Fan, J.S.; Feng, J.C. Exploring the application of sustainable poly(propylene carbonate) copolymer in toughening epoxy thermosets. ACS Sustain. Chem. Eng. 2015, 3, 2077–2083. [Google Scholar] [CrossRef]
- Yang, J.; Pan, H.W.; Li, X.; Sun, S.L.; Zhang, H.L.; Dong, L.S. A study on the mechanical, thermal properties and crystallization behavior of poly(lactic acid)/thermoplastic poly(propylene carbonate) polyurethane blends. RSC Adv. 2017, 7, 46183–46194. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Chen, F.; Wang, K.; Deng, H.; Zhang, Q.; Bai, H.W.; Fu, Q. A promising alternative to conventional polyethylene with poly(propylene carbonate) reinforced by graphene oxide nanosheets. J. Mater. Chem. 2011, 21, 17627–17630. [Google Scholar] [CrossRef]
- Bian, J.; Wei, X.W.; Lin, H.L.; Gong, S.J.; Zhang, H.; Guan, Z.P. Preparation and characterization of modified graphite oxide/poly(propylene carbonate) composites by solution intercalation. Polym. Degrad. Stab. 2011, 96, 1833–1840. [Google Scholar] [CrossRef]
- Shi, X.D.; Gan, Z.H. Preparation and characterization of poly(propylene carbonate)/montmorillonite nanocomposites by solution intercalation. Eur. Polym. J. 2007, 43, 4852–4858. [Google Scholar] [CrossRef]
- Zhai, L.P.; Li, G.F.; Xu, Y.; Xiao, M.; Wang, S.J.; Meng, Y.Z. Poly(propylene carbonate)/aluminum flake composite films with enhanced gas barrier properties. J. Appl. Polym. Sci. 2015, 132, 13–22. [Google Scholar] [CrossRef]
- Liao, J.G.; Li, Y.Q.; Zou, Q.; Duan, X.Z.; Yang, Z.P.; Xie, Y.F.; Liu, H.H. Preparation, characterization and properties of nano-hydroxyapatite/polypropylene carbonate biocomposite. Mat. Sci. Eng. C. 2016, 63, 285–291. [Google Scholar] [CrossRef]
- Chen, L.J.; Qin, Y.S.; Wang, X.H.; Zhao, X.J.; Wang, F.S. Plasticizing while toughening and reinforcing poly(propylene carbonate) using low molecular weight urethane: Role of hydrogen-bonding interaction. Polymer 2011, 52, 4873–4880. [Google Scholar] [CrossRef]
- Qin, Y.S.; Chen, L.J.; Wang, X.H.; Zhao, X.J.; Wang, F.S. Enhanced mechanical performance of poly(propylene carbonate) via hydrogen bonding interaction with o-lauroyl chitosan. Carbohydr. Polym. 2011, 84, 329–334. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, R.K.Y.; Cao, Y.X.; Meng, Y.Z. Essential work of fracture analysis for starch filled poly(propylene carbonate) composites. Mater. Des. 2007, 28, 1934–1939. [Google Scholar] [CrossRef]
- Qi, X.D.; Jing, M.F.; Liu, Z.W.; Dong, P.; Liu, T.Y.; Fu, Q. Microfibrillated cellulose reinforced bio-based poly(propylene carbonate) with dual-responsive shape memory properties. RSC Adv. 2016, 6, 7560–7567. [Google Scholar] [CrossRef]
- Hao, Y.P.; Ge, H.H.; Han, L.J.; Liang, H.Y.; Zhang, H.L.; Dong, L.S. Thermal, mechanical, and rheological properties of poly(propylene carbonate) cross-linked with polyaryl polymethylene isocyanate. Polym. Bull. 2013, 70, 1991–2003. [Google Scholar] [CrossRef]
- Qin, Y.S.; Ma, Q.W.; Wang, X.H.; Sun, J.Z.; Zhao, X.J.; Wang, F.S. Electron-beam irradiation on poly(propylene carbonate) in the presence of polyfunctional monomers. Polym. Degrad. Stab. 2007, 92, 1942–1947. [Google Scholar] [CrossRef]
- Xia, L.; Chen, L.B. Silicone modified poly(propylene carbonate). Polym. Mater. Sci. Eng. 2003, 19, 202–204. [Google Scholar]
- Song, P.F.; Mao, X.D.; Liu, X.J.; Ji, X.Q.; Zhang, X.F.; Wang, R.M. Study on synthesis and properties of terpolymers derived from carbon dioxide, propylene oxide and γ-glycidyloxypropyltrimethoxysilane. Mater. Rev. 2013, 27, 82–84. [Google Scholar]
- Okada, A.; Kikuchi, S.; Yamada, T. Alternating copolymerization of propylene oxide/alkylene oxide and carbon dioxide: Tuning thermal properties of polycarbonates. Chem. Lett. 2011, 40, 209–211. [Google Scholar] [CrossRef]
- Gao, L.J.; Feng, J.Y. A one-step strategy for thermally and mechanically reinforced pseudo-interpenetrating poly(propylene carbonate) networks by terpolymerization of CO2, propylene oxide and pyromellitic dianhydride. J. Mater. Chem. A. 2013, 1, 3556–3560. [Google Scholar] [CrossRef]
- Chen, X.G.; Wang, L.Y.; Feng, J.Y.; Huang, X.L.; Guo, X.Z.; Chen, J.; Xiao, Z.Y.; Liang, X.J.; Gao, L.J. Enhanced poly(propylene carbonate) with thermoplastic networks: A one-pot synthesis from carbon dioxide, propylene oxide, and a carboxylic dianhydride. Polymers 2018, 10, 552. [Google Scholar] [CrossRef] [PubMed]
- Song, P.F.; Mao, X.D.; Zhang, X.F.; Zhu, X.G.; Wang, R.M. A one-step strategy for cross-linkable aliphatic polycarbonates with high degradability derived from CO2, propylene oxide and itaconic anhydride. RSC Adv. 2014, 4, 9503–9508. [Google Scholar] [CrossRef]
- Tao, Y.H.; Wang, X.H.; Zhao, X.J.; Li, J.; Wang, F.S. Double propagation based on diepoxide, a facile route to high molecular weight poly(propylene carbonate). Polymer 2006, 47, 7368–7373. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Zhang, H.Y.; Ding, H.N.; Liu, B.Y.; Wang, X.H. One-pot synthesis and postpolymerization functionalization of cyclic carbonate/epoxide-difunctional polycarbonates prepared by regioselective diepoxide/CO2 copolymerization. Polym. Chem. 2016, 7, 4453–4457. [Google Scholar] [CrossRef]
- Hilf, J.; Scharfenberg, M.; Poon, J.; Moers, C.; Frey, H. Aliphatic polycarbonates based on carbon dioxide, furfuryl glycidyl ether, and glycidyl methyl ether: Reversible functionalization and cross-linking. Macromol. Rapid Commun. 2015, 36, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Nademi, F.; Zohuriaan-Mehr, M.J.; Rostami, A. An efficient fully bio-based reactive diluent for epoxy thermosets: 2-[(Oxiran-2-ylmethoxy) methyl] furan versus a petroleum-based counterpart. J. Appl. Polym. Sci. 2017, 134, 44957. [Google Scholar] [CrossRef]
- Ree, M.; Bae, J.Y.; Jung, J.H.; Shin, T.J. A new copolymerization process leading to poly(propylene carbonate) with a highly enhanced yield from carbon dioxide and propylene oxide. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 1863–1876. [Google Scholar] [CrossRef]
- Lee, N.J.; Kang, N.I.; Cho, W.J.; Kim, S.H.; Kang, K.T.; Theodorakis, E.A. Synthesis and biological activity of medium range molecular weight polymers containing exo-3,6-epoxy-1,2,3,6-tetrahydrophthalimidocaproic acid. Polym. Int. 2001, 50, 1010–1015. [Google Scholar] [CrossRef]
- Tian, Q.; Rong, M.Z.; Zhang, M.Q.; Yuan, Y.C. Synthesis and characterization of epoxy with improved thermal remendability based on Diels-Alder reaction. Polym. Int. 2010, 59, 1339–1345. [Google Scholar] [CrossRef]
- Gao, L.J.; Luo, Y.C.; Lin, Y.J.; Su, T.; Su, R.P.; Feng, J.Y. Silica-supported zinc glutarate catalyst synthesized by rheological phase reaction used in the copolymerization of carbon dioxide and propylene oxide. J. Polym. Res. 2015, 22, 220. [Google Scholar] [CrossRef]
- SDBSWeb. Available online: http://sdbs.db.aist.go.jp (accessed on 4 May 2019).
- Li, X.H.; Meng, Y.Z.; Zhu, Q.; Tjong, S.C. Thermal decomposition characteristics of poly(propylene carbonate) using TG/IR and Py-GC/MS techniques. Polym. Degrad. Stab. 2003, 81, 157–165. [Google Scholar] [CrossRef]
- Hou, J.Q.; Qiao, L.J.; Wei, J.; Hu, Y.X.; Wang, X.H.; Zhao, X.J.; Wang, F.S. Copolymerization of furfuryl glycidyl ether and carbon dioxide. Acta Polym. Sin. 2009, 10, 1007–1011. [Google Scholar] [CrossRef]




| Sample | Feed Molar Ratio of MA, FGE, and PO | Yield (g Copolymer/g ZnGA) | Mn (g/mol) | Mw/Mn | Gel (%) |
|---|---|---|---|---|---|
| PPC | 0:0:100 | 26.5 | 74503 | 4.5 | 0 |
| PPC-MF-1 | 1:1:100 | 45.4 | 87443 | 3.7 | 11.0 ± 0.8 |
| PPC-MF-2 | 2:2:100 | 47.3 | 84525 | 4.3 | 17.4 ± 1.1 |
| PPC-MF-3 | 3:3:100 | 50.8 | 85705 | 4.4 | 24.2 ± 1.4 |
| PPC-MF-4 | 4:4:100 | 56.5 | 99367 | 4.1 | 26.1 ± 1.5 |
| Sample | Tensile Strength/MPa | Elongation at Break/% |
|---|---|---|
| PPC | 11.0 ± 1.2 | 559 ± 13 |
| PPC-MF-1 | 6.6 ± 0.4 | 513 ± 9 |
| PPC-MF-2 | 6.8 ± 0.3 | 429 ± 6 |
| PPC-MF-3 | 6.8 ± 0.5 | 334 ± 4 |
| PPC-MF-4 | 8.3 ± 0.6 | 273 ± 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Chen, X.; Liang, X.; Guo, X.; Huang, X.; Chen, C.; Wan, X.; Deng, R.; Wu, Q.; Wang, L.; et al. A Novel One-Pot Synthesis of Poly(Propylene Carbonate) Containing Cross-Linked Networks by Copolymerization of Carbon Dioxide, Propylene Oxide, Maleic Anhydride, and Furfuryl Glycidyl Ether. Polymers 2019, 11, 881. https://doi.org/10.3390/polym11050881
Gao L, Chen X, Liang X, Guo X, Huang X, Chen C, Wan X, Deng R, Wu Q, Wang L, et al. A Novel One-Pot Synthesis of Poly(Propylene Carbonate) Containing Cross-Linked Networks by Copolymerization of Carbon Dioxide, Propylene Oxide, Maleic Anhydride, and Furfuryl Glycidyl Ether. Polymers. 2019; 11(5):881. https://doi.org/10.3390/polym11050881
Chicago/Turabian StyleGao, Lijun, Xianggen Chen, Xiangjun Liang, Xiuzhi Guo, Xianling Huang, Caifen Chen, Xiaodan Wan, Ruyu Deng, Qifeng Wu, Lingyun Wang, and et al. 2019. "A Novel One-Pot Synthesis of Poly(Propylene Carbonate) Containing Cross-Linked Networks by Copolymerization of Carbon Dioxide, Propylene Oxide, Maleic Anhydride, and Furfuryl Glycidyl Ether" Polymers 11, no. 5: 881. https://doi.org/10.3390/polym11050881